A Comprehensive Report of Intrinsically Disordered Regions in Inherited Retinal Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Identification
Databases and Metrics
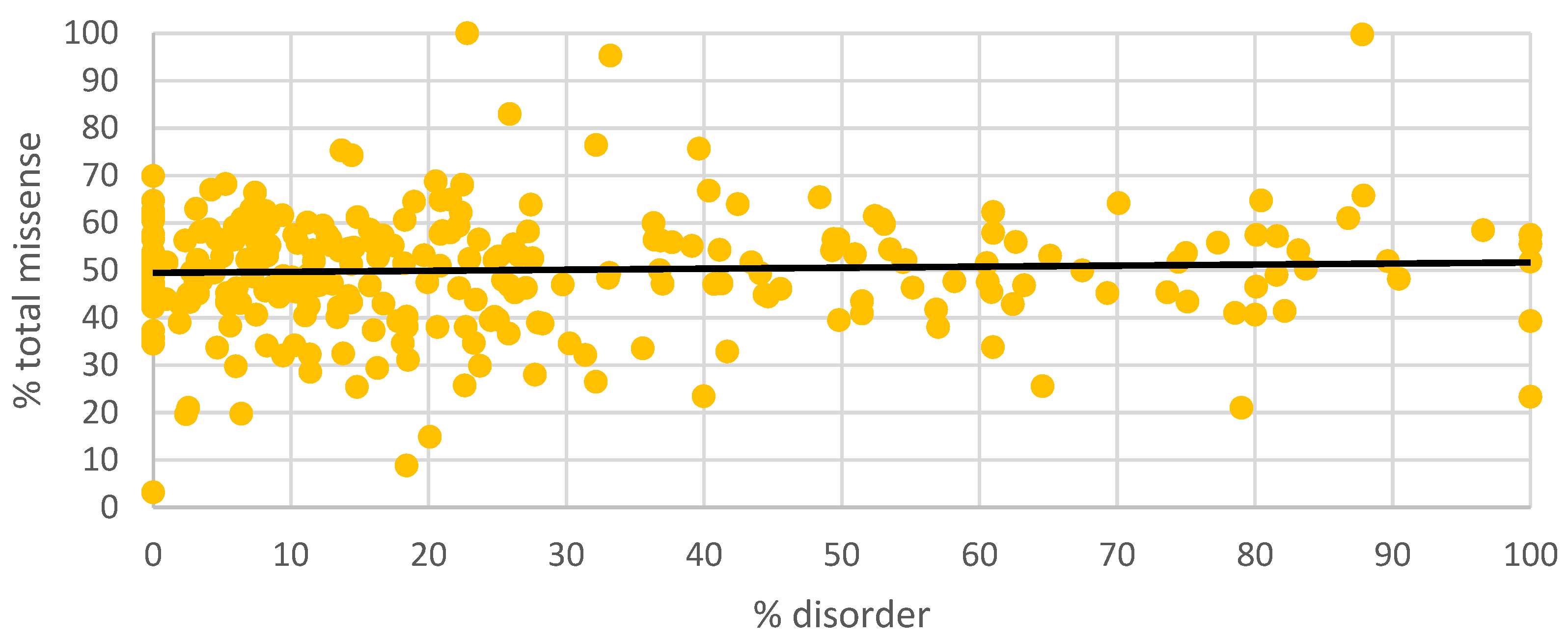
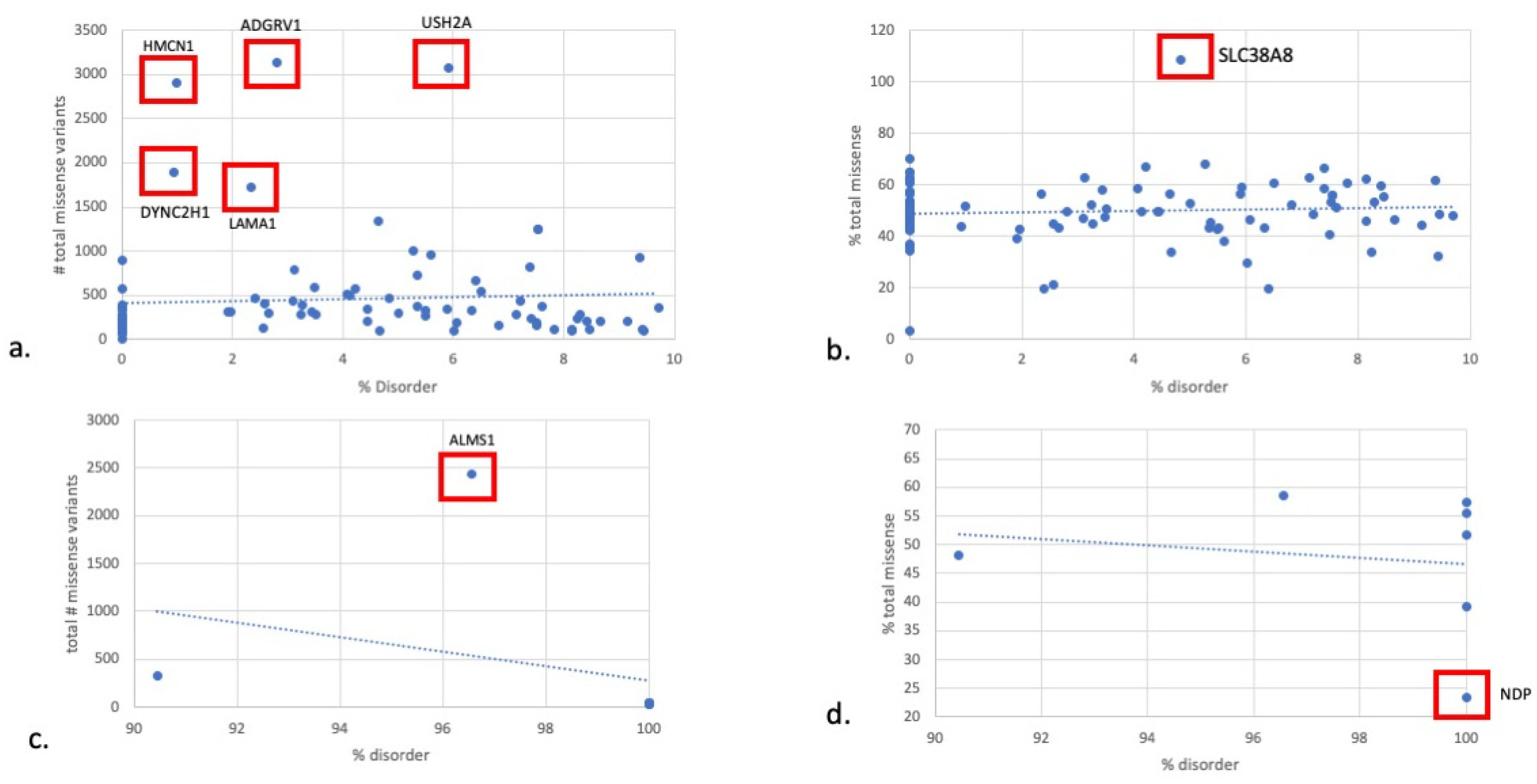
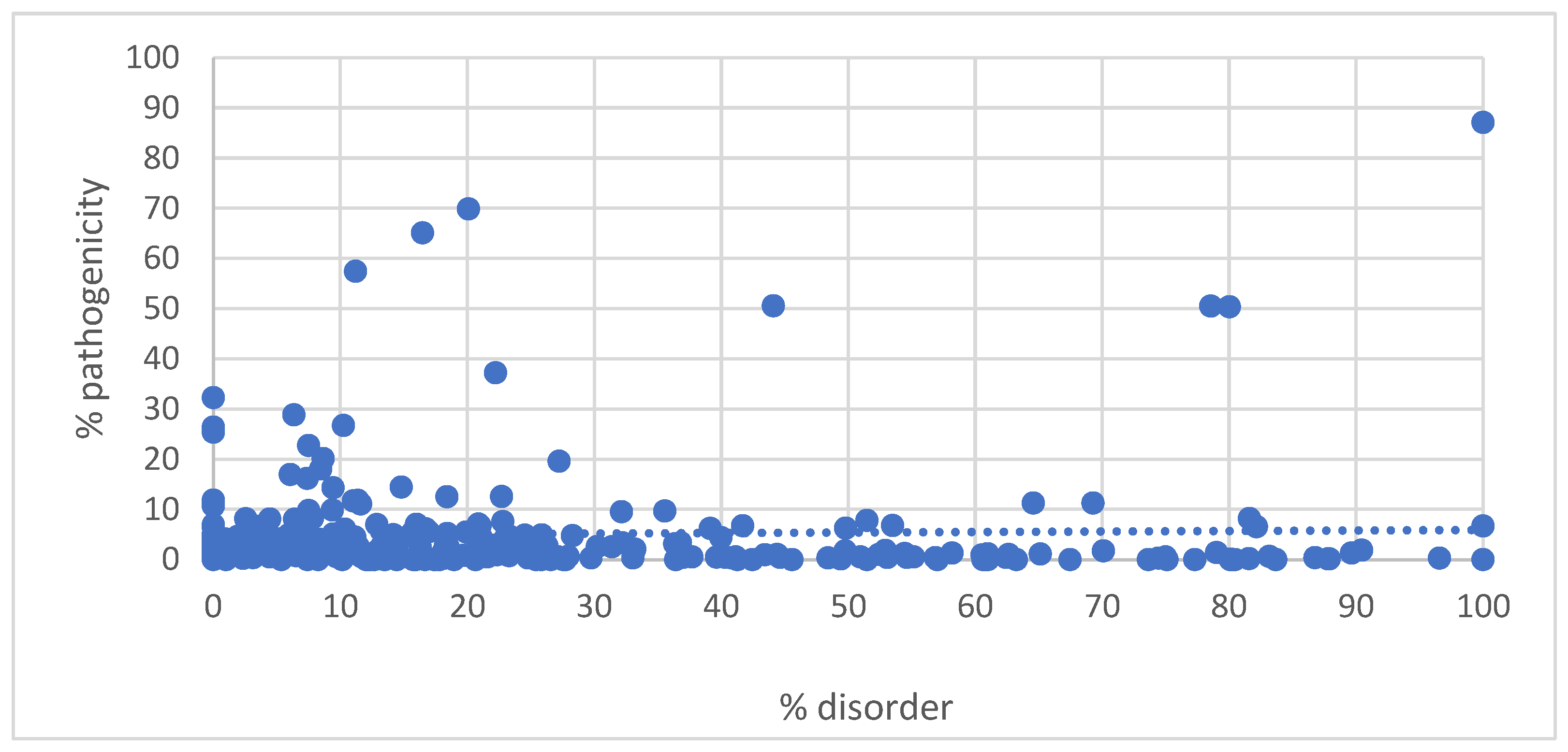
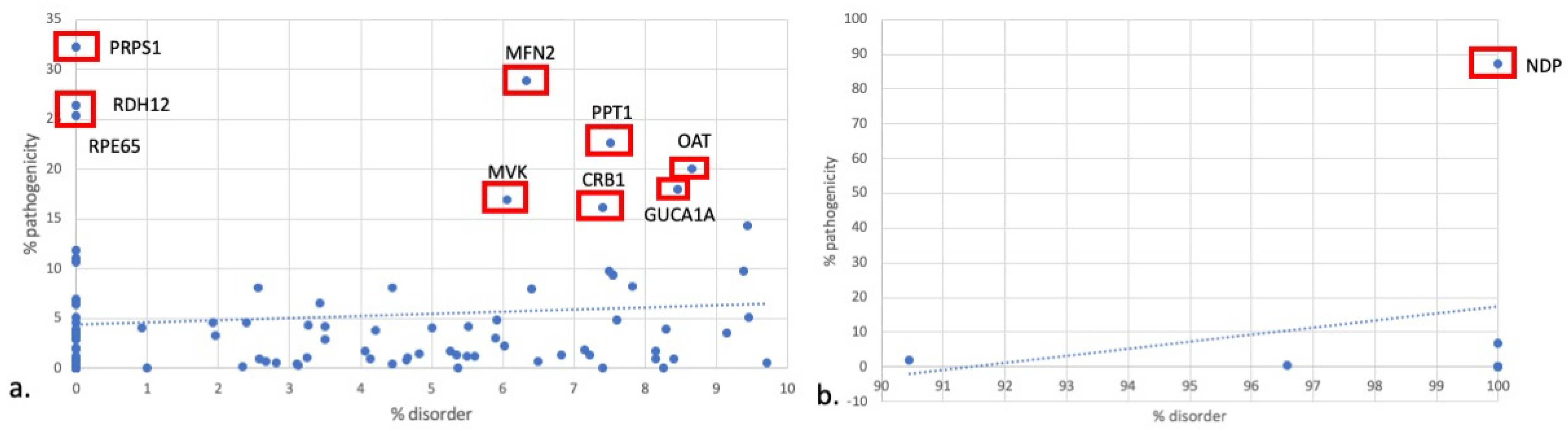
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georgiou, M.; Fujinami, K.; Michaelides, M. Inherited retinal diseases: Therapeutics, clinical trials and end points—A review. Clin. Experiment. Ophthalmol. 2021, 49, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.E.; Dyson, H.J. Intrinsically unstructured proteins: Re-assessing the protein structure-function paradigm. J. Mol. Biol. 1999, 293, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Bondos, S.E.; Dunker, A.K.; Uversky, V.N. Intrinsically disordered proteins play diverse roles in cell signaling. Cell Commun. Signal. CCS 2022, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Procopio, R.; Pulido, J.S.; Gunton, K.B. Initial Investigations of Intrinsically Disordered Regions in Inherited Retinal Diseases. Int. J. Mol. Sci. 2023, 24, 1060. