Abstract
The contribution of human genes to the variability of disease outcomes has been shown to be important across infectious diseases. Studies have shown mutations within specific human genes are associated with variable COVID-19 outcomes. We focused on the SARS-CoV-2 receptors/co-receptors to identify the role of specific polymorphisms within ACE2, TMPRSS2, NRP1 and CD147. Polymorphisms within ACE2 (rs2285666), TMPRSS2 (rs12329760), CD147 (rs8259) and NRP1 (rs10080) have been shown to associate with COVID-19 severity. Using cryopreserved samples from COVID-19-positive African, European and South Asian individuals within South Africa, we determined genotype frequencies. The genetic variant rs2285666 was associated with COVID-19 severity with an ethnic bias. African individuals with a CC genotype demonstrate more severe COVID-19 outcomes (OR = 7.5; 95% CI 1.164–80.89; p = 0.024) compared with those with a TT genotype. The expressions of ACE2 and SARS-CoV-2 viral load were measured using droplet digital PCR. Our results demonstrate rs2285666 and rs10080 were significantly associated with increased SARS-CoV-2 viral load and worse outcomes in certain ethnicities. This study demonstrates two important findings. Firstly, SARS-CoV-2 viral load is significantly lower in Africans compared with individuals of European and South Asian descent (p = 0.0002 and p < 0.0001). Secondly, SARS-CoV-2 viral load associates with specific SARS-CoV-2 receptor variants. A limited number of studies have examined the receptor/co-receptor genes within Africa. This study investigated genetic variants within the SARS-CoV-2 receptor/co-receptor genes and their association with COVID-19 severity and SARS-CoV-2 viral load across different ethnicities. We provide a genetic basis for differences in COVID-19 severity across ethnic groups in South Africa, further highlighting the importance of further investigation to determine potential therapeutic targets and to guide vaccination strategies that may prioritize specific genotypes.
1. Importance
This study addresses an important knowledge gap in the genetic understanding of COVID-19 severity in South Africa. We show that the frequency of genetic mutations within viral receptor genes is variable across different ethnic groups and may influence COVID-19 severity. More importantly, we show that SARS-CoV-2 viral load varies on the basis of genetic variants across ethnic groups in South Africa. This study highlights the importance for further investigation of the role of genetics in determining potential therapeutic targets and to guide COVID-19 vaccination strategies and the allocation of limited resources.
2. Introduction
Coronavirus disease 19 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Early in the COVID-19 pandemic, there was considerable concern over regions with a high burden of infectious diseases, such as Africa [1,2]. Furthermore, it was predicted that African regions would be impacted far more than European and American regions due to weaker healthcare infrastructure and socioeconomic factors [3]. As of 19 April 2023, the World Health Organization (WHO) has reported 7,584,874 confirmed cases and 178,597 deaths due to COVID-19 in Africa. The impact of COVID-19 in Africa has been relatively lower compared with Europe, the Americas and Asia, based on confirmed cases and excess deaths reported [4,5,6]. COVID-19 severity has been variable across populations globally. It has been shown that key factors, such as age, gender and underlying comorbidities are associated with COVID-19 severity and outcomes [7].
There is growing evidence that genetic factors also contribute to the severity of COVID-19 [8,9]. Disease severity has been categorized according to the symptoms experienced by COVID-19-positive individuals. A majority of individuals experience mild or moderate symptoms, while some advance to severe disease requiring clinical intervention and ultimately succumb to the disease [10]. It has been shown that differences in immunity, gene expression and genetic diversity contribute to differences in susceptibility and severity of COVID-19 [8]. Beyond this, genetic studies on infectious diseases, such as SARS-CoV-2, are important for understanding the dynamics of disease transmission and identifying risk factors that confer greater susceptibility to severe disease and worse outcomes. Furthermore, these studies may guide the development of vaccines, drug development strategies and public health planning for the prevention and control of future disease outbreaks [8,11,12].
Several studies have examined host genetic effects on COVID-19 disease susceptibility and severity, thus identifying varying effects on disease severity between populations globally [13,14,15,16]. The genetic factors associated with COVID-19 outcomes have been widely studied within the American, European and Asian continents. However, genetic studies to understand COVID-19 severity within the African continent have been limited [17,18]. This has contributed to the gap in understanding the impact of genetic diversity and unique genomic makeup of African populations on COVID-19, as well as many other infectious diseases that disproportionately affect African individuals [19,20]. Therefore, this study aims at investigating the effect of single-nucleotide polymorphisms (SNPs) within select SARS-CoV-2 human receptor/co-receptor genes and their association with COVID-19 severity within an African cohort.
SARS-CoV-2 infection and replication requires entry into human cells, primarily those within the respiratory system [21]. The SARS-CoV-2 spike protein subunit 1 (S1) interacts with the host receptors angiotensin-converting enzyme 2 (ACE2) to gain entry into cells [22,23,24,25]. This interaction is dependent on the proteolytic cleavage of the spike protein by transmembrane serine protease 2 (TMPRSS2) to release the S1 and S2 subunits [21,26,27]. In addition to these receptors, neuropilin-1 (NRP1) and Basigin (CD147) have been shown to facilitate the entry of SARS-CoV-2 independently of ACE2 and TMPRSS2 [28,29]. Polymorphisms within these genes may influence the ability of SARS-CoV-2 to enter the human cell and be determinants for the disease severity of COVID-19 [30]. This phenomenon has been shown previously with regard to HIV infection. Samson et al. (1996) showed that a mutation within the HIV receptor chemokine receptor 5 (CCR5) conferred protection against HIV infection [31]. The mutant allele within CCR5 results in the deletion of 32 nucleotides (CCR5-∆32), which results in the production of a truncated protein, hampering viral entry [32,33].
With regard to SARS-CoV-2, SNPs within the SARS-CoV-2 host receptor genes have demonstrated detrimental and protective effects across ethnic groups [34]. Within the ACE2 and TMPRSS2 genes, rs2285666 and rs12329760, respectively, have been identified to be associated with COVID-19 severity in some studies, while others have reported no association [35,36]. These SNPs were chosen based on data from an Egyptian cohort which showed that rs2285666 (ACE2) and rs12329760 (TMPRSS2) were significantly associated with the severity of COVID-19 and select co-morbidities [36].
With regard to CD147 and NRP1, the rs8259 and rs10080 SNPs were chosen because they are found within the 3′-UTR of their respective genes. Furthermore, these SNPs are found within predicted micro-RNA binding sites, with rs8259 (CD147) being a predicted binding point for miR-492 and rs10080 (NRP1) being a predicted binding point for miR-338 [30,37,38,39]. We recently reviewed 42 SNPs within ACE2 (12), TMPRSS2 (10), NRP1 (15) and CD147 (5) and found that rs2285666 (ACE2), rs12329769 (TMPRSS2), rs10080 (NRP1) and rs8259 (CD147) are significantly associated with COVID-19 co-morbidities within European and South Asian settings [9]. We selected these SNPs as a starting point to investigate their association with COVID-19 severity and susceptibility in an African setting.
