Mitochondrial DNA in Human Diversity and Health: From the Golden Age to the Omics Era
Abstract
1. Introduction
2. The Mitochondrial Genome: Living in a Singular Intracellular Context
2.1. High Evolutionary Rate
2.2. High Copy Number per Cell
2.3. Maternal Inheritance
2.4. Absence of Recombination
3. Mitochondrial DNA Analysis: The Basis of Modern Human Population Genetics
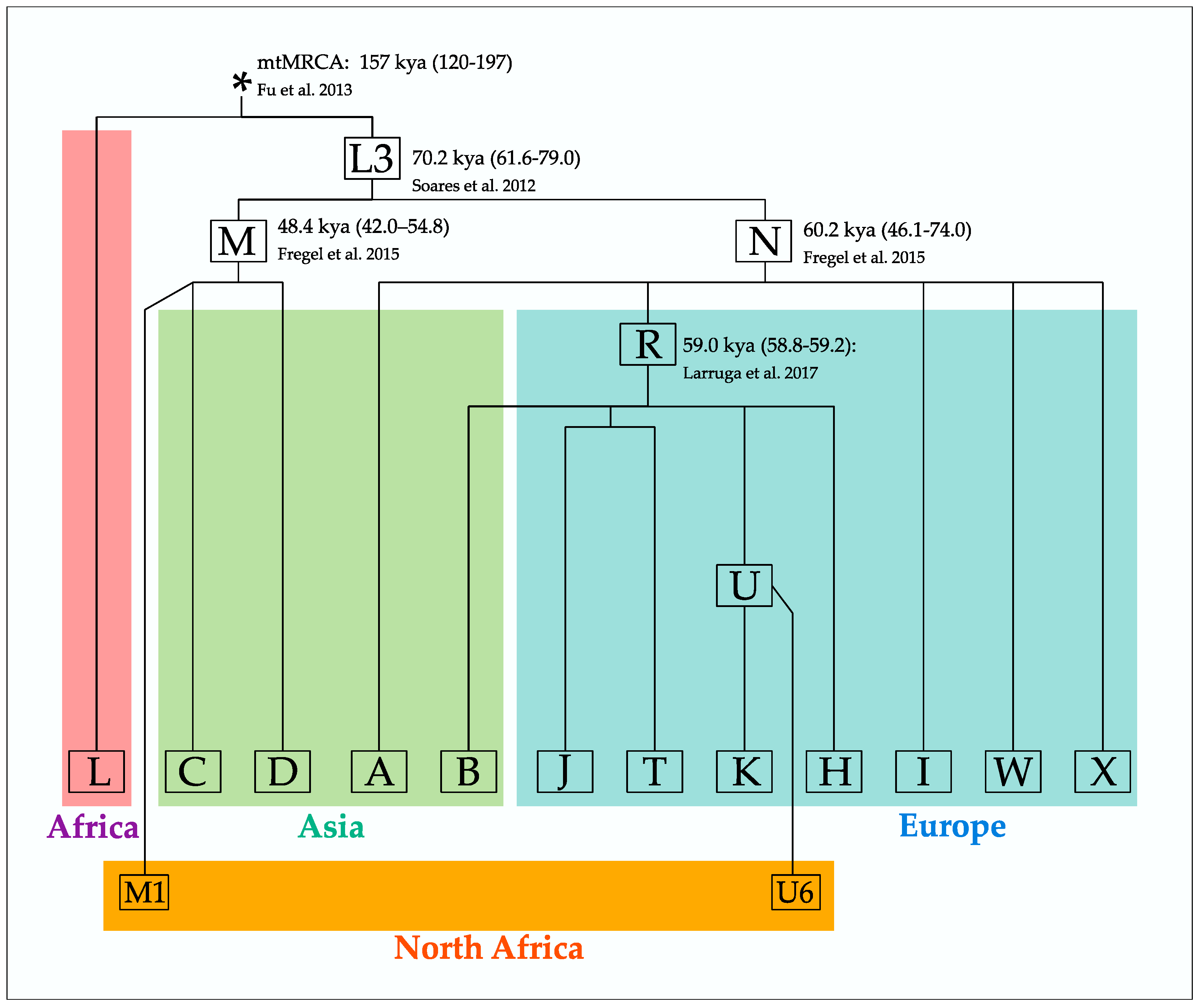
3.1. References, Nomenclature and Consensuses
3.2. Methods and Experimental Strategies
3.3. Databases and Public Repositories
| Database/Tool | Number of Samples Compiled | Type of Data | Last Update (Version) | Description | Website | Reference | Additional Tools and Resources | Scope |
|---|---|---|---|---|---|---|---|---|
| AmtDB (ancient mtDNA database) | 2548 | Mitogenomes | October 2021 | Database of ancient mitochondrial sequences, mostly from Europe. | https://amtdb.org/ | [105] | MitoPathoTool (annotation of pathological mtDNA alleles). | Population genetics and mitochondrial disease |
| EMPOP (EDNAP forensic mtDNA population database) | 48,572 | Mitogenomes, control region, HVS-I and HVS-II | November 2019 (EMPOP Release 13) | Database of mtDNA sequences. | https://empop.online/ | [106] | Haplogroup Browser; EMPcheck (validation of haplotype files); NETWORK (drawing of median networks). | Population genetics and forensics |
| gnomAD (Genome Aggregation Database) | 56,434 | Whole genome samples | November 2020 (v3.1 Mitochondrial DNA Variants) | Harmonizing exome and genome sequence data | https://gnomad.broadinstitute.org/downloads#v3-mitochondrial-dna | [107] | - | Nomenclature and notation |
| HelixMTdb | 195,983 | 14,324 Variants retrieved from mitogenomes | June 2020 | Database of mitochondrial DNA variants based on a population of ~195 k individuals. | https://www.helix.com/pages/mitochondrial-variant-database | [108] | - | Population genetics |
| HmtDB (Human Mitochondrial Genomic Resource) | 54,134 | Mitogenomes | May 2022 | Database of mitochondrial sequences annotated with population and variability data. | https://www.hmtdb.uniba.it/ | [109] | Query HmtDB (retrieving data); Genomes classification (haplogroup classification); MToolBox (pipeline to analyze human mtDNA from NGS data); HmtDB Download (download multialignments and site variability data). | Population genetics and mitochondrial disease |
| HmtVar | 54,134 | Mitogenomes | October 2022 | Explore human mitochondrial variability data and their pathological correlation. Data retrieved from twin database HmtDB | https://www.hmtvar.uniba.it/ | [110] | Query HmtVar (retrieving data); HmtVar API (retrieve variants). | Mitochondrial disease |
| MITOMAP (a human mitochondrial genome database) | 59,389 | Mostly mitogenomes (>15.4 kbp long) | January 2023 | Database of mutations (general variants and pathogenic) in human mitochondrial DNA; reference sequences. | https://www.mitomap.org/MITOMAP | [111] | Allele Search (Search MITOMAP Database for variants at given positions); MITOMASTER (haplogroup classification, variant identification, evaluation of biological significance); Marker Finder (search for variants); Sequence Finder (retrieving Hg-specific GenBank sequences); MitoTIP (pathogenicity scoring of tRNA variants) | Population genetics and mitochondrial disease |
| MSeqDR: the Mitochondrial Disease Sequence Data Resource Consortium | 316,530 | Mitogenomes | February 2021 | Genome and phenome resource to facilitate clinical diagnosis and research. | https://mseqdr.org/index.php | [112] | Disease Portal (retrieve symptoms, variants from mitochondrial diseases); HPO Browser (human phenotype ontology tree); mvTool (mtDNA variant converter). | Mitochondrial disease |
| PhyloTree | 24,275 | Mitogenomes | February 2016 (mtDNA tree Build 17) | Comprehensive and updated of human mitochondrial phylogeny. | https://www.phylotree.org/ | [113] | - | Nomenclature and notation |
| Haplogrep | - | - | April 2023 (Haplogrep 3) | Haplogroup classification service. | https://haplogrep.i-med.ac.at/ | [114] | Upload data (FASTA, VCF, txt); haplogroup classification, summary statistics, variant annotations from genome databases; phylogenies. | - |
| MitoAge | - | - | July 2015 (MitoAge Build 1.0) | Integration of mtDNA sequence data with longevity records. | https://www.mitoage.info/ | [115] | Compositional features of mitogenomes, coding and control regions and longevity records for over 900 species (including humans). | - |
| MitoScape (pipeline for obtaining mtDNA sequences from NGS data) | - | - | March 2021 | Software used for obtaining mtDNA sequence data from NGS data. | https://cavatica.sbgenomics.com/public/apps/d3b-bixu/app-publisher/mitoscape-wf | [116] | Alignment, extracting mtDNA sequences, variant calling. | - |
| Mitoverse | - | - | February 2021 | Platform for analyzing mtDNA from NGS and microarray data. | https://mitoverse.i-med.ac.at/index.html#! | [117,118] | Haplocheck (detecting contamination); mtDNA-Server (mtDNA analysis, heteroplasmy identification, contamination). | - |
3.4. Genetics, Genealogy and Public Engagement
3.5. Some Final Thoughts on the Use of mtDNA in Human Phylogeny and Phylogeography
4. Mitochondrial DNA and Health
4.1. Diseases: Main Features and Population Approaches
4.2. Aging and Longevity
5. Present and Future of Mitochondrial Studies
5.1. MtDNA in Current Human Population Genetics
5.2. The Mitochondrial Genome in Human Health
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef]
- Monzel, A.S.; Enríquez, J.A.; Picard, M. Multifaceted Mitochondria: Moving Mitochondrial Science beyond Function and Dysfunction. Nat. Metab. 2023, 5, 546–562. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial DNA Variation in Human Radiation and Disease. Cell 2015, 163, 33–38. [Google Scholar] [CrossRef]
- Mishmar, D.; Ruiz-Pesini, E.; Golik, P.; Macaulay, V.; Clark, A.G.; Hosseini, S.; Brandon, M.; Easley, K.; Chen, E.; Brown, M.D.; et al. Natural Selection Shaped Regional MtDNA Variation in Humans. Proc. Natl. Acad. Sci. USA 2003, 100, 171–176. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Mishmar, D.; Brandon, M.; Procaccio, V.; Wallace, D.C. Effects of Purifying and Adaptive Selection on Regional Variation in Human MtDNA. Science 2004, 303, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Kivisild, T.; Shen, P.; Wall, D.P.; Do, B.; Sung, R.; Davis, K.; Passarino, G.; Underhill, P.A.; Scharfe, C.; Torroni, A.; et al. The Role of Selection in the Evolution of Human Mitochondrial Genomes. Genetics 2006, 172, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Cann, R.L.; Stoneking, M.; Wilson, A.C. Mitochondrial DNA and Human Evolution. Nature 1987, 325, 31–36. [Google Scholar]
