1. Introduction
Skeletal muscles are the main meat-producing tissue of pigs and have a unique cellular structure. Skeletal muscle fibers are the largest cells in mammals, accounting for 75–90% of the skeletal muscle tissue [
1]. The number, size, and type of muscle fibers determine the basic characteristics of a muscle and its yield. Muscle fiber characteristics are closely related to pork meat color, pH, muscle tenderness, and intramuscular fat (IMF) content [
2,
3]. The IMF content affects muscle tenderness and flavor, with higher IMF content being associated with higher pork quality [
4]. The type of muscle fiber is a key factor that affects pork quality. Muscle growth after birth mainly depends on the thickening and transformation of muscle fibers [
5,
6].
Pork quality is a quantitative trait controlled by micro-efficacious polygenes and belongs to the medium and high heritability traits that play a decisive role in varieties [
7]. Meat quality traits differ significantly between breeds, especially between domestic and foreign breeds. Imported pig breeds, such as the Duroc, have a high growth rate, high glycolytic fiber content, and low IMF content [
8]. Chinese indigenous pig breeds, such as the Huai, are characterized by high IMF content; high diameter of I, IIa, and IIb muscle fibers; and a high content of oxidized fibers [
9,
10]. Huang et al. [
11] reported that Bama Xiang pigs have a high longissimus dorsi (LD) type I muscle fiber content, whereas Landrace pigs have a high glycolytic IIb muscle fiber content. Muscle fiber composition is a key factor causing differences in meat quality between Bama Xiang and Landrace pigs. The meat quality traits of different muscle groups within the same variety of pig are also significantly different. Moreover, the muscle fiber diameter and area of the LD muscle are significantly higher than those of the psoas major (PM) muscle [
12,
13]. Furthermore, the muscle fiber composition ratio of the same muscle group of the same variety or different muscle groups of the same variety affects pork meat quality. A better understanding of the genetic mechanisms underlying muscle fiber types is important for improving livestock and poultry meat quality.
Muscle growth is a complex physiological process, and a series of genes and other factors are important for accurately regulating it [
14]. Transcriptomic studies using high-throughput sequencing technology have identified several transcription factors affecting pig muscle growth and gene regulatory networks regulating muscle production by comparing Chinese and foreign pig breeds. Xu et al. [
15] compared the transcriptome data of the LD muscle of Yorkshire and Wei pigs, identified 717 differentially expressed genes (DEGs), and obtained four candidate genes related to fat metabolism (
FABP3,
PDK4,
ACSL1, and
UCP3). Shang et al. [
16] conducted transcriptomic and proteomic sequencing on Tibetan, Wujin, and White pigs. They discovered that 20 genes related to muscle fiber formation, including
CRYAB,
FSCN1, and
MAPK12, may play an important role in determining the postnatal growth rate of pigs. Zhao et al. [
17] compared the LD muscles of Tongcheng and Yorkshire pig embryos from 30 days to 5 weeks after birth and discovered that DEGs regulating muscle development showed variety-specific differential expression patterns.
In this study, transcriptomic sequencing technology was used to analyze the meat quality traits of different muscle groups from Huai and Duroc pigs to further elucidate the differences in molecular regulatory mechanisms between breeds and muscle groups. Our data could provide an important reference for subsequent improvements in livestock and poultry meat quality as well as muscle production.
2. Materials and Methods
All experimental pigs were handled in strict accordance with the good animal practices of the People’s Republic of China Ethical Procedures and Guidelines for Animals and were approved by the Animal Management and Ethics Committee of Anhui University of Science and Technology (license number 2019-002).
2.1. Animal Sampling and Meat Composition Measurements
Huai and Duroc pigs were provided by the Anhui Haoxiang Agriculture and Animal Husbandry Co., Ltd., Bozhou, China. Notably, three healthy Huai and Duroc pigs were fed under the same conditions and slaughtered at 180 days of age. LD, PM, and biceps femoris (BF) muscle tissues between the left penultimate third and fourth ribs were collected. Some samples were used for determining meat quality traits. The remaining samples were frozen in liquid nitrogen immediately after being packaged in a freezing tube and then transferred to the laboratory for storage at −80 °C until used. The IMF content was measured using Soxhlet petroleum ether extraction [
18].
The types and contents of amino acids in the LD, PM, and BF muscles of the Huai and Duroc pigs were determined according to a previously described method in
Determination of Amino Acids in National Standard Food Safety (GB 5009.124-2016) [
19].