In this study, we investigated the effect of SNPs within SARS-CoV-2 receptors and co-receptors on COVID-19 severity; furthermore, we compared the SARS-CoV-2 viral load across ethnic groups within South Africa.
3. Methods
3.1. Study Design
A cohort of SARS-CoV-2 polymerase chain reaction (PCR)-positive individuals were considered to participate in this study. SARS-CoV-2 positivity was determined using the TaqPath COVID-19 RT-PCR kit (Thermo Fisher Scientific, Waltham, MA, USA; cat no. A48102) and the QuantStudio 5 Real-Time PCR system (Applied Biosystems, Woburn, MA, USA), as per the manufacturer’s instructions.
A longitudinal cohort (SARS-CoV-2 Antibody Prevalence Study (SAP; n = 591)) of SARS-CoV-2-positive individuals were successfully recruited into this study, after signing the informed consent form. Only individuals with an RT-qPCR SARS-CoV-2-positive result were recruited. Buffy coat samples (n = 591) were collected 6 weeks, 3 months and 6 months post infection, between 2021 and 2022. However, only samples from the first time point (n = 560; 6 weeks post infection) were used in this study. All demographic and clinical data were collected after the study protocol, and consent forms were signed by each participant. All participant details and samples were collected with unique patient identifiers. The ethnic breakdown for this study was South African Black (n = 290), Indian (n = 246) and Caucasian (n = 24) individuals within South Africa. We scored each patient into two disease states (Table 1): no clinical presentations and those with clinical presentations, based on the presence of specific clinical presentations (including asymptomatic, symptomatic and oxygen required). Furthermore, we grouped ethnicity according to each of the two disease states (Table 2).

Table 1.
Stratification criteria used to score patients into no clinical presentations and clinical presentations groups.

Table 2.
The distribution of African, South Asian and European individuals with no clinical presentations and those with clinical presentations.
In addition to the buffy coat samples, SARS-CoV-2-positive nasopharyngeal swabs (n = 117) were obtained for an unbiased fraction of the total number of participants within this study.
Ethical approval for this study was obtained from the Biomedical Research Ethics Committee (BREC) at the University of KwaZulu-Natal, protocol reference number: BREC/00002648/2021.
3.2. SNPs Included in the Study
We identified rs2285666 (ACE2), rs12329760 (TMPRSS2), rs10080 (NRP1) and rs8259 (CD147). Table 3 shows the minor allele frequency (MAF) of the four SNPs within African, South Asian and European individuals included in this study. Using the Sample size Calculator, we determined the number of samples required [40]. Briefly, using the MAF frequency of the SNPs (Table 3), we determined the sample size for rs2285666 (ACE2 (n = 88)), rs12329760 (TMPRSS2), rs10080 (NRP1 (n = 62)) and rs8259 (CD147 (n = 146)), with α at 0.05 and the power determined at 80%. The calculated sample sizes are all below the number of samples recruited into the study per ethnic group (Table 2).

Table 3.
SNPs within ACE2, TMPRSS2, NRP1 and CD147 used in this study.
To investigate genetic variation within ACE2, TMPRSS2, NRP1 and CD147, DNA was extracted from 200 µL of buffy coat samples using the Quick-DNA Miniprep Plus Kit (Zymo, Irvine, CA, USA) as per the manufacturer’s instructions; the extracted DNA was standardized to 50 ng/µL and stored at −20 °C. The allelic examination of rs2285666, rs12329760, rs10080 and rs8259 was carried out using a Real-Time PCR (RT-PCR) protocol and TaqMan genotyping probes (Thermo Fisher Scientific, Waltham, MA, USA) on the QuantStudio 5 instrument as per manufacturer’s guidelines. Briefly, TaqMan Genotyping Master Mix (ThermoFisher Scientific) was prepared as a 5 µL reaction as per manufacturer guidelines, and RT-PCR was performed. RT-PCR cycling conditions and catalogue numbers for rs2285666 (C > T), rs12329760 (C > T), rs10080 (A > G) and rs8259 (T > A) are available on request.
3.3. ACE2, NRP1 and SARS-CoV-2 Viral Load Quantification
Nasopharyngeal swabs were collected and stored at 4 °C. RNA was extracted using the AmoyDx Virus/Cell RNA kit (Amoy Diagnostics, Xiamen, China) and then standardized to 20 ng/µL. Thereafter, cDNA was prepared using the Superscript VILO cDNA synthesis kit (ThermoFisher Scientific) according to manufacturer guidelines and then stored at −20 °C.
SARS-CoV-2 viral load was determined using the TaqPath COVID-19 RT-PCR kit and the QIAcuity Probe PCR kit (Qiagen, Hilden, Germany) on the QIAcuity Digital PCR instrument (QIAcuity Nanoplate 26K 24-well plate), as per the manufacturer’s specifications. Briefly, QIAcuity Probe master mix was prepared with the TaqPath COVID-19 RT-PCR kit probe and a 10 µL of sample cDNA for a total of 40 µL. SARS-CoV-2 viral load was determined and reported as copies/µL.
Gene expression of ACE2 and NRP1 was measured using QX200 ddPCR EvaGreen Supermix (Bio-Rad Laboratories, Hercules, CA, USA) on the QX200 droplet digital PCR (ddPCR) instrument, as per manufacturer’s instructions. Briefly, cDNA from nasopharyngeal swabs was added to EvaGreen master mix (total 40 µL) that was prepared according to manufacturer guidelines. Droplet generation was performed using the DG32 cartridge and gasket (Bio-Rad) set with 70 µL of droplet generation oil for EvaGreen (Bio-Rad). Primer sequences are available on request.
3.4. Statistical and Bioinformatics Analysis
GraphPad Prism 8 software was used for analysis. Mann–Whitney U test was used to compare continuous variables; categorical variables were analyzed using Fisher’s exact tests. A p-value of less than 0.05 was considered statistically significant.
4. Results
We found that a large proportion of African individuals present with no clinical presentations compared with European and South Asian individuals. In addition, South Asians account for the largest proportion of individuals with clinical presentations (Table 2).
4.1. Variants within SARS-CoV-2 Receptor Genes Are Associated with COVID-19 Severity across Ethnic Groups
We investigated the effect of SNPs within the SARS-CoV-2 receptor genes on COVID-19 severity in PCR-confirmed-positive COVID-19 individuals. The genotype frequency of each SNP was determined for each ethnic group across disease states (Supplementary Table S1). Owing to the lower sample number of European individuals (n = 24), we excluded European individuals from further genotype analysis.