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, M.; Kragelund, B.B. Functions of intrinsic disorder in transmembrane proteins. Cell. Mol. Life Sci. 2017, 74, 3205–3224. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.; Lee, Y.M.; Kuht, H.J.; Thomas, M.G.; Kim, S.J.; Lee, S.T.; Han, J. DYNC2H1 variants cause Leber congenital amaurosis without syndromic features. Clin. Genet. 2021, 100, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Rifat, Z.T.; Lohia, R.; Campbell, A.J.; Dunker, A.K.; Rahman, M.S.; Iqbal, S. Characterization of intrinsically disordered regions in proteins informed by human genetic diversity. PLoS Comput. Biol. 2022, 18, e1009911. [Google Scholar] [CrossRef] [PubMed]
- Kuht, H.J.; Han, J.; Maconachie, G.D.E.; Park, S.E.; Lee, S.T.; McLean, R.; Sheth, V.; Hisaund, M.; Dawar, B.; Sylvius, N.; et al. SLC38A8 mutations result in arrested retinal development with loss of cone photoreceptor specialization. Hum. Mol. Genet. 2020, 29, 2989–3002. [Google Scholar] [CrossRef] [PubMed]
- Toms, M.; Pagarkar, W.; Moosajee, M. Usher syndrome: Clinical features, molecular genetics and advancing therapeutics. Ther. Adv. Ophthalmol. 2020, 12, 2515841420952194. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, E.; Pennings, R.J.; te Brinke, H.; Claassen, A.; Yntema, H.G.; Hoefsloot, L.H.; Cremers, F.P.M.; Cremers, C.W.R.J.; Kremer, H. Identification of 51 novel exons of the Usher syndrome type 2A (USH2A) gene that encode multiple conserved functional domains and that are mutated in patients with Usher syndrome type II. Am. J. Hum. Genet. 2004, 74, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Fakin, A.; Bonnet, C.; Kurtenbach, A.; Mohand-Said, S.; Zobor, D.; Stingl, K.; Testa, F.; Simonelli, F.; Sahel, J.-A.; Audo, I.; et al. Characteristics of Retinitis Pigmentosa Associated with ADGRV1 and Comparison with USH2A in Patients from a Multicentric Usher Syndrome Study Treatrush. Int. J. Mol. Sci. 2021, 22, 10352. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, B.A.; Reding, M.Q.; Schimmenti, L.A. NDP-Related Retinopathies. In GeneReviews® [Internet]; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; 30 July 1999 [updated 23 March 2023]; University of Washington: Seattle, WA, USA, 1993–2023. [Google Scholar]