2.2. Total RNA Extraction and Illumina Sequencing
Total RNA was extracted from muscle tissues using the TRIzol reagent (Thermo Fisher Scientific, Carlsbad, CA, USA). The concentration, purity, and integrity of total RNA were determined using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA) and an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA), using the RNAClean XP Kit (Beckman Coulter, Inc., Kraemer Boulevard Brea, CA, USA) and the RNase-Free DNase Set (QIAGEN, GmBH, Frankfurt, Germany) for purification.
A transcriptome library was constructed by Shanghai Biotechnology Corporation (Shanghai, China). Referring to the NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA), the mRNA was purified by adsorption with Oligo (dT) magnetic beads, and fragmentation buffer was added to break the mRNA into short fragments. Subsequently, a cDNA strand was synthesized through reverse transcription using six-base random hexamers as a template, and a two-strand cDNA was synthesized by adding buffer, dNTPs, and DNA polymerase I. Thereafter, the double-stranded cDNA was purified using AMPure XP beads. The purified double-stranded cDNA was repaired at the end, and A as well as splice were added. AMPure XP beads were used for fragment size selection of double-stranded cDNA and PCR amplification was performed to construct cDNA library. Finally, all libraries were sequenced.
The raw data obtained were pretreated using Fastx, and low-quality reads, such as those with only 5′ and 3′ end joints, poly-N, or low overall quality, were removed to obtain clean reads. Using the Hisat2 (version 2.0.4) software [
20], clean sequences were compared to the Sus scrofa reference genome (Sscrofa11.1) sequences to obtain the location information on the reference genome or gene to obtain mapped reads. Gene quantification of mapping results was performed using the StringTie (version:1.3.0) software [
21]. The gene expression level is positively correlated with the abundance of transcripts, and the FPKM value is used in transcriptome sequencing to measure the expression level of each gene in the sample [
18].
The DESeq software package in R was used for differential gene expression analyses. Significant DEGs are identified between any two groups based on the following thresholds: log2lfold-changel ≥ 1 and padj-value ≤ 0.05 (Benjamini and Hochberg methods) [
22].
2.3. Functional Annotation
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses of DEGs were performed using the Bioconductor package clusterProfiler [
23]. The obtained
p values were adjusted according to Benjamini and Hochberg methods, and padj values ≤ 0.05 were considered significant.