We found different associations between individuals with no clinical and clinical presentations of COVID-19 across African and South Asian individuals for each polymorphism. The counts for genotype, odds ratio (OR), 95% confidence interval (CI) and p-values are summarized in Figure 1 below (Supplementary Table S2). For African individuals, the CC genotype compared with the TT genotype of rs2285666 is significantly detrimental towards clinical presentations of COVID-19 (OR = 7.5; 95% CI 1.164–80.89; p = 0.024, Figure 1A). However, this variant does not show significance within South Asians. Interestingly, we found the CC genotype of rs12329760 was significantly protective in African individuals (OR = 0.3134; 95% CI 0.1222–0.8045; p = 0.024 Figure 1A) but not in South Asian individuals.
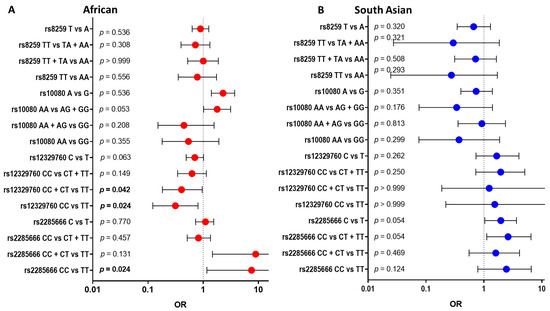
Figure 1.
Comparing COVID-19 disease states (no clinical presentations versus clinical presentations) within each of the four SNPs across African and South Asian individuals: The four SNPs included rs2285666 (ACE2), rs12329760 (TMPRSS2), rs10080 (NRP1) and rs8259 (CD147). An odds ratio (OR) of 1 is considered to have no effect (dotted line). Horizontal bars represent the 95% confidence intervals. Alleles or genotypes were used in the Fisher exact analysis, with significance accepted as p < 0.05. African individuals (A), red dots, showed rs2285666 and rs10080 as significant symptomatic effects. South Asians (B), blue dots, showed rs2285666 and rs12329760 as significant symptomatic effects.
4.2. SARS-CoV-2 Viral Load Is Different across Ethnic Groups
The variability of SARS-CoV-2 viral load between different ethnic groups has been shown previously [41]. This study examines SARS-CoV-2 viral loads in African, South Asian and European individuals within South Africa. African (mean Log VL = −0.4683) individuals have significantly lower mean SARS-CoV-2 viral loads compared with European (mean Log VL = 1.421) and South Asian (mean Log VL = −2.145) individuals, respectively (p = 0.0002 and p < 0.0001; Figure 2). However, we did not find a significant difference in SARS-CoV-2 viral load between South Asian and European individuals (p = 0.4010; Figure 2).
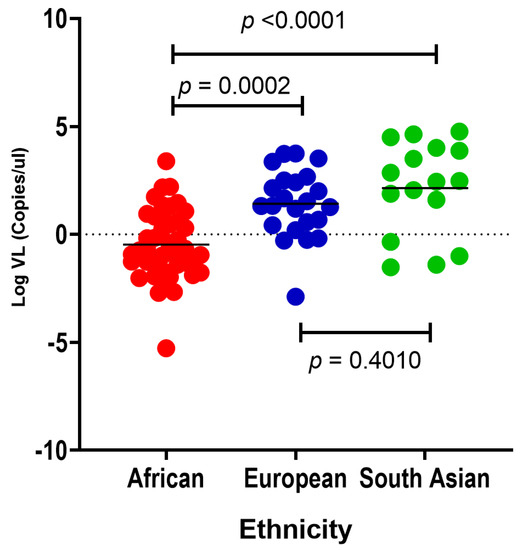
Figure 2.
A comparison of nasopharyngeal SARS-CoV-2 viral loads (copies/μL) in African (red dots, n = 43), European (blue dots, n = 23) and South Asian (green dots, n = 16) individuals. Turkey’s multiple comparisons test p-values show significant differences between the mean viral loads between African, European and South Asian individuals. The horizontal bars show the p-values for the ethnic groups being compared. African individuals have significantly lower SARS-CoV-2 viral load compared with European (p = 0.0002) and South Asian (p < 0.0001) individuals.
4.3. SARS-CoV-2 Viral Load Associates with rs2285666 (ACE2) and rs12329760 (TMPRSS2) in African Individuals
We next examined the effect of variants within the SARS-CoV-2 receptors on viral load in African individuals. To our knowledge, this is the first study to show the association of the ACE2 SNP rs2285666 and the TMPRSS2 SNP rs12329760 with SARS-CoV-2 viral load in African individuals. The number of patients within the South Asian group was too low to conduct any statistical analysis (Supplementary Figure S1). The CC genotype of rs2285666 is associated with significantly higher viral load compared with the CT + TT genotypes (p = 0.0136; Figure 3A). In addition, the CC genotype of rs12329760 is significantly associated with lower viral load compared with the CT + TT genotypes (p = 0.0361; Figure 3B). On the contrary, we did not find an association between rs10080 and rs8259 with SARS-CoV-2 viral load (p = 0.0725 and p = 0.6417; Figure 3C,D).
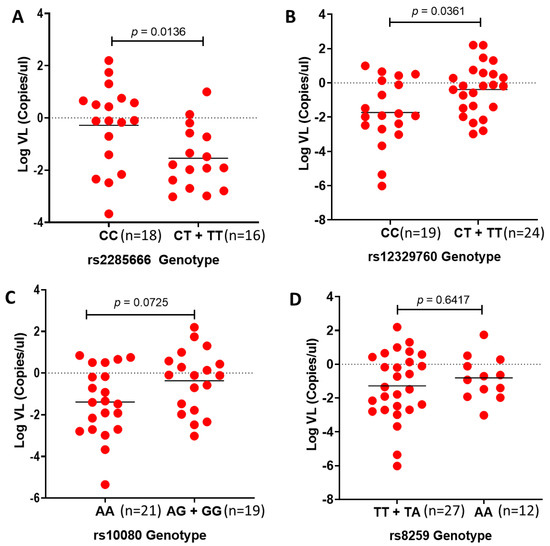
Figure 3.
Comparing SARS-CoV-2 viral loads versus rs2285666, rs12327960, rs10080 and rs8259 genotypes in African individuals: Viral loads are correlated with (A) rs2285666 and (B) rs12329760 but not (C) rs10080 and (D) rs8259 in African individuals. Mann–Whitney test p-value of comparison between homozygous and alternative genotypes.