- Chinnery, P.F.; Elliott, H.R.; Hudson, G.; Samuels, D.C.; Relton, C.L. Epigenetics, Epidemiology and Mitochondrial DNA Diseases. Int. J. Epidemiol. 2012, 41, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Russell, O.M.; Gorman, G.S.; Lightowlers, R.N.; Turnbull, D.M. Mitochondrial Diseases: Hope for the Future. Cell 2020, 181, 168–188. [Google Scholar] [CrossRef]
- Harman, D. Role of Free Radicals in Aging and Disease. Ann. N. Y. Acad. Sci. 1992, 673, 126–141. [Google Scholar]
- Chinnery, P.F. Mitochondrial DNA in Homo Sapiens. In Human Mitochondrial DNA and the Evolution of Homo Sapiens; Bandelt, H.-J., Macaulay, V., Richards, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Ballard, J.W.O.; Whitlock, M.C. The Incomplete Natural History of Mitochondria. Mol. Ecol. 2004, 13, 729–744. [Google Scholar] [CrossRef]
- Schon, E.A.; Dimauro, S.; Hirano, M. Human Mitochondrial DNA: Roles of Inherited and Somatic Mutations. Nat. Rev. Genet. 2012, 13, 878–890. [Google Scholar] [CrossRef]
- Brown, W.M.; George, M.; Wilson, A.C. Rapid Evolution of Animal Mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1971. [Google Scholar]
- Excoffier, L.; Yang, Z. Substitution Rate Variation among Sites in Mitochondrial Hypervariable Region I of Humans and Chimpanzees. Mol. Biol. Evol. 1999, 16, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Sigurğardóttir, S.; Helgason, A.; Gulcher, J.R.; Stefansson, K.; Donnelly, P. The Mutation Rate in the Human MtDNA Control Region. Am. J. Hum. Genet. 2000, 66, 1599–1609. [Google Scholar] [CrossRef]
- Endicott, P.; Ho, S.Y.W. A Bayesian Evaluation of Human Mitochondrial Substitution Rates. Am. J. Hum. Genet. 2008, 82, 895–902. [Google Scholar] [CrossRef]
- Henn, B.M.; Gignoux, C.R.; Feldman, M.W.; Mountain, J.L. Characterizing the Time Dependency of Human Mitochondrial DNA Mutation Rate Estimates. Mol. Biol. Evol. 2009, 26, 217–230. [Google Scholar] [CrossRef]
- Soares, P.; Ermini, L.; Thomson, N.; Mormina, M.; Rito, T.; Röhl, A.; Salas, A.; Oppenheimer, S.; Macaulay, V.; Richards, M.B. Correcting for Purifying Selection: An Improved Human Mitochondrial Molecular Clock. Am. J. Hum. Genet. 2009, 84, 740–759. [Google Scholar] [CrossRef]
- Loogväli, E.-L.; Kivisild, T.; Margus, T.; Villems, R. Explaining the Imperfection of the Molecular Clock of Hominid Mitochondria. PLoS ONE 2009, 4, e8260. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Mittnik, A.; Johnson, P.; Bos, K.; Lari, M.; Bollongino, R.; Sun, C.; Giemsch, L.; Schmitz, R.; Burger, J.; et al. A Revised Timescale for Human Evolution Based on Ancient Mitochondrial Genomes. Curr. Biol. 2013, 23, 553–559. [Google Scholar] [PubMed]
- Rieux, A.; Eriksson, A.; Li, M.; Sobkowiak, B.; Weinert, L.A.; Warmuth, V.; Ruiz-Linares, A.; Manica, A.; Balloux, F. Improved Calibration of the Human Mitochondrial Clock Using Ancient Genomes. Mol. Biol. Evol. 2014, 31, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, V.M. Human Molecular Evolutionary Rate, Time Dependency and Transient Polymorphism Effects Viewed through Ancient and Modern Mitochondrial DNA Genomes. Sci. Rep. 2021, 11, 5036. [Google Scholar] [CrossRef] [PubMed]
- Översti, S.; Palo, J.U. Variation in the Substitution Rates among the Human Mitochondrial Haplogroup U Sublineages. Genome Biol. Evol. 2022, 14, evac097. [Google Scholar] [CrossRef]
- Crimi, M.; Rigolio, R. The Mitochondrial Genome, a Growing Interest inside an Organelle. Int. J. Nanomed. 2008, 3, 51–57. [Google Scholar]
- Krishnan, K.J.; Greaves, L.C.; Reeve, A.K.; Turnbull, D. The Ageing Mitochondrial Genome. Nucleic Acids Res. 2007, 35, 7399–7405. [Google Scholar] [CrossRef]
- Tuppen, H.A.L.; Blakely, E.L.; Turnbull, D.M.; Taylor, R.W. Mitochondrial DNA Mutations and Human Disease. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 113–128. [Google Scholar] [CrossRef]
- Taylor, R.; Turnbull, D.M. Mitochondrial DNA Mutations in Human Disease. Nat. Rev. Genet. 2005, 6, 389–402. [Google Scholar] [CrossRef]
- Stewart, J.B.; Chinnery, P.F. The Dynamics of Mitochondrial DNA Heteroplasmy: Implications for Human Health and Disease. Nat. Rev. Genet. 2015, 16, 530–542. [Google Scholar] [CrossRef]
- Budowle, B.; Allard, M.W.; Wilson, M.R.; Chakraborty, R. Forensics and Mitochondrial DNA: Applications, Debates, and Foundations. Annu. Rev. Genom. Hum. Genet. 2003, 4, 119–141. [Google Scholar] [CrossRef]
- Pääbo, S. Ancient DNA: Extraction, Characterization, Molecular Cloning, and Enzymatic Amplification. Proc. Natl. Acad. Sci. USA 1989, 86, 1939–1943. [Google Scholar] [CrossRef]
- Amorim, A.; Fernandes, T.; Taveira, N. Mitochondrial DNA in Human Identification: A Review. PeerJ 2019, 7, e7314. [Google Scholar] [CrossRef]
- Pakendorf, B.; Stoneking, M. Mitochondrial DNA and Human Evolution. Annu. Rev. Genom. Hum. Genet. 2005, 6, 165–183. [Google Scholar] [CrossRef]
- Giles, R.E.; Blanc, H.; Cann, H.M.; Wallace, D.C. Maternal Inheritance of Human Mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1980, 77, 6715–6719. [Google Scholar] [CrossRef]
- White, D.J.; Wolff, J.N.; Pierson, M.; Gemmell, N.J. Revealing the Hidden Complexities of MtDNA Inheritance. Mol. Ecol. 2008, 17, 4925–4942. [Google Scholar] [CrossRef]
- Schwartz, M.; Vissing, J. Paternal Inheritance of Mitochondrial DNA. N. Engl. J. Med. 2002, 347, 576–580. [Google Scholar]
- Luo, S.; Valencia, C.A.; Zhang, J.; Lee, N.-C.; Slone, J.; Gui, B.; Wang, X.; Li, Z.; Dell, S.; Brown, J.; et al. Biparental Inheritance of Mitochondrial DNA in Humans. Proc. Natl. Acad. Sci. USA 2018, 115, 13039–13044. [Google Scholar] [CrossRef]
- Rius, R.; Cowley, M.J.; Riley, L.; Puttick, C.; Thorburn, D.R.; Christodoulou, J. Biparental Inheritance of Mitochondrial DNA in Humans Is Not a Common Phenomenon. Genet. Med. 2019, 21, 2823–2826. [Google Scholar] [CrossRef]
- Wei, W.; Chinnery, P.F. Inheritance of Mitochondrial DNA in Humans: Implications for Rare and Common Diseases. J. Intern. Med. 2020, 287, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Kraytsberg, Y.; Schwartz, M.; Brown, T.A.; Ebralidse, K.; Kunz, W.S.; Clayton, D.A.; Vissing, J.; Khrapko, K. Recombination of Human Mitochondrial DNA. Science 2004, 304, 981. [Google Scholar] [CrossRef] [PubMed]
- Slate, J.; Gemmell, N. Eve “n” Steve: Recombination of Human Mitochondrial DNA. Trends Ecol. Evol. 2004, 19, 561–563. [Google Scholar] [CrossRef]
- Underhill, P.A.; Kivisild, T. Use of Y Chromosome and Mitochondrial DNA Population Structure in Tracing Human Migrations. Annu. Rev. Genet. 2007, 41, 539–564. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and Organization of the Human Mitochondrial Genome. Nature 1981, 290, 457–465. [Google Scholar] [PubMed]
- Andrews, R.M.; Kubacka, I.; Chinnery, P.F.; Lightowlers, R.N.; Turnbull, D.M.; Howell, N. Reanalysis and Revision of the Cambridge Reference Sequence for Human Mitochondrial DNA. Nat. Genet. 1999, 23, 147. [Google Scholar] [CrossRef] [PubMed]
