Biological Embedding of Early-Life Adversity and a Scoping Review of the Evidence for Intergenerational Epigenetic Transmission of Stress and Trauma in Humans
Abstract
:1. Introduction
1.1. Stress as a Major Risk Factor for a Range of Non-Communicable Diseases
1.2. Early-Life Programming
1.3. Involvement of Epigenetic Mechanisms in the Biological Embedding of Stress
1.3.1. Animal Studies of Early-Life Stress
1.3.2. Post-Mortem Human Studies
1.3.3. Peripheral Biomarkers of Early-Life Stress
1.4. Transmission of Stress across Generations
1.4.1. Intergenerational and Transgenerational Inheritance
1.4.2. Findings from Animal Studies
1.4.3. Evidence in Humans—Need for a Scoping Review
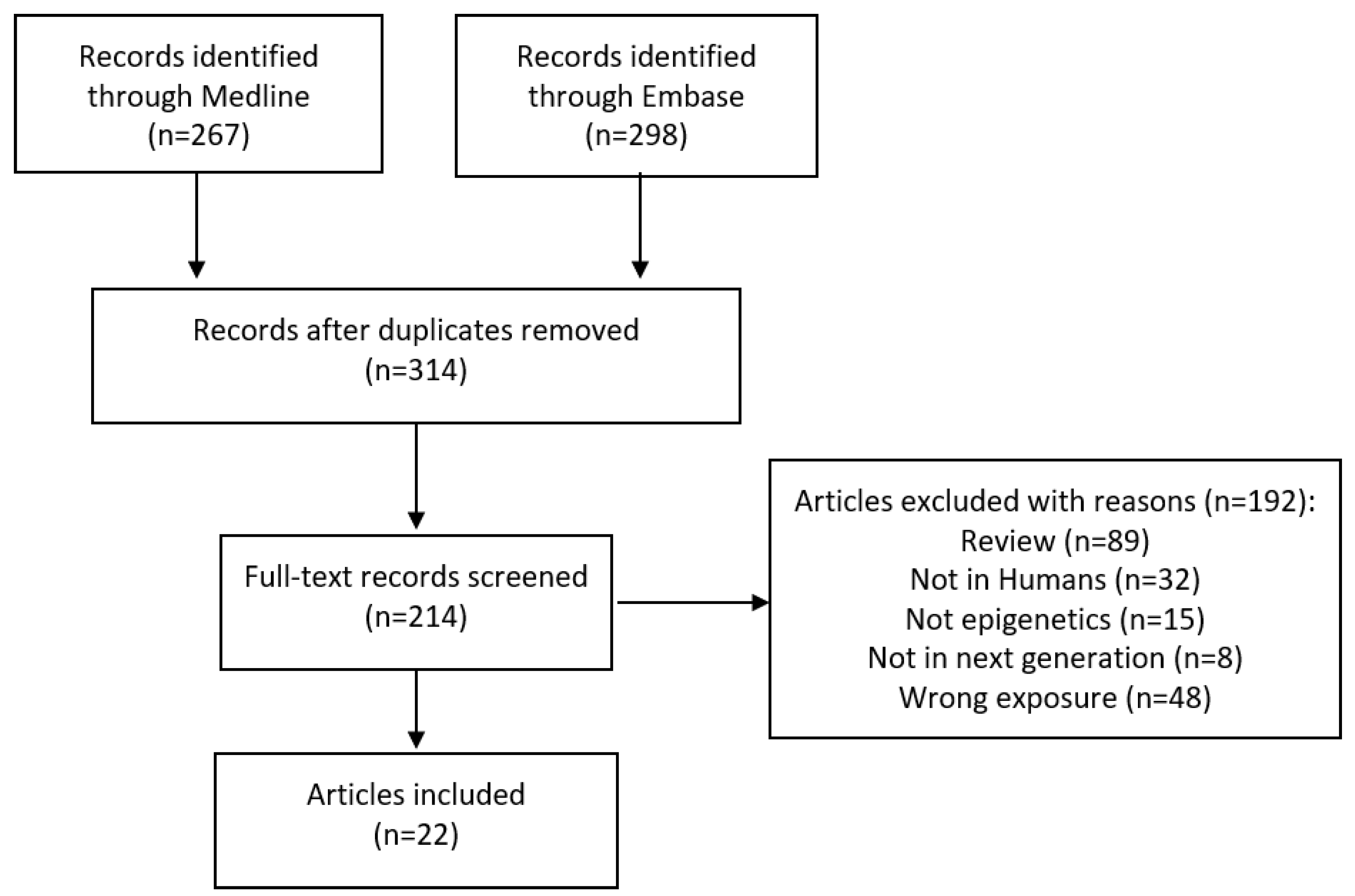
2. Materials and Methods
Search Strategy and Inclusion Criteria
3. Results
3.1. Characteristics of Included Studies
| 1st Author, Year | Study and Participant Characteristics | Exposure in G0 (Timing) | Tissue, Epigenetic Measure, Timing | Findings for Trauma-Exposed Group | Term Used |
|---|---|---|---|---|---|
| Radtke, 2011 [86] | 25 mothers and children aged 10–19 years. Germany | Intimate partner violence, pre and during pregnancy (retrospective) | Blood, 10–19 years. NR3C1 DNA methylation | In pregnancy: higher methylation; pre-pregnancy: NS | Trans |
| Perroud, 2014 [91] | 25 mothers of Tutsi ethnicity and 25 children aged 17–18 years. Rwanda (cases) or abroad (controls) | Rwandan Genocide trauma when pregnant (retrospective) | Blood, 17–18 years. NR3C1 and NR3C2 DNA methylation | Higher methylation | Trans |
| Yehuda, 2014 [95] | 80 adult offspring (conceived after trauma) and 15 matched controls. USA | Holocaust, PTSD (DSM-IV), maternal, and/or paternal (prospective) | Blood, mean 57 years. NR3C1 DNA methylation | Paternal only: higher methylation; both parents: lower methylation | Inter |
| Stroud, 2016 [100] | 153 mothers and infants from a low-income diverse sample. USA | Preconception and prenatal MDD (retrospective) | Placenta HSD11B2 DNA methylation | No significant overall associations | Inter |
| Yehuda, 2016 [83] | 22 adult offspring and 9 matched controls. USA | Holocaust, PTSD (DSM-IV), CTQ (prospective) | Blood, mean 77 years. FKBP5 DNA methylation | Holocaust: lower methylation; CTQ: NS | Inter |
| Cimino et al., 2018 [102] | 21 families with children aged 6–10 years. Italy | Psychological distress mothers and fathers (prospective) | Buccal swabs, 6–10 years. DAT DNA methylation | Higher methylation | Inter |
| Mehta, 2019 [97] | 38 male Vietnam veterans, mean age 67, 16 with PTSD. Australia or NZ | War-related PTSD, DSM-V (prospective) | Sperm, genome-wide DNA methylation | 3 CpG sites identified | Inter |
| Ramo-Fernandez, 2019 [88] | 113 mothers with newborn children. Germany | Childhood maltreatment (retrospective) | Umbilical cord blood fetal immune cells. FKBP5, NR3C1, CRHR1 DNA methylation | No significant associations | Inter |
| Bierer, 2020 [94] | 147 adult offspring (conceived after the trauma) and 31 controls. Mean age 51 years. USA | Holocaust, in childhood or adulthood (retrospective) | Blood, mean 51 years. FKBP5 DNA methylation | Exposure in childhood: Lower methylation; adulthood: NS | Inter |
| Grasso, 2020 [103] | 114 women in 3rd pregnancy trimester and newborns (24 h after delivery), USA | ACEs and adult adversity (retrospective), prenatal PTSD symptoms (prospective) | Newborn saliva, 24 h post-delivery. FKBP5 DNA methylation | Higher methylation with PTSD and threat-based ACEs | Inter |
| Pilkay, 2020 [101] | 201 mothers and newborns. USA | 20 traumatic life events (retrospective) | Umbilical cord blood. BDNF DNA methylation | Fear and males only: higher methylation. | Inter |
| Hjort, 2021 [96] | 117 women and children aged mean 12 years. Kosovo | PTSD from sexual violence during war, pregnancy (retrospective) | Blood, 6–18 years. Genome-wide DNA methylation | Nominal CpGs but not when adjusted | Inter |
| Merrill, 2021 [104] | 45 fathers and children aged 3 months. Canada | ACEs (retrospective) | Blood, 3 months. Genome-wide DNA methylation | 8 CpG sites | Inter |
