1. Introduction
The prevailing notion is that extending the crop duration for cultivation by a designated period may offset the decline in crop productivity resulting from the rise in seasonal temperatures. This has been exemplified by a few varieties of wheat in India, which manifested accelerations of 4–9 days in the onset of flowering [
1]. By sowing spring wheat before the standard planting period, it may be feasible to extend the duration of the planting intervals. For instance, initiating the planting process from one to two weeks ahead led to a significantly extended crop growth period, accompanied by the elimination of one irrigation requirement owing to the presence of residual moisture after the monsoon season. However, it was expected that an unfavorable year would lead to vulnerability and eventually reduce crop productivity [
2]. Warmer temperatures in the early stages of the growing season accelerate the occurrence of heading and anthesis during grain filling, while cooler temperatures later on lead to a delay in maturity. Irrespective of the constancy of the mean seasonal temperature, there is a positive correlation between a decrease in temperature and a reduction in yield, while an increase in yield is observed with a temperature rise [
3]. Because the monsoon season is the wettest, farmers in many northwestern and central Indian regions would prefer to sow wheat earlier in the season to capitalize on the available soil moisture. If wheat genotypes are sown earlier than the recommended planting date, then they might experience elevated temperatures during root development, leaf development, and emergence, significantly in advance. From seeding to emergence, the optimal temperature range is between 20.4 and 23.6 °C, with the highest temperatures recorded reaching between 31.8 and 33.6 °C [
4]. Root development during the vegetative stage thrives at an ideal temperature of 20 °C, whereas shoot growth prefers significantly lower temperatures [
5]. Based on a research study, it was determined that the most favorable temperature range for the emergence of leaves is between 21.3 and 24.3 °C [
6], whereas it is 22 °C according to another study [
7].
There is a lack of understanding about how genes regulate the early stages of heat tolerance and the adaptive response. Many of the key genes involved in adaptation have been identified in recent years. These include genes involved in the vernalization reaction (
Vrn), photoperiod sensitivity (
Ppd), and “earliness per se” (
Eps) in terms of the growth rate [
8,
9,
10,
11]. However, there are significant differences in our understanding of the implications of potential allele combinations for environment-specific adaptations. On the one hand, the
Vrn genes regulate the distinction between spring and winter wheat by specifying the number of chilling hours necessary for the wheat plant to initiate flowering. On the other hand, the
Ppd genes are crucial in postponing the flowering time during spring, once the vernalization requirement has been met. The effects of the
Eps loci may enable more nuanced adjustments to the plant’s life cycle, facilitating regional adaptation. Several areas possibly harboring
Eps genes have been mapped out by Quantitative trait locus quantitative trait loci (QTL) investigations [
12,
13]. Allelic heterogeneity appears to be prevalent in various germplasms for specific QTLs, including those found on chromosomes 4B, 6A, and 7D. The genes
Vrn,
Ppd, and
Eps also contribute to epistatic interactions [
14,
15]. As a result, numerous combinations of alleles are likely to influence the regulation of growth patterns and optimal adaptation to particular climates.
Planting wheat before the season starts has several adverse effects on the plant’s growth and development. Early planting may increase the risk of unproductive vegetative growth, reduce tillering, and make plants more susceptible to drought in dry conditions. The high soil temperatures in the planting stage may reduce the germination percentage and coleoptile length. Furthermore, the deeply planted seed may not have a long enough coleoptile to split through the soil surface, leading to lower emergence and less adequate stand establishment. The early planting of traditional and short-duration varieties, with photoperiod insensitivity, leads to early heading and maturity, resulting in yield reduction. Therefore, selecting advanced breeding lines suitable for early planting may be difficult but it is not impossible, as diverse germplasms can give these variations to show a better adaptive capacity in early planting.