2.4. Real-Time Quantitative PCR
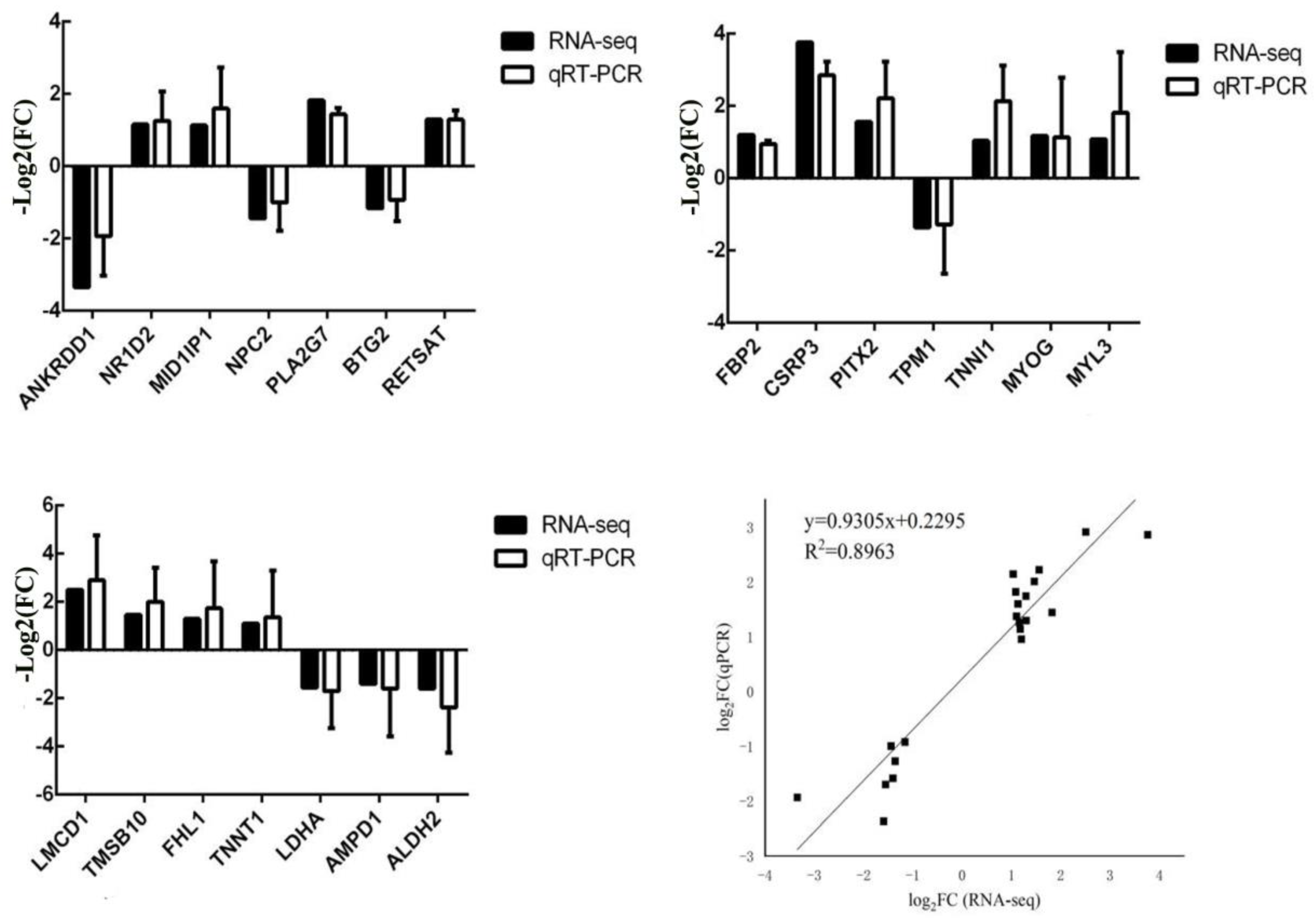
The differential expression patterns of genes detected in the transcriptome data were verified using RT-qPCR analyses. Notably, 21 DEGs were associated with muscle development.
Total RNA (50 µg) was extracted from LD, leg, and waist muscle tissue samples from different pig breeds using a TRIzol kit (Thermo Fisher, Shanghai, China). qPCR was performed on SYBR Green I-treated samples using Roche 96. Each qPCR assay was performed in a 20 µL volume consisting of 2 µL DNA template, 0.4 µL up- and downstream primers, 10 µL SYBR Premix Ex Taq II reverse transcriptase (Thermo Fisher, Shanghai, China), and 7.2 µL RNase-free ddH
2O. The fluorescence quantitative results were calculated using the 2
−ΔΔCt method, where ΔCt = Ct (target gene) − Ct (internal reference gene). Statistical analyses were performed using IBM SPASS 20.0 software, and the results are expressed as mean ± SD. The primer sequences are listed in
Table S1. All samples were tested three times, and negative controls were set up for each test.
4. Discussion
Water and IMF, the most common traits of pork, contribute to pork juiciness, and the IMF content is highly correlated with flavor. The oxidized muscle fibers (MyHC I and MyHC Пa) had lower glycogen content, higher myoglobin and phospholipid content, and higher aerobic metabolic capacity than those of MyHC IIb. The metabolic activity and contractile characteristics of MyHC IIx were between those of the oxidized and glycolytic muscle fibers. In addition, the expression levels of MyHC I, MyHC IIa, and MyHC IIx were positively correlated with pH, flesh color, marblework, and IMF content, whereas MyHC IIb expression was inversely correlated with these parameters [
25,
26]. When the proportion of oxidized muscle fibers is high, the muscle fiber diameter is smaller, the flesh color and marbling scores are higher, the drip loss is reduced, and the IMF content as well as water retention are higher [
27,
28]. In this study, the L-value, drip loss, cooking loss, and hardness of the LD, PM, and BF muscles of the Huai pigs were shown to be significantly lower than those of the Duroc pigs (
p < 0.05). In contrast, the hydraulic, elasticity, and IMF contents of the Huai pigs were significantly higher than those of the Duroc pigs (
p < 0.05), which is consistent with results from previous studies [
29,
30]. Therefore, the meat quality of Huai pigs is better than that of Duroc pigs, and the different types of muscle fibers directly affect pork quality.