| Nwanaji-Enwerem, 2021 [92] | 238 women and children. Mexican–American | ACEs up to 18 years (retrospective) | Blood, 7, 9, and 14 years. Epigenetic age acceleration (8 measures) | Age acceleration (Horvath and Intrinsic) | Inter |
| Ramo-Fernandez, 2021 [89] | 113 mothers and newborn children. Germany | Childhood maltreatment (retrospective) | Umbilical cord blood fetal immune cells. OXTR DNA methylation | No significant associations | Inter |
| Cordero, 2022 [98] | 48 mothers and children aged 12–42 months. Switzerland. | Interpersonal violence-related PTSD (retrospective) | Saliva, 1–2 years. NR3C1 DNA methylation | Higher methylation | Inter |
| Folger, 2022 [105] | 53 mother–child pairs, pregnancy and infant development study. USA | ACEs (retrospective) | Buccal swabs, 1 month. Secretogranin V (SCG5) gene | Lower methylation | Inter |
| Mavioglu, 2022 [87] | 113 mother–newborn dyads, 1st week after birth. Germany | Childhood maltreatment (retrospective) | Umbilical cord blood and buccal swabs (n = 68). DNMT1 methylation | No significant associations | Inter |
| Moore, 2022 [106] | Pregnant women <22 weeks gestation and 12-week infants (124 buccal, 92 blood). Canada | ACEs (retrospective) | Blood or buccal, 3 months. Genome-wide DNA methylation | Numerous nominally but not adjusted significant CpGs | Inter |
| Musanabaganwa, 2022 [90] | 33 mothers of Tutsi ethnicity and 26 children aged 17–18 years. Rwanda (cases) or abroad (controls) | Rwandan Genocide trauma, pregnancy (retrospective) | Blood, 17–18 years. Genome-wide DNA methylation | 16 differentially methylated regions identified | Inter |
| Mendonca, 2023 [99] | 60 mothers with children aged 6–12 years. Brazil | Major depression, pregnancy (retrospective) | Buccal, 6–12 years. FKBP5 and NR3C1 DNA methylation | Lower FKBP5 methylation | Inter |
| Scorza, 2023 [93] | 896 mothers and infants just after birth. UK | ACEs, cumulative score (retrospective) | Umbilical cord blood at birth. Genome-wide DNA methylation | 5 CpG sites, but only in male offspring | Inter |
3.2. Summary of Findings
4. Discussion
4.1. Summary of Findings
4.2. Limitations and Challenges
4.3. Future Research Needed
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McEwen, B.S. Protective and damaging effects of stress mediators: The good and bad sides of the response to stress. Metabolism 2002, 51 (Suppl. S1), 2–4. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological stress and disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef]
- Nilaweera, D.; Phyo, A.Z.Z.; Teshale, A.B.; Htun, H.L.; Wrigglesworth, J.; Gurvich, C.; Freak-Poli, R.; Ryan, J. Lifetime posttraumatic stress disorder as a predictor of mortality: A systematic review and meta-analysis. BMC Psychiatry 2023, 23, 229. [Google Scholar] [CrossRef]
- Brotman, D.J.; Golden, S.H.; Wittstein, I.S. The cardiovascular toll of stress. Lancet 2007, 370, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Glozier, N.; Tofler, G.H.; Colquhoun, D.M.; Bunker, S.J.; Clarke, D.M.; Hare, D.L.; Hickie, I.B.; Tatoulis, J.; Thompson, D.R.; Wilson, A.; et al. Psychosocial risk factors for coronary heart disease. Med. J. Aust. 2013, 199, 179–180. [Google Scholar] [CrossRef]
- Marcovecchio, M.L.; Chiarelli, F. The effects of acute and chronic stress on diabetes control. Sci. Signal 2012, 5 Pt 10. [Google Scholar] [CrossRef]
- Nilaweera, D.; Freak-Poli, R.; Ritchie, K.; Chaudieu, I.; Ancelin, M.L.; Ryan, J. The long-term consequences of trauma and posttraumatic stress disorder symptoms on later life cognitive function and dementia risk. Psychiatry Res. 2020, 294, 113506. [Google Scholar] [CrossRef] [PubMed]
- Nilaweera, D.; Gurvich, C.; Freak-Poli, R.; Woods, R.; Owen, A.; Murray, A.; Orchard, S.G.; Britt, C.; Wu, Z.; McNeil, J.; et al. Adverse events in older adults and the risk of dementia and cognitive decline. J. Affect. Disord. Rep. 2023, 13, 100592. [Google Scholar] [CrossRef]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef]