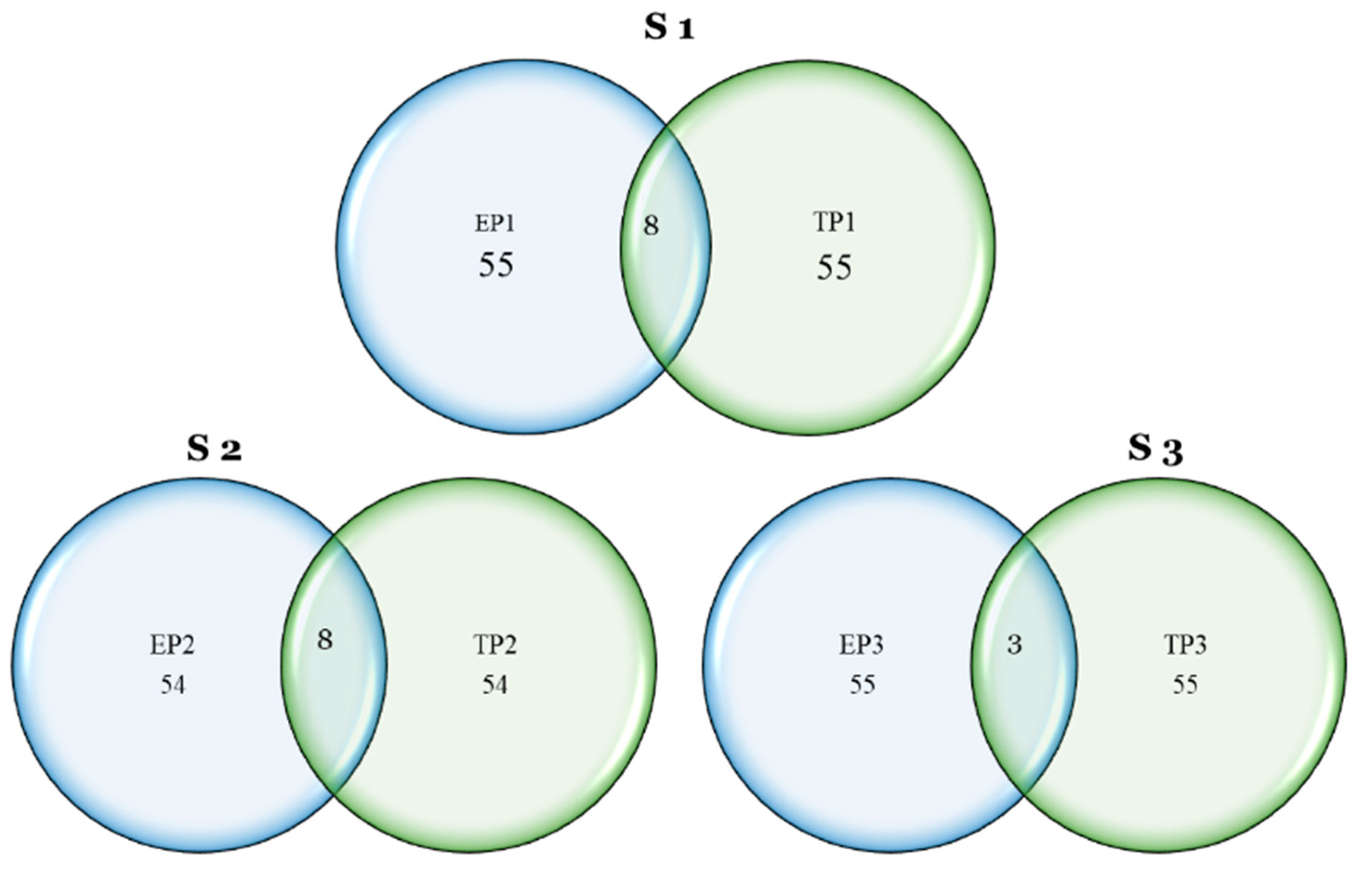
The mapping of agronomic and phenological traits is a continuous process in wheat development. QTL mapping has been widely used in wheat breeding to understand the probable genetic control of loci, genes, or even genome segments in biotic and abiotic stress adaptation or resistance. Early wheat establishment was initiated at the BISA in Ludhiana, India, in the 2017 season, and, after three years of extensive research, several data points were generated. The early-planted wheat lines had three theoretical types of adaptation: (a) suitable for early- as well as timely-planted conditions, (b) suitable for early planting but not suitable for timely planting, and (c) suitable for timely planting but not suitable for early planting. This adaptation expresses phenotypically with variation in morpho-physiological traits, and genotypically with the effect of different genes. Diseases may also be a limiting factor for early-established plants.
With this perspective in mind, the current study focused on finding wheat lines for early establishment by analyzing agro-morphological features and mapping genes or QTLs associated with early-adaptation features. A few agro-morphological traits supporting genotypes for excellent performance in early planting have already been identified in our previous study, along with the ideotype selection procedure [
16]. The major research questions to identify QTLs associated with adaptation to early planting for this study are as follows:
- (a)
Can we identify the major QTLs important for the early establishment of wheat?