For same breed animals, the type of muscle fibers in different muscle groups also varies greatly. Most muscles are composed of both oxidized and glycolytic fibers. Deep muscles have a higher proportion of oxidized fibers than shallow muscles. The BF muscle is the largest muscle tissue, with a higher oxidation capacity and lower drip loss than the LD muscle. In addition, there are important differences in IMF content between the two muscles [
31]. In pigs, the LD and BF are mainly composed of fast glycolytic fibers. The PM is mainly composed of rapidly oxidizing and intermediate fibers; therefore, the proportion of oxidized muscle fibers in the PM is significantly higher than that in the LD and BF muscles [
32,
33]. Furthermore, the IMF content in the PM is significantly higher than that in the LD and BF muscles. We also discovered that the IMF content of the PM muscle of Huai and Duroc pigs was significantly higher than that of the LD and BF muscles, respectively.
Proteins are synthesized from amino acids, and the category as well as content of amino acids directly affect the nutritional value and flavor of pig meat. Therefore, small amino acids are strongly related to the quality and flavor of pig meat. Flavor is a major determinant of meat quality and consumer purchasing decision. Amino acids that can present a special flavor are called flavor amino acids and include glutamic acid, aspartic acid, phenylalanine, alanine, glycine, and tyrosine. The content of these flavor amino acids has been suggested to be particularly important in the quality and taste of pork [
34,
35]. Glutamic acid forms umami, buffering acid, and base taste flavors and plays an important role in flavor amino acids [
36]. Fatty acids, phospholipids, and flavor amino acids affect muscle flavor traits [
37]. The content of flavor substances in oxidized muscle tissue is higher than that in glycolytic muscle tissue, which further confirms that muscles with a higher proportion of oxidized muscle fibers have a preferable flavor [
38,
39]. The content of flavor amino acids in the LD and BF muscles of the Huai pigs was significantly higher than that in the Duroc pigs (
p < 0.05), which is consistent with previous results [
19,