- Morris, M.C.; Compas, B.E.; Garber, J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clin. Psychol. Rev. 2012, 32, 301–315. [Google Scholar] [CrossRef]
- Olff, M.; Güzelcan, Y.; de Vries, G.J.; Assies, J.; Gersons, B.P. HPA- and HPT-axis alterations in chronic posttraumatic stress disorder. Psychoneuroendocrinology 2006, 31, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Liu, X.; Liu, Y.; Xue, C.; Zhang, L. A meta-analysis of risk factors for depression in adults and children after natural disasters. BMC Public Health 2014, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.; Wang, Y.; Liu, L.; Zhang, X.; Li, B.; Cui, R. The Effects of Psychological Stress on Depression. Curr. Neuropharmacol. 2015, 13, 494–504. [Google Scholar] [CrossRef]
- Belvederi Murri, M.; Pariante, C.; Mondelli, V.; Masotti, M.; Atti, A.R.; Mellacqua, Z.; Antonioli, M.; Ghio, L.; Menchetti, M.; Zanetidou, S.; et al. HPA axis and aging in depression: Systematic review and meta-analysis. Psychoneuroendocrinology 2014, 41, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Beluche, I.; Chaudieu, I.; Norton, J.; Carrière, I.; Boulenger, J.P.; Ritchie, K.; Ancelin, M.L. Persistence of abnormal cortisol levels in elderly persons after recovery from major depression. J. Psychiatr. Res. 2009, 43, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Maniam, J.; Antoniadis, C.; Morris, M.J. Early-Life Stress, HPA Axis Adaptation, and Mechanisms Contributing to Later Health Outcomes. Front. Endocrinol. 2014, 5, 73. [Google Scholar] [CrossRef]
- Ancelin, M.L.; Scali, J.; Norton, J.; Ritchie, K.; Dupuy, A.M.; Chaudieu, I.; Ryan, J. Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression. Psychoneuroendocrinology 2017, 77, 90–94. [Google Scholar] [CrossRef]
- Nievergelt, C.M.; Maihofer, A.X.; Klengel, T.; Atkinson, E.G.; Chen, C.Y.; Choi, K.W.; Coleman, J.R.I.; Dalvie, S.; Duncan, L.E.; Gelernter, J.; et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat. Commun. 2019, 10, 4558. [Google Scholar] [CrossRef]
- Ryan, J.; Chaudieu, I.; Ancelin, M.L.; Saffery, R. Biological underpinnings of trauma and post-traumatic stress disorder: Focusing on genetics and epigenetics. Epigenomics 2016, 8, 1553–1569. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A. Developmental origins of disease paradigm: A mechanistic and evolutionary perspective. Pediatr. Res. 2004, 56, 311–317. [Google Scholar] [CrossRef]
- Smith, K.E.; Pollak, S.D. Early life stress and development: Potential mechanisms for adverse outcomes. J. Neurodev. Disord. 2020, 12, 34. [Google Scholar] [CrossRef]
- Doom, J.R.; Cicchetti, D.; Rogosch, F.A. Longitudinal patterns of cortisol regulation differ in maltreated and nonmaltreated children. J. Am. Acad. Child. Adolesc. Psychiatry 2014, 53, 1206–1215. [Google Scholar] [CrossRef]
- Dong, M.; Giles, W.H.; Felitti, V.J.; Dube, S.R.; Williams, J.E.; Chapman, D.P.; Anda, R.F. Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation 2004, 110, 1761–1766. [Google Scholar] [CrossRef]
- Dube, S.R.; Fairweather, D.; Pearson, W.S.; Felitti, V.J.; Anda, R.F.; Croft, J.B. Cumulative childhood stress and autoimmune diseases in adults. Psychosom. Med. 2009, 71, 243–250. [Google Scholar] [CrossRef]
- Johnson, J.; Chaudieu, I.; Ritchie, K.; Scali, J.; Ancelin, M.L.; Ryan, J. The extent to which childhood adversity and recent stress influence all-cause mortality risk in older adults. Psychoneuroendocrinology 2020, 111, 104492. [Google Scholar] [CrossRef]
- Aristizabal, M.J.; Anreiter, I.; Halldorsdottir, T.; Odgers, C.L.; McDade, T.W.; Goldenberg, A.; Mostafavi, S.; Kobor, M.S.; Binder, E.B.; Sokolowski, M.B.; et al. Biological embedding of experience: A primer on epigenetics. Proc. Natl. Acad. Sci. USA 2020, 117, 23261–23269. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef]
- Januar, V.; Saffery, R.; Ryan, J. Epigenetics and depressive disorders: A review of current progress and future directions. Int. J. Epidemiol. 2015, 44, 1364–1387. [Google Scholar] [CrossRef]