- (b)
What are the QTLs associated with higher performances in both planting conditions?
- (c)
Are those QTLs linked with major genes underlying adaptation to early planting?
4. Discussion
Seasonal variations often influence adaptation by changing the temperature and photoperiod. Three different sets of genotypes were grown in different seasons, with varying weather conditions. The photo-growing degree days were measured to standardize the temperature and photoperiod with calendar days. It was evident that the photo-growing degree days reduced the data variability by minimizing the calendar-day difference with the PGDD calculation in phenological traits [
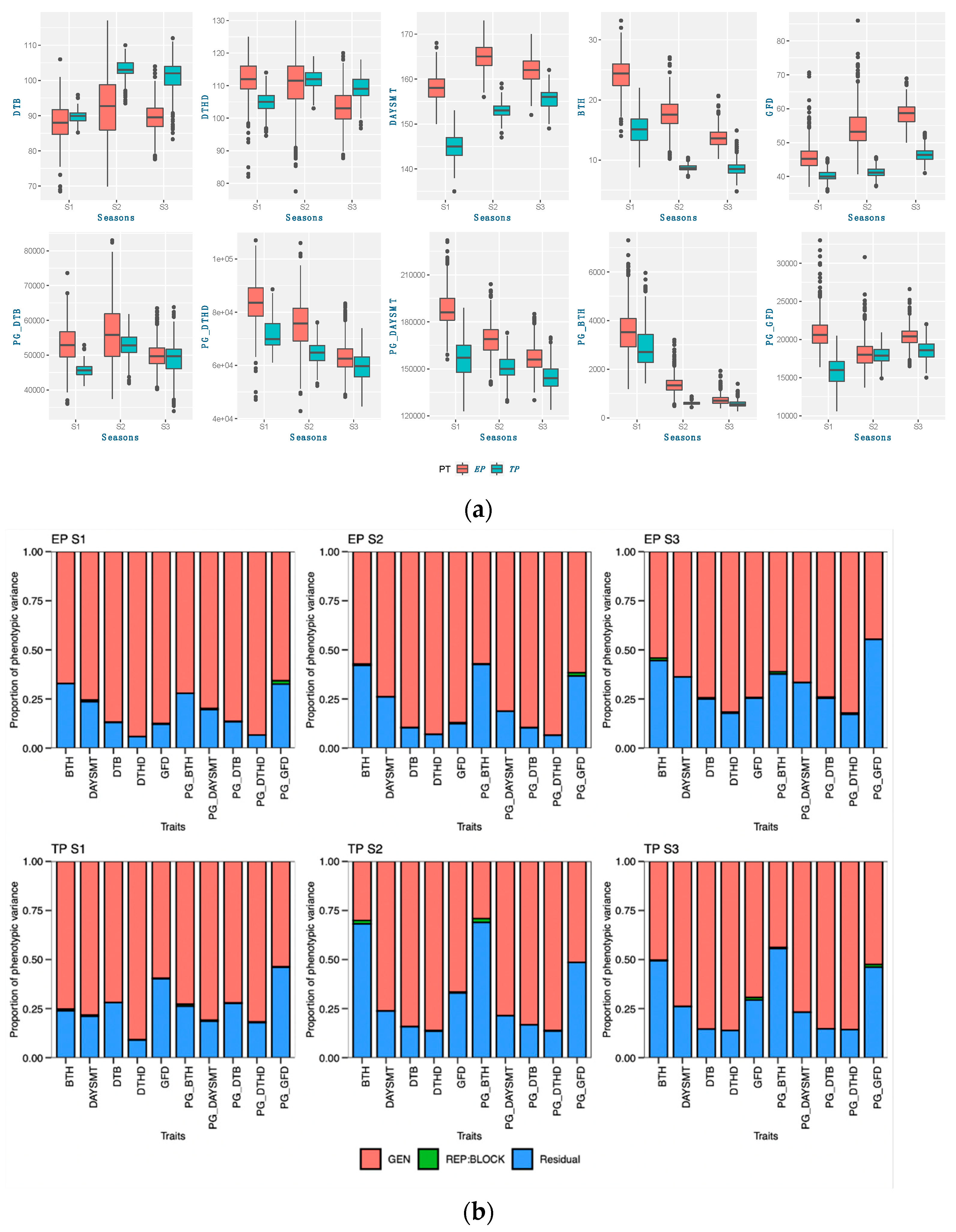