- Behar, D.M.; van Oven, M.; Rosset, S.; Metspalu, M.; Loogväli, E.-L.; Silva, N.M.; Kivisild, T.; Torroni, A.; Villems, R. A “Copernican” Reassessment of the Human Mitochondrial DNA Tree from Its Root. Am. J. Hum. Genet. 2012, 90, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Pennarun, E.; Kivisild, T.; Metspalu, E.; Metspalu, M.; Reisberg, T.; Behar, D.M.; Jones, S.C.; Villems, R. Divorcing the Late Upper Palaeolithic Demographic Histories of MtDNA Haplogroups M1 and U6 in Africa. BMC Evol. Biol. 2012, 12, 234. [Google Scholar] [CrossRef]
- Barbieri, C.; Vicente, M.; Rocha, J.; Mpoloka, S.W.; Stoneking, M.; Pakendorf, B. Ancient Substructure in Early MtDNA Lineages of Southern Africa. Am. J. Hum. Genet. 2013, 92, 285–292. [Google Scholar] [CrossRef]
- Brotherton, P.; Haak, W.; Templeton, J.; Brandt, G.; Soubrier, J.; Jane Adler, C.; Richards, S.M.; Sarkissian, C.D.; Ganslmeier, R.; Friederich, S.; et al. Neolithic Mitochondrial Haplogroup H Genomes and the Genetic Origins of Europeans. Nat. Commun. 2013, 4, 1764. [Google Scholar] [CrossRef]
- Sarno, S.; Boattini, A.; Carta, M.; Ferri, G.; Alù, M.; Yao, D.Y.; Ciani, G.; Pettener, D.; Luiselli, D. An Ancient Mediterranean Melting Pot: Investigating the Uniparental Genetic Structure and Population History of Sicily and Southern Italy. PLoS ONE 2014, 9, e96074. [Google Scholar] [CrossRef]
- De Fanti, S.; Barbieri, C.; Sarno, S.; Sevini, F.; Vianello, D.; Tamm, E.; Metspalu, E.; van Oven, M.; Hübner, A.; Sazzini, M.; et al. Fine Dissection of Human Mitochondrial DNA Haplogroup HV Lineages Reveals Paleolithic Signatures from European Glacial Refugia. PLoS ONE 2015, 10, e0144391. [Google Scholar] [CrossRef]
- Olivieri, A.; Pala, M.; Gandini, F.; Hooshiar Kashani, B.; Perego, U.A.; Woodward, S.R.; Grugni, V.; Battaglia, V.; Semino, O.; Achilli, A.; et al. Mitogenomes from Two Uncommon Haplogroups Mark Late Glacial/Postglacial Expansions from the Near East and Neolithic Dispersals within Europe. PLoS ONE 2013, 8, e70492. [Google Scholar] [CrossRef]
- Gandini, F.; Achilli, A.; Pala, M.; Bodner, M.; Brandini, S.; Huber, G.; Egyed, B.; Ferretti, L.; Gómez-Carballa, A.; Salas, A.; et al. Mapping Human Dispersals into the Horn of Africa from Arabian Ice Age Refugia Using Mitogenomes. Sci. Rep. 2016, 6, 25472. [Google Scholar] [CrossRef]
- Posth, C.; Wißing, C.; Kitagawa, K.; Pagani, L.; van Holstein, L.; Racimo, F.; Wehrberger, K.; Conard, N.J.; Kind, C.J.; Bocherens, H.; et al. Deeply Divergent Archaic Mitochondrial Genome Provides Lower Time Boundary for African Gene Flow into Neanderthals. Nat. Commun. 2017, 8, 16046. [Google Scholar] [CrossRef]
- Sahakyan, H.; Hooshiar Kashani, B.; Tamang, R.; Kushniarevich, A.; Francis, A.; Costa, M.D.; Pathak, A.K.; Khachatryan, Z.; Sharma, I.; van Oven, M.; et al. Origin and Spread of Human Mitochondrial DNA Haplogroup U7. Sci. Rep. 2017, 7, 46044. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.G.; Heinze, A.; Chan, E.K.F.; Smith, A.B.; Hayes, V.M. First Ancient Mitochondrial Human Genome from a Prepastoralist Southern African. Genome Biol. Evol. 2014, 6, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Fregel, R.; Cabrera, V.; Larruga, J.M.; Abu-Amero, K.K.; González, A.M. Carriers of Mitochondrial DNA Macrohaplogroup N Lineages Reached Australia around 50,000 Years Ago Following a Northern Asian Route. PLoS ONE 2015, 10, e0129839. [Google Scholar] [CrossRef]
- Hervella, M.; Svensson, E.M.; Alberdi, A.; Günther, T.; Izagirre, N.; Munters, A.R.; Alonso, S.; Ioana, M.; Ridiche, F.; Soficaru, A.; et al. The Mitogenome of a 35,000-Year-Old Homo Sapiens from Europe Supports a Palaeolithic Back-Migration to Africa. Sci. Rep. 2016, 6, 25501. [Google Scholar] [CrossRef]
- Bandelt, H.-J.; Kloss-Brandstätter, A.; Richards, M.B.; Yao, Y.-G.; Logan, I. The Case for the Continuing Use of the Revised Cambridge Reference Sequence (RCRS) and the Standardization of Notation in Human Mitochondrial DNA Studies. J. Hum. Genet. 2014, 59, 66–77. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: Retrospect and Prospect. J. Biogeogr. 2009, 36, 3–15. [Google Scholar] [CrossRef]
- Torroni, A.; Schurr, T.G.; Cabell, M.F.; Brown, M.D.; Neel, J.V.; Larsen, M.; Smith, D.G.; Vullo, C.M.; Wallace, D.C. Asian Affinities and Continental Radiation of the Four Founding Native American MtDNAs. Am. J. Hum. Genet. 1993, 53, 563–590. [Google Scholar]
- Hernández, C.L.; Calderón, R. Matrilineal Heritage in Southern Iberia Reveals Deep Genetic Links between Continents. Coll. Antropol. 2017, 41, 1–10. [Google Scholar] [PubMed]
- Torroni, A.; Schurr, T.G.; Yang, C.-C.; Szathmary, E.J.E.; Williams, R.C.; Schanfield, M.S.; Troup, G.A.; Knowler, W.C.; Lawrence, D.N.; Weiss, K.M.; et al. Native American Mitochondrial DNA Analysis Indicates That the Amerind and the Nadene Populations Were Founded by Two Independent Migrations. Genetics 1992, 130, 153–162. [Google Scholar] [CrossRef]
- Torroni, A.; Lott, M.T.; Cabell, M.F.; Chen, Y.-S.; Lavergne, L.; Wallace, D.C. MtDNA and the Origin of Caucasians: Identification of Ancient Caucasian-Specific Haplogroups, One of Which Is Prone to a Recurrent Somatic Duplication in the D-Loop Region. Am. J. Hum. Genet. 1994, 55, 760–776. [Google Scholar] [PubMed]
- Torroni, A.; Huoponen, K.; Francalacci, P.; Petrozzi, M.; Morelli, L.; Scozzari, R.; Obinu, D.; Savontaus, M.-L.; Wallace, D.C. Classification of European MtDNAs from an Analysis of Three European Populations. Genetics 1996, 144, 1835–1850. [Google Scholar]
- Olivieri, A.; Achilli, A.; Pala, M.; Battaglia, V.; Fornarino, S.; Al-Zahery, N.; Scozzari, R.; Cruciani, F.; Behar, D.M.; Dugoujon, J.M.; et al. The MtDNA Legacy of the Levantine Early Upper Palaeolithic in Africa. Science 2006, 314, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.; Alshamali, F.; Pereira, J.B.; Fernandes, V.; Silva, N.M.; Afonso, C.; Costa, M.D.; Musilová, E.; Macaulay, V.; Richards, M.B.; et al. The Expansion of MtDNA Haplogroup L3 within and out of Africa. Mol. Biol. Evol. 2012, 29, 915–927. [Google Scholar] [CrossRef]
- Larruga, J.M.; Marrero, P.; Abu-Amero, K.K.; Golubenko, M.V.; Cabrera, V.M. Carriers of Mitochondrial DNA Macrohaplogroup R Colonized Eurasia and Australasia from a Southeast Asia Core Area. BMC Evol. Biol. 2017, 17, 115. [Google Scholar] [CrossRef]
- Richards, M.B.; Macaulay, V.A.; Bandelt, H.-J.; Sykes, B.C. Phylogeography of Mitochondrial DNA in Western Europe. Ann. Hum. Genet. 1998, 62, 241–260. [Google Scholar] [CrossRef]
- Kivisild, T.; Metspalu, M.; Bandelt, H.-J.; Richards, M.; Villems, R. The World MtDNA Phylogeny. In Human Mitochondrial DNA and the Evolution of Homo Sapiens; Bandelt, H.-J., Macaulay, V., Richards, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- The Y Chromosome Consortium. A Nomenclature System for the Tree of Human Y-Chromosomal Binary Haplogroups. Genome Res. 2002, 12, 339–348. [Google Scholar] [CrossRef]