- Kuan, P.F.; Waszczuk, M.A.; Kotov, R.; Marsit, C.J.; Guffanti, G.; Gonzalez, A.; Yang, X.; Koenen, K.; Bromet, E.; Luft, B.J. An epigenome-wide DNA methylation study of PTSD and depression in World Trade Center responders. Transl. Psychiatry 2017, 7, e1158. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Miller, O.; Bruenig, D.; David, G.; Shakespeare-Finch, J. A Systematic Review of DNA Methylation and Gene Expression Studies in Posttraumatic Stress Disorder, Posttraumatic Growth, and Resilience. J. Trauma. Stress 2020, 33, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Cutfield, W.; Hofman, P.; Hanson, M.A. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum. Dev. 2005, 81, 51–59. [Google Scholar] [CrossRef]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Boersma, G.J.; Lee, R.S.; Cordner, Z.A.; Ewald, E.R.; Purcell, R.H.; Moghadam, A.A.; Tamashiro, K.L. Prenatal stress decreases Bdnf expression and increases methylation of Bdnf exon IV in rats. Epigenetics 2014, 9, 437–447. [Google Scholar] [CrossRef]
- Matrisciano, F.; Tueting, P.; Dalal, I.; Kadriu, B.; Grayson, D.R.; Davis, J.M.; Nicoletti, F.; Guidotti, A. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology 2013, 68, 184–194. [Google Scholar] [CrossRef]
- Elliott, E.; Ezra-Nevo, G.; Regev, L.; Neufeld-Cohen, A.; Chen, A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 2010, 13, 1351–1353. [Google Scholar] [CrossRef]
- Kember, R.L.; Dempster, E.L.; Lee, T.H.; Schalkwyk, L.C.; Mill, J.; Fernandes, C. Maternal separation is associated with strain-specific responses to stress and epigenetic alterations to Nr3c1, Avp, and Nr4a1 in mouse. Brain Behav. 2012, 2, 455–467. [Google Scholar] [CrossRef]
- Sterrenburg, L.; Gaszner, B.; Boerrigter, J.; Santbergen, L.; Bramini, M.; Elliott, E.; Chen, A.; Peeters, B.W.; Roubos, E.W.; Kozicz, T. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS ONE 2011, 6, e28128. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Hara, K.; Kobayashi, A.; Otsuki, K.; Yamagata, H.; Hobara, T.; Suzuki, T.; Miyata, N.; Watanabe, Y. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron 2011, 69, 359–372. [Google Scholar] [CrossRef]
- McGowan, P.O.; Sasaki, A.; D’Alessio, A.C.; Dymov, S.; Labonté, B.; Szyf, M.; Turecki, G.; Meaney, M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Labonté, B.; Azoulay, N.; Yerko, V.; Turecki, G.; Brunet, A. Epigenetic modulation of glucocorticoid receptors in posttraumatic stress disorder. Transl. Psychiatry 2014, 4, e368. [Google Scholar] [CrossRef]
- Davies, M.N.; Volta, M.; Pidsley, R.; Lunnon, K.; Dixit, A.; Lovestone, S.; Coarfa, C.; Harris, R.A.; Milosavljevic, A.; Troakes, C.; et al. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 2012, 13, R43. [Google Scholar] [CrossRef]
- Essex, M.J.; Boyce, W.T.; Hertzman, C.; Lam, L.L.; Armstrong, J.M.; Neumann, S.M.; Kobor, M.S. Epigenetic vestiges of early developmental adversity: Childhood stress exposure and DNA methylation in adolescence. Child Dev. 2013, 84, 58–75. [Google Scholar] [CrossRef]
- Kundakovic, M.; Gudsnuk, K.; Herbstman, J.B.; Tang, D.; Perera, F.P.; Champagne, F.A. DNA methylation of BDNF as a biomarker of early-life adversity. Proc. Natl. Acad. Sci. USA 2015, 112, 6807–6813. [Google Scholar] [CrossRef] [PubMed]
- Suderman, M.; Borghol, N.; Pappas, J.J.; Pinto Pereira, S.M.; Pembrey, M.; Hertzman, C.; Power, C.; Szyf, M. Childhood abuse is associated with methylation of multiple loci in adult DNA. BMC Med. Genom. 2014, 7, 13. [Google Scholar] [CrossRef]
- Provençal, N.; Suderman, M.J.; Guillemin, C.; Vitaro, F.; Côté, S.M.; Hallett, M.; Tremblay, R.E.; Szyf, M. Association of childhood chronic physical aggression with a DNA methylation signature in adult human T cells. PLoS ONE 2014, 9, e89839. [Google Scholar] [CrossRef]
- Klengel, T.; Mehta, D.; Anacker, C.; Rex-Haffner, M.; Pruessner, J.C.; Pariante, C.M.; Pace, T.W.; Mercer, K.B.; Mayberg, H.S.; Bradley, B.; et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013, 16, 33–41. [Google Scholar] [CrossRef]