40]. Therefore, the amino acid content might represent the main factor that causes differences in the flavor, odor, and meat quality between the Huai and Duroc pigs.
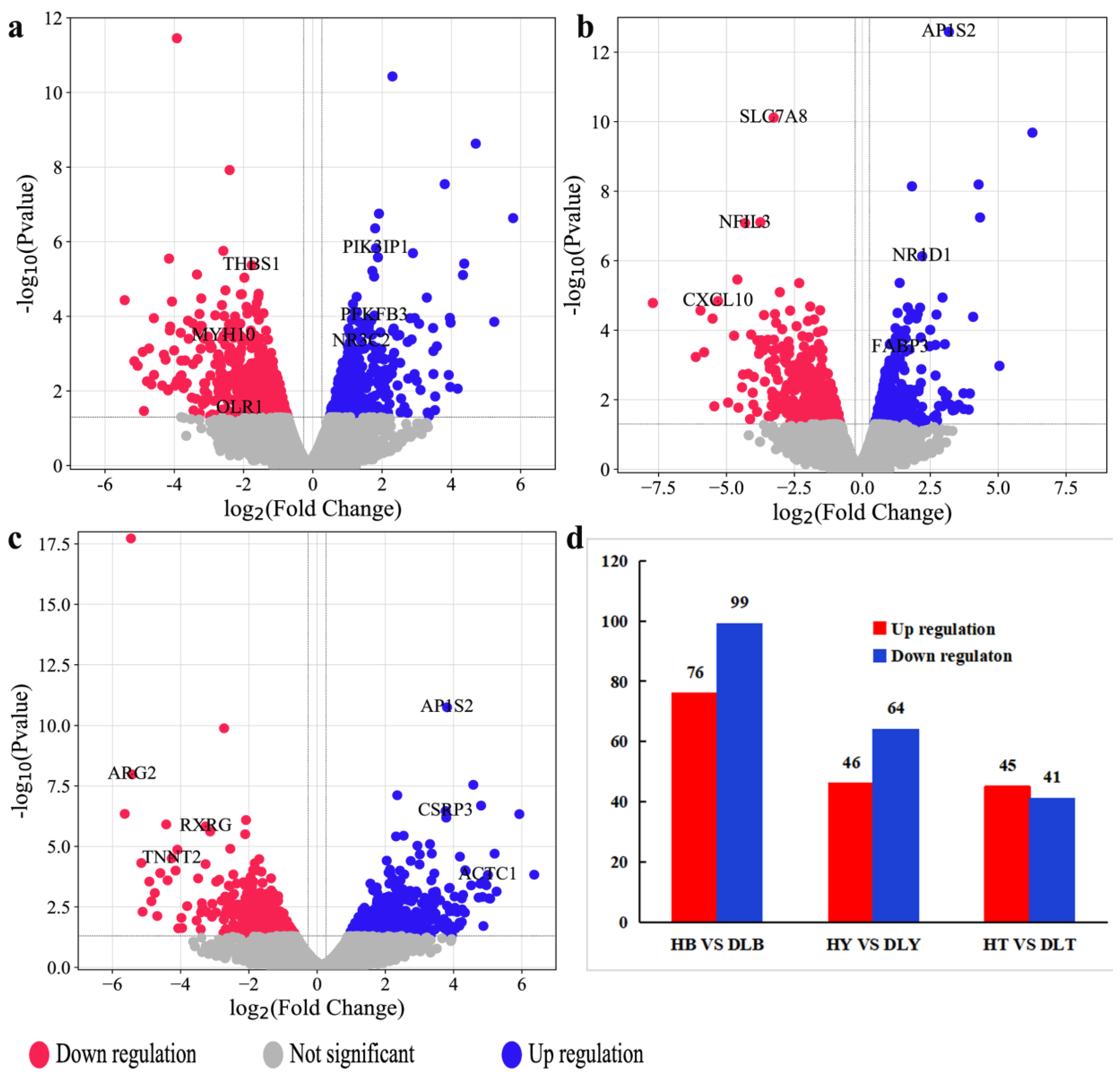
Animal muscle development is a complex process involving multiple coregulating genes and signaling pathways. GO enrichment analysis of the DEGs from different muscle groups of Huai and Duroc pigs revealed the biological processes closely related to muscle development, IMF, and lipid metabolism, including muscle tissue development, striated muscle cell differentiation, lipid metabolism regulation, and myofibril formation. Notably, seven DEGs (
CSRP3,
LMOD2,
NR3C2,
CXCL10,
ACTC1,
MYH10, and
SPTLC3) related to meat quality, muscle development, and lipid metabolism were identified during these significantly enriched biological processes. Compared to the levels in Duroc pigs,
CSRP3,
LMOD2, and
NR3C2 levels were significantly upregulated in the different muscle groups of the Huai pigs, whereas
CXCL10,
ACTC1,
MYH10, and
SPTLC3 levels were significantly downregulated. In addition, the expression levels of the same DEG differed across muscle groups within the same breed.
CXCL10 and
MYH10 are closely associated with muscle growth and development [
41,
42].
ACTC1 is a key gene involved in early myogenesis that also plays an important role in the growth and development of embryonic muscles; its low expression is conducive to muscle growth and development [
43,
44].
CSRP3 and
LMOD2 play crucial roles in muscle fiber composition, can affect the distribution of muscle fiber types as well as meat quality, and have important positive regulatory effects [
45,
46].
SPTLC3 and
NR3C2 play regulatory roles in lipid metabolism [
47,
48]. Our results showed that Huai pork was of higher quality than Duroc pork, based on six key regulatory genes identified to be related to pork quality traits and muscle development.
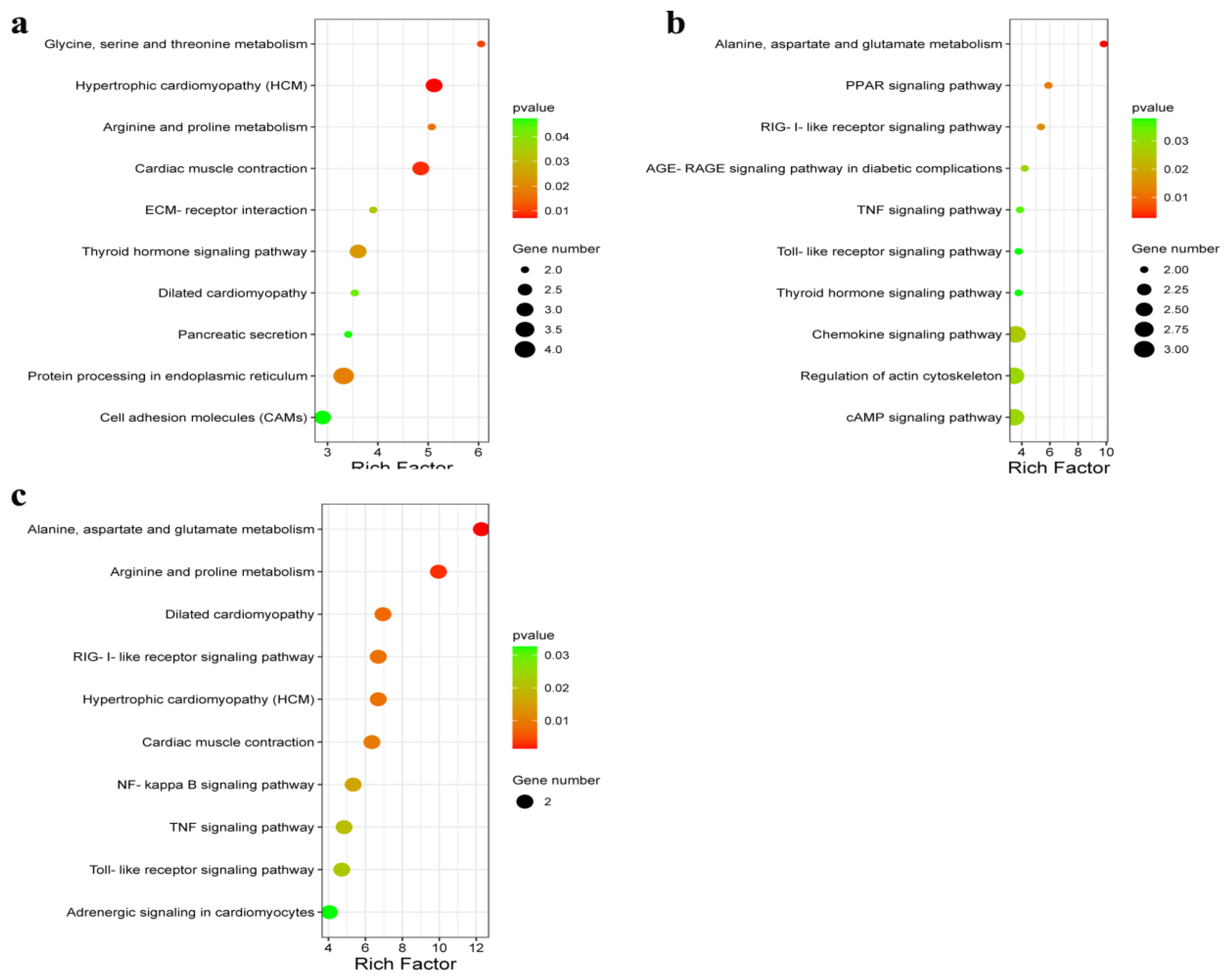
In this study, KEGG pathway analysis was used to assess the DEGs enriched in different muscle groups of Huai and Duroc pigs. The significantly enriched pathways were mainly associated with amino acid metabolism, PPAR signaling pathway, cAMP signaling pathway, and ECM–receptor interaction. The PPAR signaling pathway plays an important role in carbohydrate and lipid metabolism as well as muscle development and growth [