4.4. ACE2 and NRP1 Expression and SARS-CoV-2 Viral Load in African Individuals
Due to sample limitations, the measurement of mRNA expression was prioritized to ACE2 and NRP1, since these genes showed significance and they serve as receptors to the virus and not co-receptors. ACE2 is known as the key mediator of SARS-CoV-2 infection. Studies have shown that the binding affinity of spike glycoprotein to ACE2 is 20-fold higher than that of its predecessor, SARS-CoV [42]. Furthermore, due to the association of rs2285666 and SARS-CoV-2 viral load in African individuals, we investigated the effect of ACE2 expression on SARS-CoV-2 viral load in nasopharyngeal swabs at the time of infection. Interestingly, we found that ACE2 negatively correlates with SARS-CoV-2 viral load (r = −0.3488; p = 0.0020; Figure 4A) across ethnic groups. In addition, we found no correlation between ACE2 copies and rs2285666 (Figure 4B). Lastly, we found no significant correlation between NRP1 expression and SARS-CoV-2 viral load (r = −0.0587; p = 0.6529; Figure 4C). In addition, NRP1 expression trends with rs10080 genotypes across South African individuals; however, these results were not significant (AA vs. GG; p = 0.3691; Figure 4D).
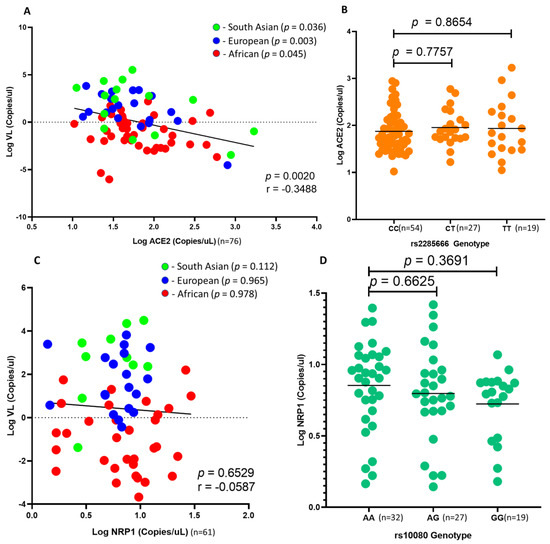
Figure 4.
Comparing SARS-CoV-2 viral load with ACE2 and NRP1 gene expression. A comparison between ACE2 and NRP1 gene expression against rs2285666 and rs10080 genotypes in South African individuals: (A) There is a significant linear correlation of SARS-CoV-2 viral load with ACE2 expression across African (red dots), European (blue dots) and South Asian (green dots) individuals (p = 0.045, p = 0.003 and p = 0.036). However, ACE2 expression does not correlate with rs2285666 genotypes (B). SARS-CoV-2 viral load does not correlate with NRP1 expression (C); in addition, NRP1 expression does not associate with rs10080 genotypes (D) in South African individuals.
5. Discussion
This study aimed to investigate the effect of SNPs with SARS-CoV-2 receptors and co-receptors on COVID-19 severity; furthermore, we compared SARS-CoV-2 viral load across ethnic groups within Durban, South Africa. To our knowledge, this is the first study to evaluate SARS-CoV-2 receptor variants and viral load within a South African setting. We observed that specific variants (rs2285666 and rs10080) are significantly associated with increased SARS-CoV-2 viral load and worse outcomes in certain ethnicities. This study identified two significant findings. Firstly, we identified that Africans have significantly lower SARS-CoV-2 viral loads compared with European and South Asian individuals. Secondly, SARS-CoV-2 viral load correlates with specific SARS-CoV-2 receptor variants in African individuals.
Previous studies have highlighted differences in SARS-CoV-2 viral loads across different ethnic groups. Peterson et al. (2021) showed that average viral loads (lower cyclic thresholds/numbers) were lower in African Americans compared with Caucasian American and Asian individuals [41]. In line with previous reports, we show that SARS-CoV-2 viral load is significantly lower in Africans compared with European and South Asian individuals, respectively. The difference in viral load between these ethnic groups may be explained by genetic variants with the SARS-CoV-2 host receptor genes (ACE2, TMPRSS2, NRP1 and CD147).
Several studies have investigated the association of rs2285666 (ACE2) and rs12329760 (TMPRSS2) with COVID-19 severity [35,36,43,44,45]. Interestingly, these studies have either confirmed [13,36,46,47,48,49,50,51] or contradicted [52,53,54,55] the association between rs2285666 and/or rs12329760 with COVID-19 severity. These differing observations have been attributed to insufficient sample sizes and variation in study designs; however, ethnic variation between cohorts and study populations can also explain these conflicting results [56].
In agreement with previous data, we found that the CC genotype of rs2285666 is significantly associated with clinical presentations of COVID-19 among individuals of African descent. Consistent with these results, we show that the CC genotype of rs2285666 is significantly associated with higher SARS-CoV-2 viral load compared with the CT + TT genotypes in individuals of African descent (p = 0.0136), further supporting the relationship between rs2285666 (CC) and severe disease. In contrast, we found no significant association between rs2285666 (CC vs. TT) and clinical presentations of COVID-19 severity for individuals of South Asian descent.
Several studies have investigated the TMPRSS2 variant (rs12329760) and COVID-19 severity across different Asian and European groups [13,46,47,48,50,54,57,58,59,60,61]. In this study, we found a significant association between the CC genotype of rs12327960 and no clinical presentations of COVID-19 in African individuals. Furthermore, we showed that the CC genotype is significantly correlated with lower SARS-CoV-2 viral loads in Africans. These findings are consistent with studies that confirm the association between rs12329760 and COVID-19 severity [36,52]. Despite the frequency of the mutant and wild-type alleles being similar across African, European and South Asian individuals, we found no association between rs12327960 and COVID-19 in South Asian individuals. We show that the CC genotype of rs12329760 has no association with COVID-19 clinical presentations in South Asian individuals (Supplementary Table S2). We attribute these observations to the small number of South Asian infected individuals with no clinical presentations (n = 26) within this study compared with the large majority who had clinical presentations of COVID-19 (n = 220).
Variants within ACE2 and TMPRSS2 have been extensively studied regarding COVID-19 severity and susceptibility. Despite subsequent studies identifying NRP1 and CD147 as additional host cell receptors for SARS-CoV-2 entry [28,29], few studies have investigated the effect of variants within these genes and COVID-19 severity [29,62,63]. As such, this study is the first to investigate the relationship between variants within the NRP1 and CD147 genes and COVID-19 severity. Our data show that the AA genotype of rs10080, a 3′-UTR variant within NRP1, is associated with clinical presentations of COVID-19 in African individuals (Supplementary Table S2) compared with the AG + GG genotypes. In addition, we show that the A allele is significantly associated with clinical presentations of COVID-19 compared with the G allele (p = 0.001). Furthermore, we show that SARS-CoV-2 viral loads are lower for African individuals with the AA genotype than the AG + GG genotypes. Our investigation of the rs8259 (CD147) showed no association with COVID-19 severity and viral load across ethnic groups. This finding is consistent with recent meta-analysis data [64].