- Palma-Gudiel, H.; Córdova-Palomera, A.; Leza, J.C.; Fañanás, L. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: A critical review. Neurosci. Biobehav. Rev. 2015, 55, 520–535. [Google Scholar] [CrossRef] [PubMed]
- Turecki, G.; Meaney, M.J. Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biol. Psychiatry 2016, 79, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tyrka, A.R.; Price, L.H.; Marsit, C.; Walters, O.C.; Carpenter, L.L. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: Preliminary findings in healthy adults. PLoS ONE 2012, 7, e30148. [Google Scholar] [CrossRef]
- Palma-Gudiel, H.; Córdova-Palomera, A.; Eixarch, E.; Deuschle, M.; Fañanás, L. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: A meta-analysis. Epigenetics 2015, 10, 893–902. [Google Scholar] [CrossRef]
- Ryan, J.; Mansell, T.; Fransquet, P.; Saffery, R. Does maternal mental well-being in pregnancy impact the early human epigenome? Epigenomics 2017, 9, 313–332. [Google Scholar] [CrossRef]
- Mulligan, C.J.; D’Errico, N.C.; Stees, J.; Hughes, D.A. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics 2012, 7, 853–857. [Google Scholar] [CrossRef]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef]
- Keenan, K.; Hipwell, A.E.; Class, Q.A.; Mbayiwa, K. Extending the developmental origins of disease model: Impact of preconception stress exposure on offspring neurodevelopment. Dev. Psychobiol. 2018, 60, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Lyons-Ruth, K.; Easterbrooks, M.A.; Cibelli, C.D. Infant attachment strategies, infant mental lag, and maternal depressive symptoms: Predictors of internalizing and externalizing problems at age 7. Dev. Psychol. 1997, 33, 681–692. [Google Scholar] [CrossRef]
- Weissman, M.M.; Talati, A.; Gameroff, M.J.; Pan, L.; Skipper, J.; Posner, J.E.; Wickramaratne, P.J. Enduring problems in the offspring of depressed parents followed up to 38 years. EClinicalMedicine 2021, 38, 101000. [Google Scholar] [CrossRef]
- Shrira, A.; Mollov, B.; Mudahogora, C. Complex PTSD and intergenerational transmission of distress and resilience among Tutsi genocide survivors and their offspring: A preliminary report. Psychiatry Res. 2019, 271, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Olino, T.M.; Pettit, J.W.; Klein, D.N.; Allen, N.B.; Seeley, J.R.; Lewinsohn, P.M. Influence of parental and grandparental major depressive disorder on behavior problems in early childhood: A three-generation study. J. Am. Acad. Child. Adolesc. Psychiatry 2008, 47, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Weissman, M.M.; Berry, O.O.; Warner, V.; Gameroff, M.J.; Skipper, J.; Talati, A.; Pilowsky, D.J.; Wickramaratne, P. A 30-Year Study of 3 Generations at High Risk and Low Risk for Depression. JAMA Psychiatry 2016, 73, 970–977. [Google Scholar] [CrossRef]
- Scharf, M. Long-term effects of trauma: Psychosocial functioning of the second and third generation of Holocaust survivors. Dev. Psychopathol. 2007, 19, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Van Steenwyk, G.; Roszkowski, M.; Manuella, F.; Franklin, T.B.; Mansuy, I.M. Transgenerational inheritance of behavioral and metabolic effects of paternal exposure to traumatic stress in early postnatal life: Evidence in the 4th generation. Environ. Epigenet. 2018, 4, dvy023. [Google Scholar] [CrossRef]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Fallet, M.; Blanc, M.; Di Criscio, M.; Antczak, P.; Engwall, M.; Guerrero Bosagna, C.; Rüegg, J.; Keiter, S.H. Present and future challenges for the investigation of transgenerational epigenetic inheritance. Environ. Int. 2023, 172, 107776. [Google Scholar] [CrossRef]
- Bohacek, J.; Mansuy, I.M. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat. Rev. Genet. 2015, 16, 641–652. [Google Scholar] [CrossRef]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef]