- Johnson, M.J.; Wallace, D.C.; Ferris, S.D.; Rattazzi, M.C.; Cavalli-Sforza, L.L. Radiation of Human Mitochondria DNA Types Analyzed by Restriction Endonuclease Cleavage Patterns. J. Mol. Evol. 1983, 19, 255–271. [Google Scholar] [PubMed]
- Vigilant, L.; Pennington, R.; Harpending, H.; Kocher, T.D.; Wilson, A.C. Mitochondrial DNA Sequences in Single Hairs from a Southern African Population. Proc. Natl. Acad. Sci. USA 1989, 86, 9350–9354. [Google Scholar] [CrossRef] [PubMed]
- Horai, S.; Hayasaka, K. Intraspecific Nucleotide Sequence Differences in the Major Noncoding Region of Human Mitochondrial DNA. Am. J. Hum. Genet. 1990, 46, 828–842. [Google Scholar] [PubMed]
- Di Rienzo, A.; Wilson, A.C. Branching Pattern in the Evolutionary Tree for Human Mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1991, 88, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Côrte-Real, H.; Forster, P.; Macaulay, V.; Wilkinson-Herbots, H.; Demaine, A.; Papiha, S.; Hedges, R.; Bandelt, H.-J.; Sykes, B. Paleolithic and Neolithic Lineages in the European Mitochondrial Gene Pool. Am. J. Hum. Genet. 1996, 59, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Parson, W.; Parsons, T.J.; Scheithauer, R.; Holland, M.M. Population Data for 101 Austrian Caucasian Mitochondrial DNA D-Loop Sequences: Application of MtDNA Sequence Analysis to a Forensic Case. Int. J. Leg. Med. 1998, 111, 124–132. [Google Scholar]
- Helgason, A.; Hickey, E.; Goodacre, S.; Bosnes, V.; Stefánsson, K.; Ward, R.; Sykes, B. MtDNA and the Islands of the North Atlantic: Estimating the Proportions of Norse and Gaelic Ancestry. Am. J. Hum. Genet. 2001, 68, 723–737. [Google Scholar] [CrossRef]
- Macaulay, V.; Richards, M.; Hickey, E.; Vega, E.; Cruciani, F.; Guida, V.; Scozzari, R.; Bonné-Tamir, B.; Sykes, B.; Torroni, A. The Emerging Tree of West Eurasian MtDNAs: A Synthesis of Control-Region Sequences and RFLPs. Am. J. Hum. Genet. 1999, 64, 232–249. [Google Scholar] [CrossRef]
- Brandstätter, A.; Parsons, T.J.; Parson, W. Rapid Screening of MtDNA Coding Region SNPs for the Identification of West European Caucasian Haplogroups. Int. J. Leg. Med. 2003, 117, 291–298. [Google Scholar] [CrossRef]
- Quintáns, B.; Álvarez-Iglesias, V.; Salas, A.; Phillips, C.; Lareu, M.V.; Carracedo, A. Typing of Mitochondrial DNA Coding Region SNPs of Forensic and Anthropological Interest Using SNaPshot Minisequencing. Forensic. Sci. Int. 2004, 140, 251–257. [Google Scholar] [CrossRef]
- Rootsi, S.; Magri, C.; Kivisild, T.; Benuzzi, G.; Help, H.; Bermisheva, M.; Kutuev, I.; Barać, L.; Pericić, M.; Balanovsky, O.; et al. Phylogeography of Y-Chromosome Haplogroup I Reveals Distinct Domains of Prehistoric Gene Flow in Europe. Am. J. Hum. Genet. 2004, 75, 128–137. [Google Scholar] [CrossRef]
- Pereira, L.; Silva, N.M.; Franco-Duarte, R.; Fernandes, V.; Pereira, J.B.; Costa, M.D.; Martins, H.; Soares, P.; Behar, D.M.; Richards, M.; et al. Population Expansion in the North African Late Pleistocene Signalled by Mitochondrial DNA Haplogroup U6. BMC Evol. Biol. 2010, 10, 390. [Google Scholar] [CrossRef]
- Schönberg, A.; Theunert, C.; Li, M.; Stoneking, M.; Nasidze, I. High-Throughput Sequencing of Complete Human MtDNA Genomes from the Caucasus and West Asia: High Diversity and Demographic Inferences. Eur. J. Hum. Genet. 2011, 19, 988–994. [Google Scholar] [CrossRef]
- Behar, D.M.; Harmant, C.; Manry, J.; van Oven, M.; Haak, W.; Martinez-Cruz, B.; Salaberria, J.; Oyharçabal, B.; Bauduer, F.; Comas, D.; et al. The Basque Paradigm: Genetic Evidence of a Maternal Continuity in the Franco-Cantabrian Region since Pre-Neolithic Times. Am. J. Hum. Genet. 2012, 90, 486–493. [Google Scholar] [CrossRef]
- Fernandes, V.; Alshamali, F.; Alves, M.; Costa, M.D.; Pereira, J.B.; Silva, N.M.; Cherni, L.; Harich, N.; Cerny, V.; Soares, P.; et al. The Arabian Cradle: Mitochondrial Relicts of the First Steps along the Southern Route out of Africa. Am. J. Hum. Genet. 2012, 90, 347–355. [Google Scholar] [CrossRef]
- Pala, M.; Olivieri, A.; Achilli, A.; Accetturo, M.; Metspalu, E.; Reidla, M.; Tamm, E.; Karmin, M.; Reisberg, T.; Hooshiar Kashani, B.; et al. Mitochondrial DNA Signals of Late Glacial Recolonization of Europe from near Eastern Refugia. Am. J. Hum. Genet. 2012, 90, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Achilli, A.; Perego, U.A.; Lancioni, H.; Olivieri, A.; Gandini, F.; Hooshiar Kashani, B.; Battaglia, V.; Grugni, V.; Angerhofer, N.; Rogers, M.P.; et al. Reconciling Migration Models to the Americas with the Variation of North American Native Mitogenomes. Proc. Natl. Acad. Sci. USA 2013, 110, 14308–14313. [Google Scholar] [CrossRef]
- Hernández, C.L.; Soares, P.; Dugoujon, J.M.; Novelletto, A.; Rodríguez, J.N.; Rito, T.; Oliveira, M.; Melhaoui, M.; Baali, A.; Pereira, L.; et al. Early Holocenic and Historic MtDNA African Signatures in the Iberian Peninsula: The Andalusian Region as a Paradigm. PLoS ONE 2015, 10, e0139784. [Google Scholar] [CrossRef]
- Torroni, A.; Rengo, C.; Guida, V.; Cruciani, F.; Sellitto, D.; Coppa, A.; Luna Calderon, F.; Simionati, B.; Valle, G.; Richards, M.; et al. Do the Four Clades of the MtDNA Haplogroup L2 Evolve at Different Rates? Am. J. Hum. Genet. 2001, 69, 1348–1356. [Google Scholar] [CrossRef]
- Taylor, R.W.; Taylor, G.A.; Durham, S.E.; Turnbull, D.M. The Determination of Complete Human Mitochondrial DNA Sequences in Single Cells: Implications for the Study of Somatic Mitochondrial DNA Point Mutations. Nucleic Acids Res. 2001, 29, e74. [Google Scholar] [CrossRef] [PubMed]
- Maca-Meyer, N.; González, A.M.; Larruga, J.M.; Flores, C.; Cabrera, V.M. Major Genomic Mitochondrial Lineages Delineate Early Human Expansions. BMC Genet. 2001, 2, 13. [Google Scholar] [CrossRef]
- Li, M.; Schönberg, A.; Schaefer, M.; Schroeder, R.; Nasidze, I.; Stoneking, M. Detecting Heteroplasmy from High-Throughput Sequencing of Complete Human Mitochondrial DNA Genomes. Am. J. Hum. Genet. 2010, 87, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.X.; Sivakumar, I.K.A.; Maragh, S.; Veeramachaneni, V.; Hariharan, R.; Parulekar, M.; Fredrikson, K.M.; Harkins, T.T.; Lin, J.; Feldman, A.B.; et al. Next-Generation Sequencing of Human Mitochondrial Reference Genomes Uncovers High Heteroplasmy Frequency. PLoS Comput. Biol. 2012, 8, e1002737. [Google Scholar] [CrossRef]
- Gunnarsdóttir, E.D.; Li, M.; Bauchet, M.; Finstermeier, K.; Stoneking, M. High-throughput sequencing of complete human mtDNA genomes from the Philippines. Genome Res. 2010, 21, 1–11. [Google Scholar] [CrossRef]
- De Fanti, S.; Vianello, D.; Giuliani, C.; Quagliariello, A.; Cherubini, A.; Sevini, F.; Iaquilano, N.; Franceschi, C.; Sazzini, M.; Luiselli, D. Massive Parallel Sequencing of Human Whole Mitochondrial Genomes with Ion Torrent Technology: An Optimized Workflow for Anthropological and Population Genetics Studies. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2017, 28, 843–850. [Google Scholar] [CrossRef]