We also investigated the difference in gene expression of ACE2 and NRP1 among South African individuals. Our data showed no significant difference in gene expression of ACE2 and NRP1 across African, European and South Asian individuals (Supplementary Figure S2). We further investigated the relationship between SARS-CoV-2 viral load and ACE2 and NRP1 expression using ddPCR (as copies/μL). We found no significant difference in ACE2 and NRP1 expression across African, European and South Asian individuals (Supplementary Figure S3). We showed that NRP1 expression is lower in individuals that are homozygous GG compared with homozygous AA (Supplementary Figure S1B). In addition, NRP1 expression is negatively correlated with SARS-CoV-2 viral load. It is known that rs10080 is within the 3′-UTR of NRP1, and it has been reported that the G allele facilitates the binding of miR-338, thus downregulating the expression of NRP1 [30,37,38]. Further studies are required to validate the epigenetic effect of miR-338 on rs10080 regulation of NRP1 expression.
Previous studies report a significant positive correlation between transmembrane ACE2 expression and SARS-CoV-2 viral load in nasopharyngeal swabs [65,66]. However, Nikiforuk et al. showed that (2021) nasopharyngeal expression of ACE2 plays a dual role in SARS-CoV-2-infected individuals [65]. The study showed that the transmembrane isoform of ACE2 is positively correlated, while the soluble isoforms of ACE2 are negatively correlated with viral RNA load after adjusting for age, biological sex and TMPRSS2 mRNA. In line with these findings, we observed a significant negative correlation between SARS-CoV-2 viral loads and soluble ACE2 expression in nasopharyngeal swabs. In addition, Gutierrez-Chamorro et al. (2021) showed that ACE2 enzymatic activity and gene expression decreased over time. Furthermore, Fajnzylber et al. (2020) and To et al. (2020) showed that SARS-CoV-2 viral loads decreased post-symptom onset across different tissues [67,68]. Within South Africa, the delay in testing for COVID-19 may influence our results.
These data are not unexpected due to the use of human recombinant soluble ACE2 (hrsACE2) for the treatment of COVID-19. A recent case report showed that hrsACE2 was able to reduce SARS-CoV-2 load by a factor of 1000–5000 in in vitro experiments [69]. It is well established that ACE2 is a target of regulated intramembrane proteolysis (RIP) by ADAM17, thus releasing a soluble ACE2 C-terminal [70,71].
The difference in viral load between these ethnic groups cannot be explained by the specific genetic variants examined here with ACE2, TMPRSRS2, NRP1 and CD147 genes alone. We suggest that differences in immunity, genetics and epigenetics contribute to the observed differences. For instance, the delta-32 mutation within CCR5 confers protection against HIV infection within Caucasian individuals because the mutation is found at a much higher frequency than in Africans and South Asians. In addition, we have shown that DNA methylation of BST-2 affects BST-2 expression. The study showed that BST-2 levels inhibit the production of HIV-1 by hindering the release of viral progeny [72]. Therefore, this study provides an understanding of polymorphisms within the SARS-CoV-2 receptors and co-receptors within a South African cohort.
A core limitation of this study is the low sample size. In addition, the distribution of age within the cohort is skewed. This study only considers individuals older than 18 years of age; furthermore, a large number of individuals within this cohort are middle-aged. Moreover, we note that host gene expression does not necessarily reflect post-transcriptional regulation. However, it has been shown that ACE2 transcript expression does reflect protein expression in upper airway tissues [73].
For future studies, we suggest more collaborative work within Africa to fully understand the genetic contribution to SARS-CoV-2 disease severity. This study shows that South Africa provides a diverse setting for host genetic studies. In addition, we support the use of genetic variant testing as a model for infectious disease prioritization. Bubar et al. (2021) suggested the use of a strategy using age and serostatus to prioritize vaccine roll-out and prioritization [74]. We suggest a point-of-care strategy using genotypic data to prioritize vaccination for individuals with genetic variants which are associated with increased susceptibility and severity of disease. This strategy will reduce the burden on the availability of vaccines within resource-limited areas. This strategy has been recently suggested by Bruce and Johnson (2022), who showed that individuals with genetic risk factors should be prioritized for vaccination drives [75].
6. Conclusions
Despite early predictions that the COVID-19 pandemic would affect African countries far worse than other well-resourced countries due to the lack of healthcare infrastructure and the high level of poverty, Africa was not as severely affected by the pandemic compared with other European and Asian settings based on reported data. In this study, we show that SARS-CoV-2 viral loads are significantly different across ethnic groups in South Africa. We found that Black African individuals had significantly lower viral loads compared with European and South Asian individuals. In addition, we showed that SNPs within ACE2, TMPRSS2, NRP1 and CD147 have variable associations with COVID-19 severity across these ethnic groups. This study provides strong evidence that the severity of COVID-19 is dependent on host genetic factors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14091798/s1, Figure S1: SARS-CoV-2 viral load and Genotypes in South Asian individuals; Figure S2: The expression of (A) ACE2 and (B) NRP1 across ethnic groups in South Africa; Figure S3: Linear regression comparison of ACE2 expression and SARS-CoV-2 viral load against age. (A) Nasopharyngeal ACE2 expression negatively correlates with age (p = 0.2079), while (B) SARS-CoV-2 viral load positively correlates with age (p = 0.0916). However, both comparisons are not significant; Table S1: Genotype frequencies of ACE2, TMPRSS2, NRP1 and CD147 SNPs in patients with and without clinical presentations across ethnic groups; Table S2: A summary of the comparison of COVID-19 disease states (No clinical presentations versus clinical presentations) within each of the four SNPs across African and South Asian individuals.
Author Contributions
Conceptualization, V.R.; Data curation, A.N.; Writing—original draft, T.A. (Theolan Adimulam); Writing—review & editing, T.A. (Thilona Arumugam) and K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was supported by the South African Medical Research Council with funds received from the South African Department of Science and Technology. V.R. was funded as a FLAIR Research Fellow (the Future Leader in African Independent Research (FLAIR)) Fellowship Programme, which was a partnership between the African Academy of Sciences (AAS) and the Royal Society that was funded by the UK Government as part of the Global Challenge Research Fund (GCRF) [Grant # FLAIR-FLR\R1\190204] supported by the South African Medical Research Council (SAMRC) with funds from the Department of Science and Technology (DST); and V.R. was also supported in part through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative (Grant # DEL-15-006) by the AAS. This is supported by The South African Medical Research Council through the Self-initiated Research Grant. T.A. (Thilona Arumugam) is funded by South African Medical Research Council Self-Initiated Research Grant and L’ORÉAL UNESCO for Woman in Science South African Young Talent fellow. T.A. (Theolan Adimulam) is funded by the Poliomyelitis Research Foundation (PRF) Bursary. A.N. is supported by K43TW011437 and the National Institutes of Health, National Institutes of Allergy and Infectious Disease (NIAID) and the South African Medical Research Council through R01AI152142-01.