- Perez, M.F.; Lehner, B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 2019, 21, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Yeshurun, S.; Hannan, A.J. Transgenerational epigenetic influences of paternal environmental exposures on brain function and predisposition to psychiatric disorders. Mol. Psychiatry 2019, 24, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L.; Lubin, F.D.; Funk, A.J.; Sweatt, J.D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry 2009, 65, 760–769. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Fontaine, A.; MacCallum, P.E.; Drover, J.; Blundell, J. Stress Across Generations: DNA Methylation as a Potential Mechanism Underlying Intergenerational Effects of Stress in Both Post-traumatic Stress Disorder and Pre-clinical Predator Stress Rodent Models. Front. Behav. Neurosci. 2019, 13, 113. [Google Scholar] [CrossRef]
- Bale, T.L. Lifetime stress experience: Transgenerational epigenetics and germ cell programming. Dialogues Clin. Neurosci. 2014, 16, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Morgan, C.P.; Adrian Leu, N.; Shetty, A.; Cisse, Y.M.; Nugent, B.M.; Morrison, K.E.; Jašarević, E.; Huang, W.; Kanyuch, N.; et al. Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment. Nat. Commun. 2020, 11, 1499. [Google Scholar] [CrossRef]
- Mychasiuk, R.; Harker, A.; Ilnytskyy, S.; Gibb, R. Paternal stress prior to conception alters DNA methylation and behaviour of developing rat offspring. Neuroscience 2013, 241, 100–105. [Google Scholar] [CrossRef]
- Pang, T.Y.C.; Short, A.K.; Bredy, T.W.; Hannan, A.J. Transgenerational paternal transmission of acquired traits: Stress-induced modification of the sperm regulatory transcriptome and offspring phenotypes. Curr. Opin. Behav. Sci. 2017, 14, 140–147. [Google Scholar] [CrossRef]
- De Quervain, D.; Schwabe, L.; Roozendaal, B. Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nat. Rev. Neurosci. 2017, 18, 7–19. [Google Scholar] [CrossRef]
- Moisiadis, V.G.; Constantinof, A.; Kostaki, A.; Szyf, M.; Matthews, S.G. Prenatal Glucocorticoid Exposure Modifies Endocrine Function and Behaviour for 3 Generations Following Maternal and Paternal Transmission. Sci. Rep. 2017, 7, 11814. [Google Scholar] [CrossRef]
- Short, A.K.; Fennell, K.A.; Perreau, V.M.; Fox, A.; O’Bryan, M.K.; Kim, J.H.; Bredy, T.W.; Pang, T.Y.; Hannan, A.J. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl. Psychiatry 2016, 6, e837. [Google Scholar] [CrossRef]
- Gapp, K.; Bohacek, J.; Grossmann, J.; Brunner, A.M.; Manuella, F.; Nanni, P.; Mansuy, I.M. Potential of Environmental Enrichment to Prevent Transgenerational Effects of Paternal Trauma. Neuropsychopharmacology 2016, 41, 2749–2758. [Google Scholar] [CrossRef]
- Yehuda, R.; Daskalakis, N.P.; Bierer, L.M.; Bader, H.N.; Klengel, T.; Holsboer, F.; Binder, E.B. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol. Psychiatry 2016, 80, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Radtke, K.M.; Ruf, M.; Gunter, H.M.; Dohrmann, K.; Schauer, M.; Meyer, A.; Elbert, T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl. Psychiatry 2011, 1, e21. [Google Scholar] [CrossRef]
- Mavioglu, R.N.; Ramo-Fernandez, L.; Gumpp, A.M.; Kolassa, I.T.; Karabatsiakis, A. A history of childhood maltreatment is associated with altered DNA methylation levels of DNA methyltransferase 1 in maternal but not neonatal mononuclear immune cells. Front. Psychiatry 2022, 13, 945343. [Google Scholar] [CrossRef] [PubMed]
- Ramo-Fernandez, L.; Boeck, C.; Koenig, A.M.; Schury, K.; Binder, E.B.; Gundel, H.; Fegert, J.M.; Karabatsiakis, A.; Kolassa, I.T. The effects of childhood maltreatment on epigenetic regulation of stress-response associated genes: An intergenerational approach. Sci. Rep. 2019, 9, 983. [Google Scholar] [CrossRef]
- Ramo-Fernandez, L.; Gumpp, A.M.; Boeck, C.; Krause, S.; Bach, A.M.; Waller, C.; Kolassa, I.T.; Karabatsiakis, A. Associations between childhood maltreatment and DNA methylation of the oxytocin receptor gene in immune cells of mother-newborn dyads. Transl. Psychiatry 2021, 11, 449. [Google Scholar] [CrossRef]