- Emenecker, R.J.; Griffith, D.; Holehouse, A.S. metapredict: A fast, accurate, and easy-to-use predictor of consensus disorder and structure. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.E.; Pulido, J.S.; da Palma, M.M.; Procopio, R.; Hufnagel, R.B.; Reynolds, M. A Comprehensive Report of Intrinsically Disordered Regions in Inherited Retinal Diseases. Genes 2023, 14, 1601. https://doi.org/10.3390/genes14081601
Lee KE, Pulido JS, da Palma MM, Procopio R, Hufnagel RB, Reynolds M. A Comprehensive Report of Intrinsically Disordered Regions in Inherited Retinal Diseases. Genes. 2023; 14(8):1601. https://doi.org/10.3390/genes14081601
Chicago/Turabian StyleLee, Karen E., Jose S. Pulido, Mariana M. da Palma, Rebecca Procopio, Robert B. Hufnagel, and Margaret Reynolds. 2023. "A Comprehensive Report of Intrinsically Disordered Regions in Inherited Retinal Diseases" Genes 14, no. 8: 1601. https://doi.org/10.3390/genes14081601
APA StyleLee, K. E., Pulido, J. S., da Palma, M. M., Procopio, R., Hufnagel, R. B., & Reynolds, M. (2023). A Comprehensive Report of Intrinsically Disordered Regions in Inherited Retinal Diseases. Genes, 14(8), 1601. https://doi.org/10.3390/genes14081601