Institutional Review Board Statement
Ethical approval for this study was obtained from the Biomedical Research Ethics Committee (BREC) at the University of KwaZulu-Natal, protocol reference number: BREC/00002648/2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We would like to thank Tevan Naidoo and Anmol Gokul for their assistance with the curation of the SAP cohort.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moore, M.; Gelfeld, B.; Okunogbe, A.; Paul, C. Identifying Future Disease Hot Spots: Infectious Disease Vulnerability Index. Rand Health Q 2017, 6, 5. [Google Scholar] [PubMed]
- Lone, S.A.; Ahmad, A. COVID-19 pandemic—An African perspective. Emerg. Microbes Infect 2020, 9, 1300–1308. [Google Scholar] [CrossRef]
- Massinga Loembé, M.; Tshangela, A.; Salyer, S.J.; Varma, J.K.; Ouma, A.E.O.; Nkengasong, J.N. COVID-19 in Africa: The spread and response. Nat. Med. 2020, 26, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Farhaan, S.V.; Juan Carlos, N.; Jennifer, R.M.; Osman, K.; Alan, P.; Stephen, L.J.; Faisal, M.; Sostman, H.D.; Robert, P.; Julia, D.A.; et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: Analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open 2020, 10, e039849. [Google Scholar]
- Robertson, M.M.; Shamsunder, M.G.; Brazier, E.; Mantravadi, M.; Zimba, R.; Rane, M.S.; Westmoreland, D.A.; Parcesepe, A.M.; Maroko, A.R.; Kulkarni, S.G.; et al. Racial/Ethnic Disparities in Exposure, Disease Susceptibility, and Clinical Outcomes during COVID-19 Pandemic in National Cohort of Adults, United States. Emerg. Infect. Dis. 2022, 28, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Rentsch, C.T.; Morton, C.E.; Hulme, W.J.; Schultze, A.; MacKenna, B.; Eggo, R.M.; Bhaskaran, K.; Wong, A.Y.S.; Williamson, E.J.; et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: An observational cohort study using the OpenSAFELY platform. Lancet 2021, 397, 1711–1724. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Kwok, A.J.; Mentzer, A.; Knight, J.C. Host genetics and infectious disease: New tools, insights and translational opportunities. Nat. Rev. Genet. 2021, 22, 137–153. [Google Scholar] [CrossRef]
- Adimulam, T.; Arumugam, T.; Gokul, A.; Ramsuran, V. Genetic Variants within SARS-CoV-2 Human Receptor Genes May Contribute to Variable Disease Outcomes in Different Ethnicities. Int. J. Mol. Sci. 2023, 24, 8711. [Google Scholar] [CrossRef]
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 20 April 2023).
- Doeschl-Wilson, A.B.; Davidson, R.; Conington, J.; Roughsedge, T.; Hutchings, M.R.; Villanueva, B. Implications of host genetic variation on the risk and prevalence of infectious diseases transmitted through the environment. Genetics 2011, 188, 683–693. [Google Scholar] [CrossRef]
- Frodsham, A.J.; Hill, A.V.S. Genetics of infectious diseases. Hum. Mol. Genet. 2004, 13 (Suppl. S2), R187–R194. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhao, J.; Martin, W.; Kallianpur, A.; Chung, M.K.; Jehi, L.; Sharifi, N.; Erzurum, S.; Eng, C.; Cheng, F. New insights into genetic susceptibility of COVID-19: An ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020, 18, 216. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, H.; Edahiro, R.; Takano, T.; Nishihara, H.; Shirai, Y.; Sonehara, K.; Tanaka, H.; Azekawa, S.; Mikami, Y.; Lee, H.; et al. DOCK2 is involved in the host genetics and biology of severe COVID-19. Nature 2022, 609, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Diz-de Almeida, S.; López de Heredia, M.; Quintela, I.; Ceballos, F.C.; Pita, G.; Lorenzo-Salazar, J.M.; González-Montelongo, R.; Gago-Domínguez, M.; Sevilla Porras, M.; et al. Novel genes and sex differences in COVID-19 severity. Hum. Mol. Genet. 2022, 31, 3789–3806. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Gkizarioti, Z.; Patrinos, G.P.; Tsakris, A. Human genetic factors associated with susceptibility to SARS-CoV-2 infection and COVID-19 disease severity. Hum. Genom. 2020, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, D.; Bush, S.; Bannerman, R.; Downs, P.; Shu’aibu, J.; Boko-Collins, P.; Radvan, I.; Wohlgemuth, L.; Boyton, C. Neglected tropical diseases activities in Africa in the COVID-19 era: The need for a “hybrid” approach in COVID-endemic times. Infect. Dis. Poverty 2021, 10, 1. [Google Scholar] [CrossRef]
- Molyneux, D.H.; Aboe, A.; Isiyaku, S.; Bush, S. COVID-19 and neglected tropical diseases in Africa: Impacts, interactions, consequences. Int. Health 2020, 12, 367–372. [Google Scholar] [CrossRef]
- Campbell, M.C.; Tishkoff, S.A. African genetic diversity: Implications for human demographic history, modern human origins, and complex disease mapping. Annu. Rev. Genom. Hum. Genet. 2008, 9, 403–433. [Google Scholar] [CrossRef]
- Winkler, C. Is there a genetic basis for health disparities in human immunodeficiency virus disease? Mt. Sinai J. Med. 2010, 77, 149–159. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The major cell entry receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Samavati, L.; Uhal, B.D. ACE2, much more than just a receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020, 10, 317. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Mayi, B.S.; Leibowitz, J.A.; Woods, A.T.; Ammon, K.A.; Liu, A.E.; Raja, A. The role of Neuropilin-1 in COVID-19. PLoS Pathog. 2021, 17, e1009153. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.-Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.-X.; Gong, L. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Hashemi, S.M.A.; Thijssen, M.; Hosseini, S.Y.; Tabarraei, A.; Pourkarim, M.R.; Sarvari, J. Human gene polymorphisms and their possible impact on the clinical outcome of SARS-CoV-2 infection. Arch. Virol. 2021, 166, 2089–2108. [Google Scholar] [CrossRef]