- Musanabaganwa, C.; Wani, A.H.; Donglasan, J.; Fatumo, S.; Jansen, S.; Mutabaruka, J.; Rutembesa, E.; Uwineza, A.; Hermans, E.J.; Roozendaal, B.; et al. Leukocyte methylomic imprints of exposure to the genocide against the Tutsi in Rwanda: A pilot epigenome-wide analysis. Epigenomics 2022, 14, 11–25. [Google Scholar] [CrossRef]
- Perroud, N.; Rutembesa, E.; Paoloni-Giacobino, A.; Mutabaruka, J.; Mutesa, L.; Stenz, L.; Malafosse, A.; Karege, F. The Tutsi genocide and transgenerational transmission of maternal stress: Epigenetics and biology of the HPA axis. World J. Biol. Psychiatry 2014, 15, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Nwanaji-Enwerem, J.C.; Van Der Laan, L.; Kogut, K.; Eskenazi, B.; Holland, N.; Deardorff, J.; Cardenas, A. Maternal adverse childhood experiences before pregnancy are associated with epigenetic aging changes in their children. Aging 2021, 13, 25653–25669. [Google Scholar] [CrossRef]
- Scorza, P.; Duarte, C.S.; Lee, S.; Wu, H.; Posner, J.; Baccarelli, A.; Monk, C. Epigenetic Intergenerational Transmission: Mothers’ Adverse Childhood Experiences and DNA Methylation. J. Am. Acad. Child. Adolesc. Psychiatry, 2023; in press. [Google Scholar]
- Bierer, L.M.; Bader, H.N.; Daskalakis, N.P.; Lehrner, A.; Provencal, N.; Wiechmann, T.; Klengel, T.; Makotkine, I.; Binder, E.B.; Yehuda, R. Intergenerational Effects of Maternal Holocaust Exposure on FKBP5 Methylation. Am. J. Psychiatry 2020, 177, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Daskalakis, N.P.; Lehrner, A.; Desarnaud, F.; Bader, H.N.; Makotkine, I.; Flory, J.D.; Bierer, L.M.; Meaney, M.J. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am. J. Psychiatry 2014, 171, 872–880. [Google Scholar] [CrossRef]
- Hjort, L.; Rushiti, F.; Wang, S.J.; Fransquet, P.; Krasniqi, S.P.; Çarkaxhiu, S.I.; Arifaj, D.; Xhemaili, V.D.; Salihu, M.; A Leku, N.; et al. Intergenerational effects of maternal post-traumatic stress disorder on offspring epigenetic patterns and cortisol levels. Epigenomics 2021, 13, 967–980. [Google Scholar] [CrossRef]
- Mehta, D.; Pelzer, E.S.; Bruenig, D.; Lawford, B.; McLeay, S.; Morris, C.P.; Gibson, J.N.; Young, R.M.; Voisey, J. DNA methylation from germline cells in veterans with PTSD. J. Psychiatr. Res. 2019, 116, 42–50. [Google Scholar] [CrossRef]
- Cordero, M.I.; Stenz, L.; Moser, D.A.; Rusconi Serpa, S.; Paoloni-Giacobino, A.; Schechter, D.S. The relationship of maternal and child methylation of the glucocorticoid receptor NR3C1 during early childhood and subsequent child psychopathology at school-age in the context of maternal interpersonal violence-related post-traumatic stress disorder. Front. Psychiatry 2022, 13, 919820. [Google Scholar] [CrossRef]
- Mendonca, M.S.; Mangiavacchi, P.M.; Mendes, A.V.; Loureiro, S.R.; Martin-Santos, R.; Gloria, L.S.; Marques, W.; De Marco, S.P.G.; Kanashiro, M.M.; Hallak, J.E.C.; et al. DNA methylation in regulatory elements of the FKBP5 and NR3C1 gene in mother-child binomials with depression. J. Affect. Disord. 2023, 331, 287–299. [Google Scholar] [CrossRef]
- Stroud, L.R.; Papandonatos, G.D.; Parade, S.H.; Salisbury, A.L.; Phipps, M.G.; Lester, B.M.; Padbury, J.F.; Marsit, C.J. Prenatal Major Depressive Disorder, Placenta Glucocorticoid and Serotonergic Signaling, and Infant Cortisol Response. Psychosom. Med. 2016, 78, 979–990. [Google Scholar] [CrossRef]
- Pilkay, S.R.; Combs-Orme, T.; Tylavsky, F.; Bush, N.; Smith, A.K. Maternal trauma and fear history predict BDNF methylation and gene expression in newborns. PeerJ 2020, 8, e8858. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Cerniglia, L.; Ballarotto, G.; Marzilli, E.; Pascale, E.; D’Addario, C.; Adriani, W.; Tambelli, R. DNA methylation at the DAT promoter and risk for psychopathology: Intergenerational transmission between school-age youths and their parents in a community sample. Front. Psychiatry 2018, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Grasso, D.J.; Drury, S.; Briggs-Gowan, M.; Johnson, A.; Ford, J.; Lapidus, G.; Scranton, V.; Abreu, C.; Covault, J. Adverse childhood experiences, posttraumatic stress, and FKBP5 methylation patterns in postpartum women and their newborn infants. Psychoneuroendocrinology 2020, 114, 104604. [Google Scholar] [CrossRef]