49]. Alternatively, cAMP signaling promotes lipid metabolism and differentiation [
50]. ECM–receptor interactions form a network with pathways related to lipid metabolism, thereby affecting IMF deposition [
51,
52]. In our analysis, Huai pigs exhibited higher expression levels of
PIM1 and
FABP3 and lower expression levels of
OLR1,
TNNT2, and
THBS1 than those observed in Duroc pigs.
PIM1 actively regulates myoblast function and skeletal muscle regeneration [
53].
FABP3 and
OLR1 play important roles in fat metabolism [
54,
55].
TNNT2 is positively correlated with IMF content [
56].
THBS1 plays an important role in animal muscle growth and development [
57]. These differences might have led to increased muscle mass in the Huai pigs compared to that observed in Duroc pigs.
5. Conclusions
Significant differences in meat quality and muscle fiber content were identified between different muscle groups of Huai and Duroc pigs. The proportion of oxidized muscle fibers in the different muscle groups of Huai pigs was significantly higher than that in Duroc pigs. The proportion of oxidized muscle fibers in the PM was significantly higher than that in the LD and BF muscles, whereas the IMF content in the PM was significantly higher than that in the LD and BF muscles. The differences in muscle fiber type and IMF content in muscles from Huai and Duroc pigs might impact meat quality traits, as reflected by the demand for different pork products and the need to improve pork yield.
In this study, the transcriptomes of LD, PM, and BF of Huai and Duroc pigs were comprehensively assessed. There were significant differences in meat quality, muscle fiber content, and IMF content between the two breeds. Several candidate genes (CSRP3, LMOD2, NR3C2, CXCL10, ACTC1, MYH10, SPTLC3) related to meat quality traits, muscle fibers, and IMF were identified. Our findings provide new insights into the regulatory mechanism of different meat quality traits associated with Huai and Duroc pigs and a theoretical reference for marker-assisted selection breeding based on DEGs to improve pork yield and quality in later stages.