- Samson, M.; Libert, F.; Doranz, B.J.; Rucker, J.; Liesnard, C.; Farber, C.-M.; Saragosti, S.; Lapouméroulie, C.; Cognaux, J.; Forceille, C. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 1996, 382, 722–725. [Google Scholar] [CrossRef]
- de Silva, E.; Stumpf, M.P.H. HIV and the CCR5-Δ32 resistance allele. FEMS Microbiol. Lett. 2004, 241, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martinson, J.J.; Chapman, N.H.; Rees, D.C.; Liu, Y.-T.; Clegg, J.B. Global distribution of the CCR5 gene 32-basepair deletion. Nat. Genet. 1997, 16, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikova, I.G.; Haralambieva, I.H.; Crooke, S.N.; Poland, G.A.; Kennedy, R.B. The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunol. Rev. 2020, 296, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Arendt-Nielsen, L.; Díaz-Gil, G.; Gómez-Esquer, F.; Gil-Crujera, A.; Gómez-Sánchez, S.M.; Ambite-Quesada, S.; Palomar-Gallego, M.A.; Pellicer-Valero, O.J.; Giordano, R. Genetic Association between ACE2 (rs2285666 and rs2074192) and TMPRSS2 (rs12329760 and rs2070788) Polymorphisms with Post-COVID Symptoms in Previously Hospitalized COVID-19 Survivors. Genes 2022, 13, 1935. [Google Scholar] [CrossRef]
- Abdelsattar, S.; Kasemy, Z.A.; Ewida, S.F.; Abo-Elsoud, R.A.A.; Zytoon, A.A.; Abdelaal, G.A.; Abdelgawad, A.S.; Khalil, F.O.; Kamel, H.F.M. ACE2 and TMPRSS2 SNPs as Determinants of Susceptibility to, and Severity of, a COVID-19 Infection. Br. J. Biomed. Sci. 2022, 79, 10238. [Google Scholar] [CrossRef]
- Fan, S.-H.; Shen, Z.-Y.; Xiao, Y.-M. Functional polymorphisms of the neuropilin 1 gene are associated with the risk of tetralogy of Fallot in a Chinese Han population. Gene 2018, 653, 72–79. [Google Scholar] [CrossRef]
- Agúndez, J.A.; García-Martín, E.; Martínez, C.; Benito-León, J.; Millán-Pascual, J.; Díaz-Sánchez, M.; Calleja, P.; Pisa, D.; Turpín-Fenoll, L.; Alonso-Navarro, H.; et al. Heme Oxygenase-1 and 2 Common Genetic Variants and Risk for Multiple Sclerosis. Sci. Rep. 2016, 6, 20830. [Google Scholar] [CrossRef]
- Wu, L.-S.; Li, F.-F.; Sun, L.-D.; Li, D.; Su, J.; Kuang, Y.-H.; Chen, G.; Chen, X.-P.; Chen, X. A miRNA-492 binding-site polymorphism in BSG (basigin) confers risk to psoriasis in central south Chinese population. Hum. Genet. 2011, 130, 749–757. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics, 7th, ed.; Brooks/Cole: Boston, MA, USA, 2011. [Google Scholar]
- Petersen, J.; Jhala, D. Ethnicity, Comorbid Medical Conditions, and SARS-CoV-2 Test Cycle Thresholds in the Veteran Population. J. Racial Ethn. Health Disparities 2021, 9, 1775–1782. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Najafi, M.; Mahdavi, M.R. Association investigations between ACE1 and ACE2 polymorphisms and severity of COVID-19 disease. Mol. Genet. Genom. 2023, 298, 27–36. [Google Scholar] [CrossRef]
- Beyranvand, S.; Davoodian, P.; Alizade, H.; Gouklani, H.; Nejatizadeh, A.; Eftekhar, E.; Nikpoor, A.R. Study of frequency and inheritance model of ACE1 I/D and ACE2 rs2285666 polymorphisms in COVID-19 patients with varying severity of lung involvement and its effect on serum cytokines levels. Cell Biol. Int. 2023, 47, 731–741. [Google Scholar] [CrossRef]
- Keikha, M. and M. Karbalaei, Global distribution of ACE1 (rs4646994) and ACE2 (rs2285666) polymorphisms associated with COVID-19: A systematic review and meta-analysis. Microb. Pathog. 2022, 172, 105781. [Google Scholar] [CrossRef]
- Latini, A.; Agolini, E.; Novelli, A.; Borgiani, P.; Giannini, R.; Gravina, P.; Smarrazzo, A.; Dauri, M.; Andreoni, M.; Rogliani, P.; et al. COVID-19 and Genetic Variants of Protein Involved in the SARS-CoV-2 Entry into the Host Cells. Genes 2020, 11, 1010. [Google Scholar] [CrossRef]
- Singh, H.; Choudhari, R.; Nema, V.; Khan, A.A. ACE2 and TMPRSS2 polymorphisms in various diseases with special reference to its impact on COVID-19 disease. Microb. Pathog. 2021, 150, 104621. [Google Scholar] [CrossRef]
- Dos Santos Nascimento, I.J.; da Silva-Júnior, E.F.; de Aquino, T.M. Molecular Modeling Targeting Transmembrane Serine Protease 2 (TMPRSS2) as an Alternative Drug Target Against Coronaviruses. Curr. Drug Targets 2022, 23, 240–259. [Google Scholar] [CrossRef]
- Khalilzadeh, F.; Sakhaee, F.; Sotoodehnejadnematalahi, F.; Zamani, M.S.; Ahmadi, I.; Anvari, E.; Fateh, A. Angiotensin-converting enzyme 2 rs2285666 polymorphism and clinical parameters as the determinants of COVID-19 severity in Iranian population. Int. J. Immunogenet. 2022, 49, 325–332. [Google Scholar] [CrossRef]
- Beheshti Shirazi, S.S.; Sakhaee, F.; Sotoodehnejadnematalahi, F.; Zamani, M.S.; Ahmadi, I.; Anvari, E.; Fateh, A. rs12329760 Polymorphism in Transmembrane Serine Protease 2 Gene and Risk of Coronavirus Disease 2019 Mortality. Biomed. Res. Int. 2022, 2022, 7841969. [Google Scholar] [CrossRef]
- Sabater Molina, M.; Nicolás Rocamora, E.; Bendicho, A.I.; Vázquez, E.G.; Zorio, E.; Rodriguez, F.D.; Gil Ortuño, C.; Rodríguez, A.I.; Sánchez-López, A.J.; Jara Rubio, R. Polymorphisms in ACE, ACE2, AGTR1 genes and severity of COVID-19 disease. PLoS ONE 2022, 17, e0263140. [Google Scholar] [CrossRef]
- Wulandari, L.; Hamidah, B.; Pakpahan, C.; Damayanti, N.S.; Kurniati, N.D.; Adiatmaja, C.O.; Wigianita, M.R.; Soedarsono; Husada, D.; Tinduh, D.; et al. Initial study on TMPRSS2 p.Val160Met genetic variant in COVID-19 patients. Hum. Genom. 2021, 15, 29. [Google Scholar] [CrossRef]