- Merrill, S.M.; Moore, S.R.; Gladish, N.; Giesbrecht, G.F.; Dewey, D.; Konwar, C.; MacIssac, J.L.; Kobor, M.S.; Letourneau, N.L. Paternal adverse childhood experiences: Associations with infant DNA methylation. Dev. Psychobiol. 2021, 63, e22174. [Google Scholar] [CrossRef]
- Folger, A.T.; Nidey, N.; Ding, L.; Ji, H.; Yolton, K.; Ammerman, R.T.; Bowers, K.A. Association Between Maternal Adverse Childhood Experiences and Neonatal SCG5 DNA Methylation-Effect Modification by Prenatal Home Visiting. Am. J. Epidemiol. 2022, 191, 636–645. [Google Scholar] [CrossRef]
- Moore, S.R.; Merrill, S.M.; Sekhon, B.; MacIsaac, J.L.; Kobor, M.S.; Giesbrecht, G.F.; Letourneau, N. Infant DNA methylation: An early indicator of intergenerational trauma? Early Hum. Dev. 2022, 164, 105519. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.L.; Gladish, N.; Gatev, E.; Jones, M.J.; Chen, Y.; MacIsaac, J.L.; Tworoger, S.S.; Austin, S.B.; Tanrikut, C.; Chavarro, J.E.; et al. Exposure to childhood abuse is associated with human sperm DNA methylation. Transl. Psychiatry 2018, 8, 194. [Google Scholar] [CrossRef]
- Serpeloni, F.; Radtke, K.; de Assis, S.G.; Henning, F.; Nätt, D.; Elbert, T. Grandmaternal stress during pregnancy and DNA methylation of the third generation: An epigenome-wide association study. Transl. Psychiatry 2017, 7, e1202. [Google Scholar] [CrossRef]
- Bohacek, J.; Mansuy, I.M. A guide to designing germline-dependent epigenetic inheritance experiments in mammals. Nat. Methods 2017, 14, 243–249. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; Provost, A.C.; Hunter, R.G.; Guffanti, G. Noncoding RNAs: Stress, Glucocorticoids, and Posttraumatic Stress Disorder. Biol. Psychiatry 2018, 83, 849–865. [Google Scholar] [CrossRef]
- Dickson, D.A.; Paulus, J.K.; Mensah, V.; Lem, J.; Saavedra-Rodriguez, L.; Gentry, A.; Pagidas, K.; Feig, L.A. Reduced levels of miRNAs 449 and 34 in sperm of mice and men exposed to early life stress. Transl. Psychiatry 2018, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Daxinger, L.; Whitelaw, E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012, 13, 153–162. [Google Scholar] [CrossRef]
- Jawaid, A.; Jehle, K.L.; Mansuy, I.M. Impact of Parental Exposure on Offspring Health in Humans. Trends Genet. 2021, 37, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Teperek, M.; Simeone, A.; Gaggioli, V.; Miyamoto, K.; Allen, G.E.; Erkek, S.; Kwon, T.; Marcotte, E.M.; Zegerman, P.; Bradshaw, C.R.; et al. Sperm is epigenetically programmed to regulate gene transcription in embryos. Genome Res. 2016, 26, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Manikkam, M.; Haque, M.M.; Zhang, B.; Savenkova, M.I. Epigenetic transgenerational inheritance of somatic transcriptomes and epigenetic control regions. Genome Biol. 2012, 13, R91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, A.; Ryan, J. Biological Embedding of Early-Life Adversity and a Scoping Review of the Evidence for Intergenerational Epigenetic Transmission of Stress and Trauma in Humans. Genes 2023, 14, 1639. https://doi.org/10.3390/genes14081639
Zhou A, Ryan J. Biological Embedding of Early-Life Adversity and a Scoping Review of the Evidence for Intergenerational Epigenetic Transmission of Stress and Trauma in Humans. Genes. 2023; 14(8):1639. https://doi.org/10.3390/genes14081639
Chicago/Turabian StyleZhou, Aoshuang, and Joanne Ryan. 2023. "Biological Embedding of Early-Life Adversity and a Scoping Review of the Evidence for Intergenerational Epigenetic Transmission of Stress and Trauma in Humans" Genes 14, no. 8: 1639. https://doi.org/10.3390/genes14081639
APA StyleZhou, A., & Ryan, J. (2023). Biological Embedding of Early-Life Adversity and a Scoping Review of the Evidence for Intergenerational Epigenetic Transmission of Stress and Trauma in Humans. Genes, 14(8), 1639. https://doi.org/10.3390/genes14081639