- Strobl, C.; Churchill Cihlar, J.; Lagacé, R.; Wootton, S.; Roth, C.; Huber, N.; Schnaller, L.; Zimmermann, B.; Huber, G.; Lay Hong, S.; et al. Evaluation of Mitogenome Sequence Concordance, Heteroplasmy Detection, and Haplogrouping in a Worldwide Lineage Study Using the Precision ID MtDNA Whole Genome Panel. Forensic Sci. Int. Genet. 2019, 42, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Avila, E.; Graebin, P.; Chemale, G.; Freitas, J.; Kahmann, A.; Alho, C.S. Full MtDNA Genome Sequencing of Brazilian Admixed Populations: A Forensic-Focused Evaluation of a MPS Application as an Alternative to Sanger Sequencing Methods. Forensic Sci. Int. Genet. 2019, 42, 154–164. [Google Scholar] [CrossRef]
- Chen, R.; Aldred, M.A.; Xu, W.; Zein, J.; Bazeley, P.; Suzy, A.; Comhair, A.; Meyers, D.A.; Bleecker, E.R.; Liu, C.; et al. Comparison of Whole Genome Sequencing and Targeted Sequencing for Mitochondrial DNA. Mitochondrion 2021, 58, 303–310. [Google Scholar] [CrossRef]
- LaBerge, G.S.; Shelton, R.J.; Danielson, P.B. Forensic Utility of Mitochondrial DNA Analysis Based on Denaturing High-Performance Liquid Chromatography. Croat. Med. J. 2003, 44, 281–288. [Google Scholar] [PubMed]
- Dobrowolski, S.F.; Gray, J.; Miller, T.; Sears, M. Identifying Sequence Variants in the Human Mitochondrial Genome Using High-Resolution Melt (HRM) Profiling. Hum. Mutat. 2009, 30, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A.; Cohen, Y.; Gillespie, S.E.D.; Mambo, E.; Fukushima, N.; Hoque, M.O.; Shah, N.; Goggins, M.; Califano, J.; Sidransky, D.; et al. The Human MitoChip: A High-Throughput Sequencing Microarray for Mitochondrial Mutation Detection. Genome Res. 2004, 14, 812–819. [Google Scholar] [CrossRef]
- Hartmann, A.; Thieme, M.; Nanduri, L.K.; Stempfl, T.; Moehle, C.; Kivisild, T.; Oefner, P.J. Validation of Microarray-Based Resequencing of 93 Worldwide Mitochondrial Genomes. Hum. Mutat. 2009, 30, 115–122. [Google Scholar] [CrossRef]
- van Oven, M. PhyloTree Build 17: Growing the Human Mitochondrial DNA Tree. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, e392–e394. [Google Scholar] [CrossRef]
- Dür, A.; Huber, N.; Parson, W. Fine-Tuning Phylogenetic Alignment and Haplogrouping of Mtdna Sequences. Int. J. Mol. Sci. 2021, 22, 5747. [Google Scholar] [CrossRef] [PubMed]
- Ehler, E.; Novotný, J.; Juras, A.; Chyleński, M.; Moravčík, O.; Pačes, J. AmtDB: A Database of Ancient Human Mitochondrial Genomes. Nucleic Acids Res. 2018, 47, D29–D32. [Google Scholar] [CrossRef]
- Parson, W.; Dür, A. EMPOP-A Forensic MtDNA Database. Forensic Sci. Int. Genet. 2007, 1, 88–92. [Google Scholar] [CrossRef]
- Laricchia, K.M.; Lake, N.J.; Watts, N.A.; Shand, M.; Haessly, A.; Gauthier, L.; Benjamin, D.; Banks, E.; Soto, J.; Garimella, K.; et al. Mitochondrial DNA Variation across 56,434 Individuals in GnomAD. Genome Res. 2022, 32, 569–582. [Google Scholar] [CrossRef]
- Bolze, A.; Mendez, F.; White, S.; Tanudjaja, F.; Isaksson, M.; Jiang, R.; Rossi, A.D.; Cirulli, E.T.; Rashkin, M.; Metcalf, W.J.; et al. A Catalog of Homoplasmic and Heteroplasmic Mitochondrial DNA Variants in Humans. bioRxiv 2020, 798264. [Google Scholar] [CrossRef]
- Clima, R.; Preste, R.; Calabrese, C.; Diroma, M.A.; Santorsola, M.; Scioscia, G.; Simone, D.; Shen, L.; Gasparre, G.; Attimonelli, M. HmtDB 2016: Data Update, a Better Performing Query System and Human Mitochondrial DNA Haplogroup Predictor. Nucleic Acids Res. 2017, 45, D698–D706. [Google Scholar] [CrossRef]
- Preste, R.; Vitale, O.; Clima, R.; Gasparre, G.; Attimonelli, M. Hmtvar: A New Resource for Human Mitochondrial Variations and Pathogenicity Data. Nucleic Acids Res. 2019, 47, D1202–D1210. [Google Scholar] [CrossRef] [PubMed]
- Lott, M.T.; Leipzig, J.N.; Derbeneva, O.; Xie, H.M.; Chalkia, D.; Sarmady, M.; Procaccio, V.; Wallace, D.C. mtDNA Variation and Analysis Using Mitomap and Mitomaster. Curr. Protoc. Bioinform. 2013, 44, 1.23.1–1.23.26. [Google Scholar] [CrossRef]
- Shen, L.; Diroma, M.A.; Gonzalez, M.; Navarro-Gomez, D.; Leipzig, J.; Lott, M.T.; van Oven, M.; Wallace, D.C.; Muraresku, C.C.; Zolkipli-Cunningham, Z.; et al. MSeqDR: A Centralized Knowledge Repository and Bioinformatics Web Resource to Facilitate Genomic Investigations in Mitochondrial Disease. Hum. Mutat. 2016, 37, 540–548. [Google Scholar] [CrossRef] [PubMed]
- van Oven, M.; Kayser, M. Updated Comprehensive Phylogenetic Tree of Global Human Mitochondrial DNA Variation. Hum. Mutat. 2009, 30, E386–E394. [Google Scholar] [CrossRef]
- Schönherr, S.; Weissensteiner, H.; Kronenberg, F.; Forer, L. Haplogrep 3—An Interactive Haplogroup Classification. Nucleic Acids Res. 2023, 51, W263–W268. [Google Scholar] [CrossRef] [PubMed]
- Toren, D.; Barzilay, T.; Tacutu, R.; Lehmann, G.; Muradian, K.K.; Fraifeld, V.E. MitoAge: A Database for Comparative Analysis of Mitochondrial DNA, with a Special Focus on Animal Longevity. Nucleic Acids Res. 2016, 44, D1262–D1265. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.N.; Ennis, B.; Loneragan, B.; Tsao, N.L.; Sanchez, M.I.G.L.; Li, J.; Acheampong, P.; Tran, O.; Trounce, I.A.; Zhu, Y.; et al. MitoScape: A Big-Data, Machine-Learning Platform for Obtaining Mitochondrial DNA from next-Generation Sequencing Data. PLoS Comput. Biol. 2021, 17, e1009594. [Google Scholar] [CrossRef] [PubMed]
- Weissensteiner, H.; Forer, L.; Fuchsberger, C.; Schöpf, B.; Kloss-Brandstätter, A.; Specht, G.; Kronenberg, F.; Schönherr, S. MtDNA-Server: Next-Generation Sequencing Data Analysis of Human Mitochondrial DNA in the Cloud. Nucleic Acids Res. 2016, 44, W64–W69. [Google Scholar] [CrossRef]
- Weissensteiner, H.; Forer, L.; Fendt, L.; Kheirkhah, A.; Salas, A.; Kronenberg, F.; Schoenherr, S. Contamination Detection in Sequencing Studies Using the Mitochondrial Phylogeny. Genome Res. 2021, 31, 309–316. [Google Scholar] [CrossRef]
- Behar, D.M.; Rosset, S.; Blue-Smith, J.; Balanovsky, O.; Tzur, S.; Comas, D.; Mitchell, R.J.; Quintana-Murci, L.; Tyler-Smith, C.; Wells, R.S. The Genographic Project Public Participation Mitochondrial DNA Database. PLoS Genet. 2007, 3, e104. [Google Scholar] [CrossRef]
- Bolnick, D.A.; Fullwiley, D.; Duster, T.; Cooper, R.S.; Fujimura, J.H.; Kahn, J.; Kaufman, J.S.; Marks, J.; Morning, A.; Nelson, A.; et al. The Science and Business of Genetic Ancestry Testing. Science 2007, 318, 399–400. [Google Scholar] [CrossRef]
- Heyer, E.; Chaix, R.; Pavard, S.; Austerlitz, F. Sex-Specific Demographic Behaviours That Shape Human Genomic Variation. Mol. Ecol. 2012, 21, 597–612. [Google Scholar] [CrossRef]
- Macaulay, V.; Richards, M.B. Mitochondrial Genome Sequences and Their Phylogeographic Interpretation. Encycl. Life Sci. 2013. [Google Scholar] [CrossRef]