- Torre-Fuentes, L.; Matías-Guiu, J.; Hernández-Lorenzo, L.; Montero-Escribano, P.; Pytel, V.; Porta-Etessam, J.; Gómez-Pinedo, U.; Matías-Guiu, J.A. ACE2, TMPRSS2, and Furin variants and SARS-CoV-2 infection in Madrid, Spain. J. Med. Virol. 2021, 93, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Schönfelder, K.; Breuckmann, K.; Elsner, C.; Dittmer, U.; Fistera, D.; Herbstreit, F.; Risse, J.; Schmidt, K.; Sutharsan, S.; Taube, C.; et al. Transmembrane serine protease 2 Polymorphisms and Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection: A German Case-Control Study. Front. Genet. 2021, 12, 667231. [Google Scholar] [CrossRef] [PubMed]
- Karakaş Çelik, S.; Çakmak Genç, G.; Pişkin, N.; Açikgöz, B.; Altinsoy, B.; Kurucu İşsiz, B.; Dursun, A. Polymorphisms of ACE (I/D) and ACE2 receptor gene (Rs2106809, Rs2285666) are not related to the clinical course of COVID-19: A case study. J. Med. Virol. 2021, 93, 5947–5952. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Pandey, R.K.; Singh, P.P.; Kumar, P.; Rasalkar, A.A.; Tamang, R.; van Driem, G.; Shrivastava, P.; Chaubey, G. Most frequent South Asian haplotypes of ACE2 share identity by descent with East Eurasian populations. PLoS ONE 2020, 15, e0238255. [Google Scholar] [CrossRef] [PubMed]
- Nhung, V.P.; Ton, N.D.; Ngoc, T.T.B.; Thuong, M.T.H.; Hai, N.T.T.; Oanh, K.T.P.; Hien, L.T.T.; Thach, P.N.; Hai, N.V.; Ha, N.H. Host Genetic Risk Factors Associated with COVID-19 Susceptibility and Severity in Vietnamese. Genes 2022, 13, 1884. [Google Scholar] [CrossRef]
- Sekiya, T.; Ogura, Y.; Kai, H.; Kawaguchi, A.; Okawa, S.; Hirohama, M.; Kuroki, T.; Morii, W.; Hara, A.; Hiramatsu, Y.; et al. TMPRSS2 gene polymorphism common in East Asians confers decreased COVID-19 susceptibility. Front. Microbiol. 2022, 13, 943877. [Google Scholar] [CrossRef]
- Yaghoobi, A.; Lord, J.S.; Rezaiezadeh, J.S.; Yekaninejad, M.S.; Amini, M.; Izadi, P. TMPRSS2 polymorphism (rs12329760) and the severity of the COVID-19 in Iranian population. PLoS ONE 2023, 18, e0281750. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; Fragoso, J.M.; Sánchez-Muñoz, F.; Rojas-Velasco, G.; Ramírez-Bello, J.; López-Reyes, A.; Martínez-Gómez, L.E.; Sierra-Fernández, C.; Rodríguez-Reyna, T.; Regino-Zamarripa, N.E.; et al. Association of the Transmembrane Serine Protease-2 (TMPRSS2) Polymorphisms with COVID-19. Viruses 2022, 14, 1976. [Google Scholar] [CrossRef]
- Senapati, S.; Kumar, S.; Singh, A.K.; Banerjee, P.; Bhagavatula, S. Assessment of risk conferred by coding and regulatory variations of TMPRSS2 and CD26 in susceptibility to SARS-CoV-2 infection in human. J. Genet. 2020, 99, 53. [Google Scholar] [CrossRef]
- Chapoval, S.; Keegan, A.D. Perspectives and potential approaches for targeting neuropilin 1 in SARS-CoV-2 infection. Mol. Med. 2021, 27, 162. [Google Scholar] [CrossRef]
- Fenizia, C.; Galbiati, S.; Vanetti, C.; Vago, R.; Clerici, M.; Tacchetti, C.; Daniele, T. SARS-CoV-2 Entry: At the Crossroads of CD147 and ACE2. Cells 2021, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Kaidashev, I.; Izmailova, O.; Shlykova, O.; Kabaliei, A.; Vatsenko, A.; Ivashchenko, D.; Dudchenko, M.; Volianskyi, A.; Zelinskyy, G.; Koval, T.; et al. Polymorphism of tmprss2 (rs12329760) but not ace2 (rs4240157), tmprss11a (rs353163) and cd147 (rs8259) is associated with the severity of COVID-19 in the Ukrainian population. Acta Biomed. 2023, 94, e2023030. [Google Scholar] [PubMed]
- Nikiforuk, A.M.; Kuchinski, K.S.; Twa, D.D.W.; Lukac, C.D.; Sbihi, H.; Basham, C.A.; Steidl, C.; Prystajecky, N.A.; Jassem, A.N.; Krajden, M.; et al. The contrasting role of nasopharyngeal angiotensin converting enzyme 2 (ACE2) transcription in SARS-CoV-2 infection: A cross-sectional study of people tested for COVID-19 in British Columbia, Canada. EBioMedicine 2021, 66, 103316. [Google Scholar]
- Gutiérrez-Chamorro, L.; Riveira-Muñoz, E.; Barrios, C.; Palau, V.; Nevot, M.; Pedreño-López, S.; Senserrich, J.; Massanella, M.; Clotet, B.; Cabrera, C.; et al. SARS-CoV-2 Infection Modulates ACE2 Function and Subsequent Inflammatory Responses in Swabs and Plasma of COVID-19 Patients. Viruses 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef]
- Gonzalez, S.M.; Siddik, A.B.; Su, R.-C. Regulated Intramembrane proteolysis of ACE2: A potential mechanism contributing to COVID-19 pathogenesis? Front. Immunol. 2021, 12, 612807. [Google Scholar] [CrossRef]
- Garcia-Escobar, A.; Jimenez-Valero, S.; Galeote, G.; Jurado-Roman, A.; Garcia-Rodriguez, J.; Moreno, R. The soluble catalytic ectodomain of ACE2 a biomarker of cardiac remodelling: New insights for heart failure and COVID19. Heart Fail. Rev. 2021, 26, 961–971. [Google Scholar]
- Singh, R.; Ramsuran, V.; Naranbhai, V.; Yende-Zuma, N.; Garrett, N.; Mlisana, K.; Dong, K.L.; Walker, B.D.; Abdool Karim, S.S.; Carrington, M.; et al. Epigenetic Regulation of BST-2 Expression Levels and the Effect on HIV-1 Pathogenesis. Front. Immunol. 2021, 12, 669241. [Google Scholar] [CrossRef]
- Ortiz, M.E.; Thurman, A.; Pezzulo, A.A.; Leidinger, M.R.; Klesney-Tait, J.A.; Karp, P.H.; Tan, P.; Wohlford-Lenane, C.; McCray, P.B.; Meyerholz, D.K. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. EBioMedicine 2020, 60. [Google Scholar] [CrossRef]
- Bubar, K.M.; Reinholt, K.; Kissler, S.M.; Lipsitch, M.; Cobey, S.; Grad, Y.H.; Larremore, D.B. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 2021, 371, 916–921. [Google Scholar] [PubMed]
- Bruce, J.; Johnson, S.B. Exploring the ethics of genetic prioritisation for COVID-19 vaccines. Eur. J. Hum. Genet. 2022, 30, 875–879. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).