- Holt, I.J.; Harding, A.E.; Morgan-Hughes, J.A. Deletions of Muscle Mitochondrial DNA in Patients with Mitochondrial Myopathies. Nature 1988, 331, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C.; Singh, G.; Lott, M.T.; Hodge, J.A.; Schurr, T.G.; Lezza, A.M.; Elsas, L.J.; Nikoskelainen, E.K. Mitochondrial DNA Mutation Associated with Leber’s Hereditary Optic Neuropathy. Science 1988, 242, 1427–1430. [Google Scholar] [PubMed]
- Herrnstadt, C.; Howell, N. An Evolutionary Perspective on Pathogenic MtDNA Mutations: Haplogroup Associations of Clinical Disorders. Mitochondrion 2004, 4, 791–798. [Google Scholar] [CrossRef]
- Guyatt, A.L.; Brennan, R.R.; Burrows, K.; Guthrie, P.A.I.; Ascione, R.; Ring, S.M.; Gaunt, T.R.; Pyle, A.; Cordell, H.J.; Lawlor, D.A.; et al. A Genome-Wide Association Study of Mitochondrial DNA Copy Number in Two Population-Based Cohorts. Hum. Genom. 2019, 13, 6. [Google Scholar] [CrossRef]
- Castellani, C.A.; Longchamps, R.J.; Sun, J.; Guallar, E.; Arking, D.E. Thinking Outside the Nucleus: Mitochondrial DNA Copy Number in Health and Disease. Mitochondrion 2020, 53, 214–223. [Google Scholar] [CrossRef]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N.G. Mitochondrial DNA Copy Number in Human Disease: The More the Better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef]
- Chinnery, P.F.; Gomez-Duran, A. Oldies but Goldies MtDNA Population Variants and Neurodegenerative Diseases. Front. Neurosci. 2018, 12, 682. [Google Scholar] [CrossRef]
- O’Keefe, H.; Queen, R.; Lord, P.; Elson, J.L. What Can a Comparative Genomics Approach Tell Us about the Pathogenicity of MtDNA Mutations in Human Populations? Evol. Appl. 2019, 12, 1912–1930. [Google Scholar] [CrossRef]
- Salas, A.; García-Magariños, M.; Logan, I.; Bandelt, H.J. The Saga of the Many Studies Wrongly Associating Mitochondrial DNA with Breast Cancer. BMC Cancer 2014, 14, 659. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Smith, H.J.; Yao, P.; Mair, W.B. Causal Roles of Mitochondrial Dynamics in Longevity and Healthy Aging. EMBO Rep. 2019, 20, e48395. [Google Scholar] [CrossRef] [PubMed]
- Son, J.M.; Lee, C. Mitochondria: Multifaceted Regulators of Aging. BMB Rep. 2019, 52, 13–23. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, P1194–P1217. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Lagouge, M.; Larsson, N.G. The Role of Mitochondrial DNA Mutations and Free Radicals in Disease and Ageing. J. Intern. Med. 2013, 273, 529–543. [Google Scholar] [CrossRef]
- Zsurka, G.; Peeva, V.; Kotlyar, A.; Kunz, W.S. Is There Still Any Role for Oxidative Stress in Mitochondrial DNA-Dependent Aging? Genes 2018, 9, 175. [Google Scholar] [CrossRef]
- Kauppila, T.E.S.; Kauppila, J.H.K.; Larsson, N.G. Mammalian Mitochondria and Aging: An Update. Cell Metab. 2017, 25, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Garrido, E.; Martínez-Redondo, D.; Martín-Ruiz, C.; Gómez-Durán, A.; Ruiz-Pesini, E.; Madero, P.; Tamparillas, M.; Montoya, J.; von Zglinicki, T.; Díez-Sánchez, C.; et al. Association of Mitochondrial Haplogroup J and MtDNA Oxidative Damage in Two Different North Spain Elderly Populations. Biogerontology 2009, 10, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Pinós, T.; Nogales-Gadea, G.; Ruiz, J.R.; Rodríguez-Romo, G.; Santiago-Dorrego, C.; Fiuza-Luces, C.; Gómez-Gallego, F.; Cano-Nieto, A.; Garatachea, N.; Morán, M.; et al. Are Mitochondrial Haplogroups Associated with Extreme Longevity? A Study on a Spanish Cohort. Age 2012, 34, 227–233. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, G.; Rose, G.; Carrieri, G.; De Luca, M.; Falcone, E.; Passarino, G.; Bonafe, M.; Monti, D.; Baggio, G.; Bertolini, S.; et al. Mitochondrial DNA Inherited Variants Are Associated with Successful Aging and Longevity in Humans. FASEB J. 1999, 13, 1532–1536. [Google Scholar] [CrossRef]
- Niemi, A.K.; Hervonen, A.; Hurme, M.; Karhunen, P.J.; Jylhä, M.; Majamaa, K. Mitochondrial DNA Polymorphisms Associated with Longevity in a Finnish Population. Hum. Genet. 2003, 112, 29–33. [Google Scholar] [CrossRef]
- Alexe, G.; Fuku, N.; Bilal, E.; Ueno, H.; Nishigaki, Y.; Fujita, Y.; Ito, M.; Arai, Y.; Hirose, N.; Bhanot, G.; et al. Enrichment of Longevity Phenotype in MtDNA Haplogroups D4b2b, D4a, and D5 in the Japanese Population. Hum. Genet. 2007, 121, 347–356. [Google Scholar] [CrossRef]
- Bilal, E.; Rabadan, R.; Alexe, G.; Fuku, N.; Ueno, H.; Nishigaki, Y.; Fujita, Y.; Ito, M.; Arai, Y.; Hirose, N.; et al. Mitochondrial DNA Haplogroup D4a Is a Marker for Extreme Longevity in Japan. PLoS ONE 2008, 3, e2421. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, Y.; Fuku, N.; Tanaka, M. Mitochondrial Haplogroups Associated with Lifestyle-Related Diseases and Longevity in the Japanese Population. Geriatr. Gerontol. Int. 2010, 10, S221–S235. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, J.; Liu, M.; Wan, G.; Qi, K.; Zheng, C.; Lv, Z.; Hu, C.; Zeng, Y.; Gregory, S.G.; et al. Association of MtDNA Haplogroup F with Healthy Longevity in the Female Chuang Population, China. Exp. Gerontol. 2011, 46, 987–993. [Google Scholar] [CrossRef]
- He, Y.H.; Lu, X.; Tian, J.Y.; Yan, D.J.; Li, Y.C.; Lin, R.; Perry, B.; Chen, X.Q.; Yu, Q.; Cai, W.W.; et al. Mitochondrial DNA Plays an Equal Role in Influencing Female and Male Longevity in Centenarians. Exp. Gerontol. 2016, 83, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, M.D.; Gilbert, J.R.; Jiang, L.; Cummings, A.C.; Gallins, P.J.; Caywood, L.; Reinhart-Mercer, L.; Fuzzell, D.; Knebusch, C.; Laux, R.; et al. Mitochondrial Haplogroup X Is Associated with Successful Aging in the Amish. Hum. Genet. 2012, 131, 201–208. [Google Scholar] [CrossRef]
- Sevini, F.; Giuliani, C.; Vianello, D.; Giampieri, E.; Santoro, A.; Biondi, F.; Garagnani, P.; Passarino, G.; Luiselli, D.; Capri, M.; et al. MtDNA Mutations in Human Aging and Longevity: Controversies and New Perspectives Opened by High-Throughput Technologies. Exp. Gerontol. 2014, 56, 234–244. [Google Scholar] [CrossRef]
- Raule, N.; Sevini, F.; Li, S.; Barbieri, A.; Tallaro, F.; Lomartire, L.; Vianello, D.; Montesanto, A.; Moilanen, J.S.; Bezrukov, V.; et al. The Co-Occurrence of MtDNA Mutations on Different Oxidative Phosphorylation Subunits, Not Detected by Haplogroup Analysis, Affects Human Longevity and Is Population Specific. Aging Cell 2014, 13, 401–407. [Google Scholar] [CrossRef]
- Pan, Y.; Wen, J.; Ning, Z.; Yuan, Y.; Liu, X.; Yang, Y.; Guan, Y.; Lu, Y.; Mamatyusupu, D.; Xu, S. Comparative Genomic and Transcriptomic Analyses Reveal the Impacts of Genetic Admixture in Kazaks, Uyghurs, and Huis. Mol. Biol. Evol. 2023, 40, msad054. [Google Scholar] [CrossRef]
- Pedro, N.; Pinto, R.J.; Cavadas, B.; Pereira, L. Sub-Saharan African Information Potential to Unveil Adaptations to Infectious Disease. Hum. Mol. Genet. 2021, 30, 138–145. [Google Scholar] [CrossRef]
- Natri, H.M.; Bobowik, K.S.; Kusuma, P.; Darusallam, C.C.; Jacobs, G.S.; Hudjashov, G.; Stephen Lansing, J.; Sudoyo, H.; Banovich, N.E.; Cox, M.P.; et al. Genome-Wide DNA Methylation and Gene Expression Patterns Reflect Genetic Ancestry and Environmental Differences across the Indonesian Archipelago. PLoS Genet. 2020, 16, e1008749. [Google Scholar] [CrossRef]
- Larmuseau, M.H.D.; Ottoni, C. Mediterranean Y-Chromosome 2.0—Why the Y in the Mediterranean Is Still Relevant in the Postgenomic Era. Ann. Hum. Biol. 2018, 45, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Hellenthal, G.; Busby, G.B.J.; Band, G.; Wilson, J.F.; Capelli, C.; Falush, D.; Myers, S. A Genetic Atlas of Human Admixture History. Science 2014, 343, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, N.K.; Shapiro, B.; Green, R.E. An Ancestral Recombination Graph of Human, Neanderthal, and Denisovan Genomes. Sci. Adv. 2021, 7, eabc0776. [Google Scholar] [CrossRef]
- Wohns, A.W.; Wong, Y.; Jeffery, B.; Akbari, A.; Mallick, S.; Pinhasi, R.; Patterson, N.; Reich, D.; Kelleher, J.; McVean, G. A Unified Genealogy of Modern and Ancient Genomes. Science 2022, 375, eabi8264. [Google Scholar] [CrossRef]
- Fregel, R.; Ordóñez, A.C.; Santana-Cabrera, J.; Cabrera, V.M.; Velasco-Vázquez, J.; Alberto, V.; Moreno-Benítez, M.A.; Delgado-Darias, T.; Rodríguez-Rodríguez, A.; Hernández, J.C.; et al. Mitogenomes Illuminate the Origin and Migration Patterns of the Indigenous People of the Canary Islands. PLoS ONE 2019, 14, e0209125. [Google Scholar] [CrossRef]
- Verma, R.K.; Kalyakulina, A.; Giuliani, C.; Shinde, P.; Kachhvah, A.D.; Ivanchenko, M.; Jalan, S. Analysis of Human Mitochondrial Genome Co-Occurrence Networks of Asian Population at Varying Altitudes. Sci. Rep. 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.A.; Runfeldt, G.; Estes, R.J.; Vilar, M.G. African Mitochondrial Haplogroup L7: A 100,000-Year-Old Maternal Human Lineage Discovered through Reassessment and New Sequencing. Sci. Rep. 2022, 12, 10747. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, F.; Yu, J.; Liu, F.; Zhao, J.; Shen, H.; Zhao, B.; Jia, F.; Sun, Z.; Song, H.; et al. Strategies for Complete Mitochondrial Genome Sequencing on Ion Torrent PGMTM Platform in Forensic Sciences. Forensic Sci. Int. Genet. 2016, 22, 11–21. [Google Scholar] [CrossRef]
- Senovska, A.; Drozdova, E.; Vaculik, O.; Pardy, F.; Brzobohata, K.; Fialova, D.; Smerda, J.; Kos, P. Cost-Effective Straightforward Method for Captured Whole Mitogenome Sequencing of Ancient DNA. Forensic Sci. Int. 2021, 319, 110638. [Google Scholar] [CrossRef]
- Jorde, L.B.; Bamshad, M.J.; Watkins, W.S.; Zenger, R.; Fraley, A.E.; Krakowiak, P.A.; Carpenter, K.D.; Soodyall, H.; Jenkins, T.; Rogers, A.R. Origins and Affinities of Modern Humans: A Comparison of Mitochondrial and Nuclear Genetic Data. Am. J. Hum. Genet. 1995, 57, 523–538. [Google Scholar] [PubMed]
- Nielsen, R.; Akey, J.M.; Jakobsson, M.; Pritchard, J.K.; Tishkoff, S.; Willerslev, E. Tracing the Peopling of the World through Genomics. Nature 2017, 541, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, M.; Achilli, A.; Olivieri, A.; Perego, U.A.; Gómez-Carballa, A.; Brisighelli, F.; Lancioni, H.; Woodward, S.R.; López-Soto, M.; Carracedo, A.; et al. Reconstructing Ancient Mitochondrial DNA Links between Africa and Europe. Genome Res. 2012, 22, 821–826. [Google Scholar] [CrossRef]
- Bycroft, C.; Fernandez-Rozadilla, C.; Ruiz-Ponte, C.; Quintela-García, I.; Carracedo, Á.; Donnelly, P.; Myers, S. Patterns of Genetic Differentiation and the Footprints of Historical Migrations in the Iberian Peninsula. Nat. Commun. 2019, 10, 551. [Google Scholar] [CrossRef]
- Hernández, C.L.; Pita, G.; Cavadas, B.; López, S.; Sánchez-Martínez, L.J.; Dugoujon, J.M.; Novelletto, A.; Cuesta, P.; Pereira, L.; Calderón, R. Human Genomic Diversity Where the Mediterranean Joins the Atlantic. Mol. Biol. Evol. 2020, 37, 1041–1055. [Google Scholar] [CrossRef]
- Lopes, A.F.C. Mitochondrial Metabolism and DNA Methylation: A Review of the Interaction between Two Genomes. Clin. Epigenetics 2020, 12, 182. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S. Mitochondrial DNA in Innate Immune Responses and Inflammatory Pathology. Nat. Rev. Immunol. 2017, 17, 363–375. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Chung, J.H. Molecular Mechanisms of Mitochondrial DNA Release and Activation of the CGAS-STING Pathway. Exp. Mol. Med. 2023, 55, 510–519. [Google Scholar] [CrossRef]
- Wei, W.; Schon, K.R.; Elgar, G.; Orioli, A.; Tanguy, M.; Giess, A.; Tischkowitz, M.; Caulfield, M.J.; Chinnery, P.F. Nuclear-Embedded Mitochondrial DNA Sequences in 66,083 Human Genomes. Nature 2022, 611, 105–114. [Google Scholar] [CrossRef]
- Singh, K.K.; Choudhury, A.R.; Tiwari, H.K. Numtogenesis as a Mechanism for Development of Cancer. Semin. Cancer Biol. 2017, 47, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Zhang, W.; Yu, D.; Li, X.; Wang, H.Z.; Hu, Q.X.; Zhang, C.; Lu, W.; Ni, J.; Fang, Y.; et al. Mitochondrial DNA Haplogroup B5 Confers Genetic Susceptibility to Alzheimer’s Disease in Han Chinese. Neurobiol. Aging 2015, 36, 1604.e7–1604.e16. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Id, J.S.S.; Pienaar, I.S.; Id, J.L.E. Mitochondrial DNA Population Variation Is Not Associated with Alzheimer’s in the Japanese Population: A Consistent Finding across Global Populations. PLoS ONE 2022, 17, e0276169. [Google Scholar] [CrossRef]
- Salem, N.B.; Boussetta, S.; Rojas, I.D.; Grau, S.M.; Montrreal, L.; Mokni, N.; Mahmoud, I.; Younes, S.; Daouassi, N.; Frih, M.; et al. Mitochondrial DNA and Alzheimer’s Disease: A First Case—Control Study of the Tunisian Population. Mol. Biol. Rep. 2022, 49, 1687–1700. [Google Scholar] [CrossRef]
- van der Walt, J.M.; Dementieva, Y.A.; Martin, E.R.; Scott, W.K.; Nicodemus, K.K.; Kroner, C.C.; Welsh-Bohmer, K.A.; Saunders, A.M.; Roses, A.D.; Small, G.W.; et al. Analysis of European Mitochondrial Haplogroups with Alzheimer Disease Risk. Neurosci. Lett. 2004, 365, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.; Hinttala, R.; Majamaa, K.; Remes, A.M. Mitochondrial DNA Haplogroups in Early-Onset Alzheimer’s Disease and Frontotemporal Lobar Degeneration. Mol. Neurodegener. 2010, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Blein, S.; Bardel, C.; Danjean, V.; McGuffog, L.; Healey, S.; Barrowdale, D.; Lee, A.; Dennis, J.; Kuchenbaecker, K.B.; Soucy, P.; et al. An Original Phylogenetic Approach Identified Mitochondrial Haplogroup T1a1 as Inversely Associated with Breast Cancer Risk in BRCA2 Mutation Carriers. Breast Cancer Res. 2015, 17, 61. [Google Scholar] [CrossRef]
- Darvishi, K.; Sharma, S.; Bhat, A.K.; Rai, E.; Bamezai, R.N.K. Mitochondrial DNA G10398A Polymorphism Imparts Maternal Haplogroup N a Risk for Breast and Esophageal Cancer. Cancer Lett. 2007, 249, 249–255. [Google Scholar] [CrossRef]
- Fang, H.; Shen, L.; Chen, T.; He, J.; Ding, Z.; Wei, J.; Qu, J.; Chen, G.; Lu, J.; Bai, Y. Cancer Type-Specific Modulation of Mitochondrial Haplogroups in Breast, Colorectal and Thyroid Cancer. BMC Cancer 2010, 10, 421. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Koyanagi, A.; Vaona, A.; Demurtas, J.; Schofield, P.; Maggi, S. Mitochondrial Genetic Haplogroups and Cardiovascular Diseases: Data from the Osteoarthritis Initiative. PLoS ONE 2019, 14, e0213656. [Google Scholar] [CrossRef]
- Benn, M.; Schwartz, M.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Mitochondrial Haplogroups: Ischemic Cardiovascular Disease, Other Diseases, Mortality, and Longevity in the General Population. Circulation 2008, 117, 2492–2501. [Google Scholar] [CrossRef]
- Castro, M.G.; Huerta, C.; Reguero, J.R.; Soto, M.I.; Doménech, E.; Álvarez, V.; Gómez-Zaera, M.; Nunes, V.; González, P.; Corao, A.; et al. Mitochondrial DNA Haplogroups in Spanish Patients with Hypertrophic Cardiomyopathy. Int. J. Cardiol. 2006, 112, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.M.; Aidt, F.H.; Hedley, P.L.; Jensen, M.K.; Havndrup, O.; Kanters, J.K.; Moolman-Smook, J.C.; Larsen, S.O.; Bundgaard, H.; Christiansen, M. Mitochondrial Haplogroups Modify the Risk of Developing Hypertrophic Cardiomyopathy in a Danish Population. PLoS ONE 2013, 8, e71904. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, P.; Khan, N.A.; Rani, B.; Rani, D.S.; Selvaraj, P.; Jyothi, V.; Bahl, A.; Narasimhan, C.; Rakshak, D.; Premkumar, K.; et al. Mitochondrial DNA Variations Associated with Hypertrophic Cardiomyopathy. Mitochondrion 2014, 16, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Caggiano, M.; Barallobre-Barreiro, J.; Rego-Pérez, I.; Crespo-Leiro, M.G.; Paniagua, M.J.; Grillé, Z.; Blanco, F.J.; Doménech, N. Mitochondrial Haplogroups H and J: Risk and Protective Factors for Ischemic Cardiomyopathy. PLoS ONE 2012, 7, e44128. [Google Scholar] [CrossRef] [PubMed]
- Kofler, B.; Mueller, E.E.; Eder, W.; Stanger, O.; Maier, R.; Weger, M.; Haas, A.; Winker, R.; Schmut, O.; Paulweber, B.; et al. Mitochondrial DNA Haplogroup T Is Associated with Coronary Artery Disease and Diabetic Retinopathy: A Case Control Study. BMC Med. Genet. 2009, 10, 35. [Google Scholar] [CrossRef]
- Umbria, M.; Ramos, A.; Caner, J.; Vega, T.; Lozano, J.E.; Santos, C.; Aluja, M.P. Involvement of Mitochondrial Haplogroups in Myocardial Infarction and Stroke: A Case-Control Study in Castile and Leon (Spain) Population. Mitochondrion 2019, 44, 1–6. [Google Scholar] [CrossRef]
- Kaneva, K.; Schurr, T.G.; Tatarinova, T.V.; Buckley, J.; Merkurjev, D.; Triska, P.; Liu, X.; Done, J.; Maglinte, D.T.; Deapen, D.; et al. Mitochondrial DNA Haplogroup, Genetic Ancestry, and Susceptibility to Ewing Sarcoma. Mitochondrion 2022, 67, 6–14. [Google Scholar] [CrossRef]
- Tranah, G.J.; Santaniello, A.; Caillier, S.J.; D’Alfonso, S.; Boneschi, F.M.; Hauser, S.L.; Oksenberg, J.R. Mitochondrial DNA Sequence Variation in Multiple Sclerosis. Neurology 2015, 85, 325–330. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wang, M.; Jin, Y.; Liao, W.; Zhao, Z.; Fang, J. Mitochondrial DNA Haplogroups Participate in Osteoarthritis: Current Evidence Based on a Meta-Analysis. Clin. Rheumatol. 2020, 39, 1027–1037. [Google Scholar] [CrossRef]
- Soto-Hermida, A.; Fernández-Moreno, M.; Oreiro, N.; Fernández-López, C.; Pértega, S.; Cortés-Pereira, E.; Rego-Pérez, I.; Blanco, F.J. Mitochondrial DNA (MtDNA) Haplogroups Influence the Progression of Knee Osteoarthritis.Data from the Osteoarthritis Initiative (OAI). PLoS ONE 2014, 9, e112735. [Google Scholar] [CrossRef]
- Gaweda-Walerych, K.; Maruszak, A.; Safranow, K.; Bialecka, M.; Klodowska-Duda, G.; Czyzewski, K.; Slawek, J.; Rudzinska, M.; Styczynska, M.; Opala, G.; et al. Mitochondrial DNA Haplogroups and Subhaplogroups Are Associated with Parkinson’s Disease Risk in a Polish PD Cohort. J. Neural Transm. 2008, 115, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, D.; Marelli, C.; Achilli, A.; Goldwurm, S.; Pezzoli, G.; Barone, P.; Pellecchia, M.T.; Stanzione, P.; Brusa, L.; Bentivoglio, A.R.; et al. Mitochondrial DNA Haplogroup K Is Associated with a Lower Risk of Parkinson’s Disease in Italians. Eur. J. Hum. Genet. 2005, 13, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.; Gilyazova, I.; Ruiz-Pesini, E.; Derbeneva, O.; Khusainova, R.; Khidiyatova, I.; Magzhanov, R.; Wallace, D.C. A Mitochondrial Etiology of Neurodegenerative Diseases: Evidence from Parkinson’s Disease. Ann. N. Y. Acad. Sci. 2008, 1147, 1–20. [Google Scholar] [CrossRef]
- van der Walt, J.M.; Nicodemus, K.K.; Martin, E.R.; Scott, W.K.; Nance, M.A.; Watts, R.L.; Hubble, J.P.; Haines, J.L.; Koller, W.C.; Lyons, K.; et al. Mitochondrial Polymorphisms Significantly Reduce the Risk of Parkinson Disease. Am. J. Hum. Genet. 2003, 72, 804–811. [Google Scholar] [CrossRef]
- Feder, J.; Blech, I.; Ovadia, O.; Amar, S.; Wainstein, J.; Raz, I.; Dadon, S.; Arking, D.E.; Glaser, B.; Mishmar, D. Differences in MtDNA Haplogroup Distribution among 3 Jewish Populations Alter Susceptibility to T2DM Complications. BMC Genom. 2008, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Crispim, D.; Canani, L.H.; Gross, J.L.; Tschiedel, B.; Souto, K.E.P.; Roisenberg, I. The European-Specific Mitochondrial Cluster J/T Could Confer an Increased Risk of Insulin-Resistance and Type 2 Diabetes: An Analysis of the m.4216T> C and m.4917A> G Variants. Ann. Hum. Genet. 2006, 70, 488–495. [Google Scholar] [CrossRef] [PubMed]

| Population | N | Enriched Hg | Reference |
|---|---|---|---|
| NE Spain (>85 y) | 138 (138 controls) | J (J2) | [139] |
| C Spain (>100 y) | 65 (138 controls) | - | [140] |
| Italy (>100 y) | 212 (275 controls) | J | [141] |
| Finland (>90 y) | 225 (400 controls) | J, UK | [142] |
| Japan (>100 y) | 96 | D4a, D4b2b, D5 | [143] |
| Japan (>105 y) | 112 | D4a | [144] |
| Japan (>100 y) | 96 | D4a, D4b2b, D5 | [145] |
| S China (>100 y) | 367 (371 controls) | F (females) | [146] |
| China (>100 y) | 402 (458 controls) | - | [147] |
| Amish (US) (>80 y) | 74 | X | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, C.L. Mitochondrial DNA in Human Diversity and Health: From the Golden Age to the Omics Era. Genes 2023, 14, 1534. https://doi.org/10.3390/genes14081534
Hernández CL. Mitochondrial DNA in Human Diversity and Health: From the Golden Age to the Omics Era. Genes. 2023; 14(8):1534. https://doi.org/10.3390/genes14081534
Chicago/Turabian StyleHernández, Candela L. 2023. "Mitochondrial DNA in Human Diversity and Health: From the Golden Age to the Omics Era" Genes 14, no. 8: 1534. https://doi.org/10.3390/genes14081534
APA StyleHernández, C. L. (2023). Mitochondrial DNA in Human Diversity and Health: From the Golden Age to the Omics Era. Genes, 14(8), 1534. https://doi.org/10.3390/genes14081534