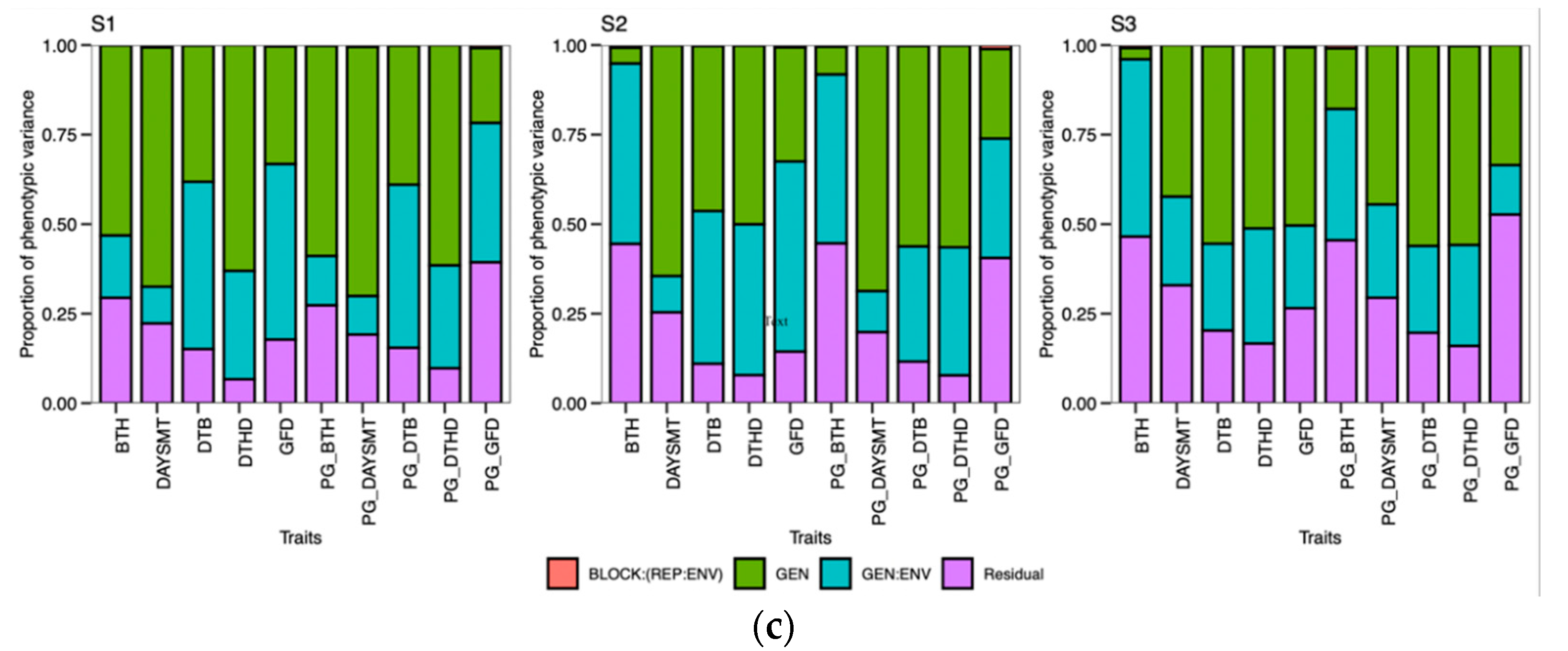
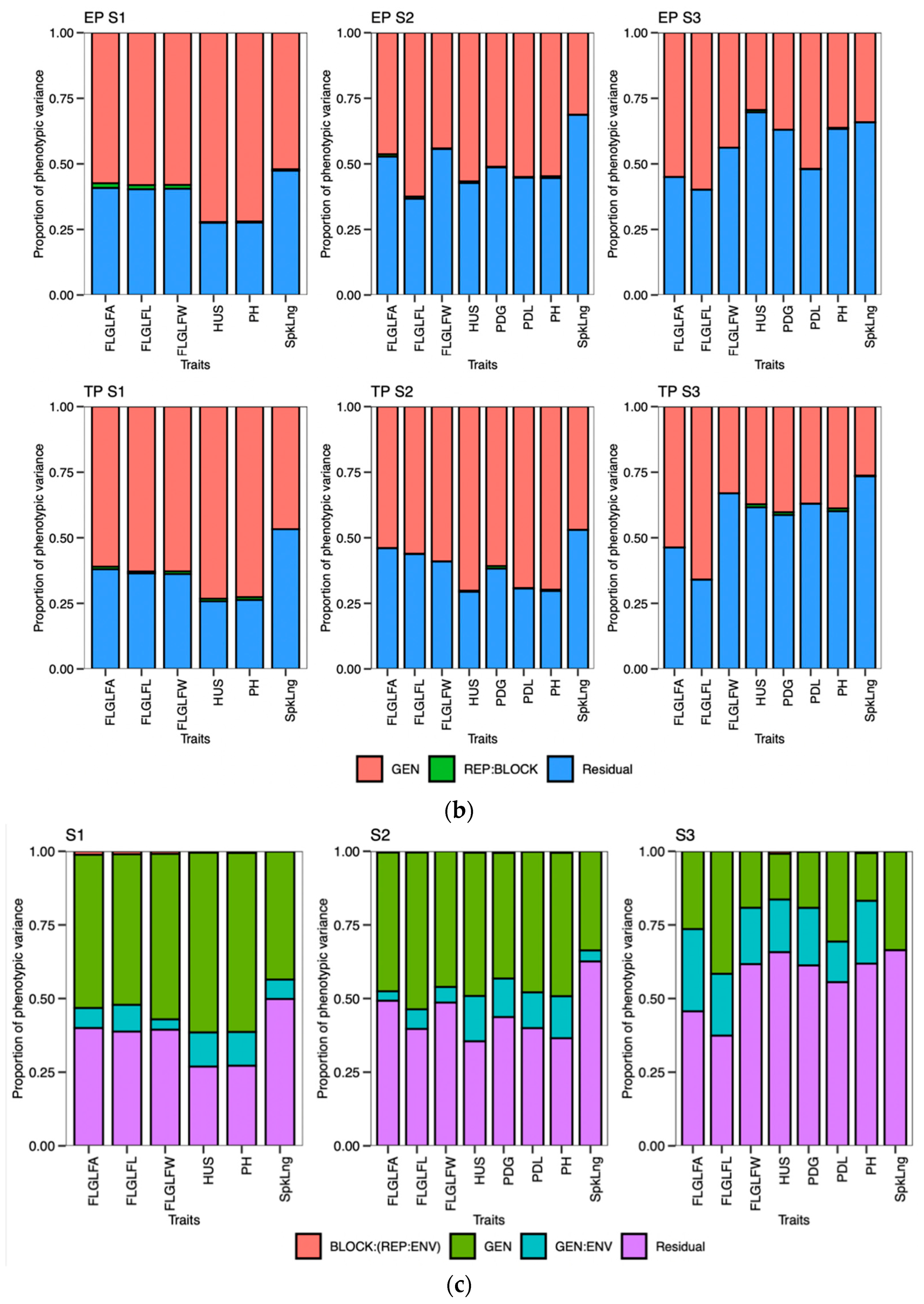
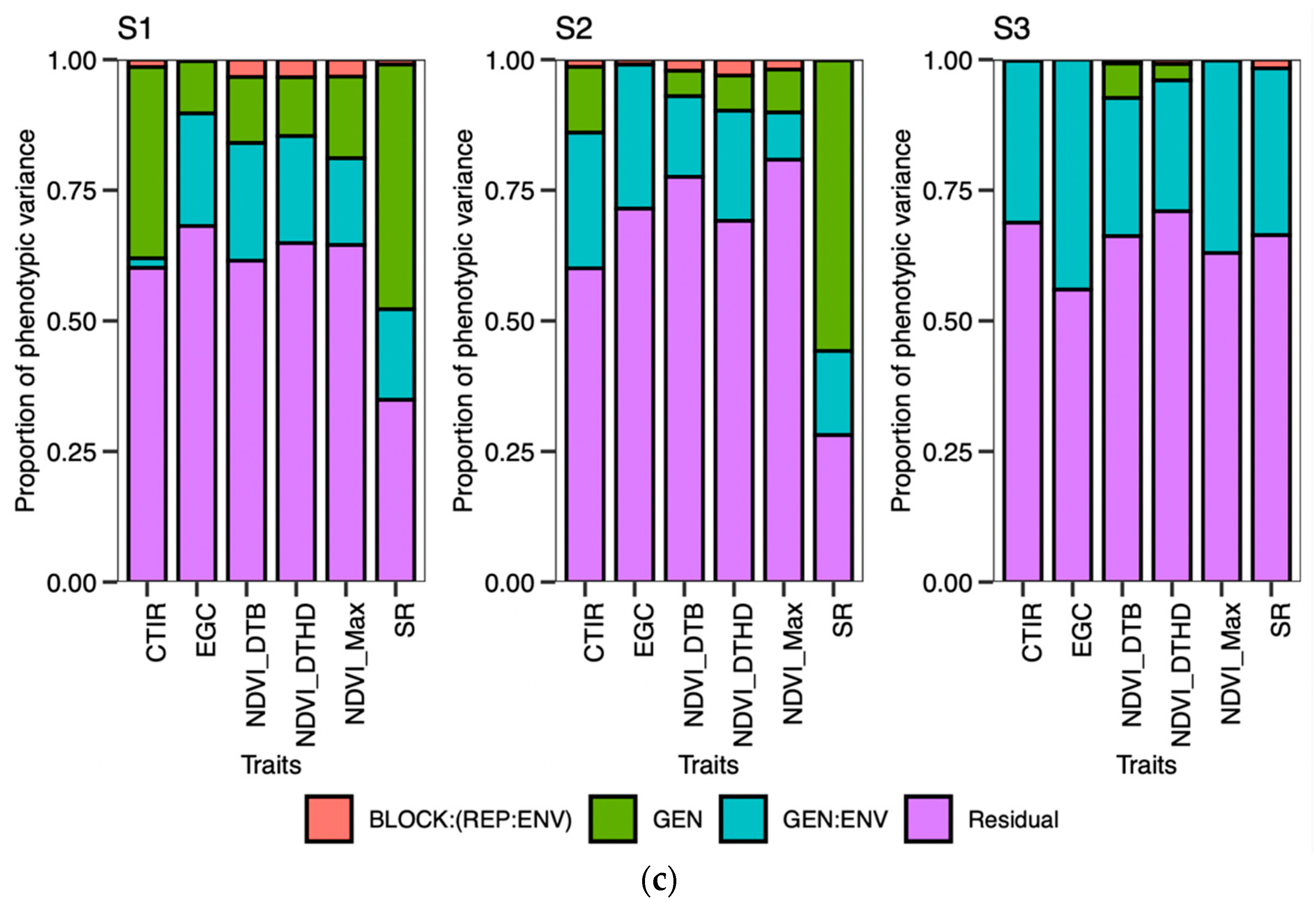
48]. Calendar days were inconsistent for the DTB and DTHD, but it was clearly evident that longer PGDDs are required more in early planting than timely planting. In season 2 (S2) and season 3 (S3), most genotypes exhibited longer days to booting (DTB) and days to heading DTHD when planted timely manner (TP). However, upon incorporating the photo growing degree days (PGDD) to calculate PG_DTB and PG_DTHD, the genotypes demonstrated shorter PGDDs values in both seasons, as compared to early planting (EP). The seasonal ambiguities were high in both these two seasons. Hailstorms along with high rainfall occurred in S2 during the booting and heading time of the timely-planted crops.
Similarly, prolonged winter in S3 caused the longer DTB and DTHD for the TP. Lower temperatures exist in both these seasons, increasing the phenological period. The addition of the cumulative effect of temperature and the photoperiod by the PGDDs minimized these seasonal ambiguities. It has been suggested that the growth and development of the crop is a function of the photoperiod and ambient temperature [
66,
67].
The global wheat-breeding program implemented by CIMMYT has demonstrated consistent genetic improvement in grain yields across diverse environments and management conditions worldwide, with a particular emphasis on South Asia [
68]. While certain traits have exhibited consistent improvement over time. Unique methodologies have been employed with the established cultivars to attain maximum grain productivity [
69]. According to a study, a considerable proportion of developing countries’ spring-wheat-growing regions (approximately 70%) either utilize CIMMYT germplasm as a parent of their varieties or produce CIMMYT germplasm as an immediate release [
68]. This practice has resulted in noteworthy economic advantages. The genotypes utilized in this investigation were sourced from an extensive collection of advanced lines produced within the wheat-breeding program of CIMMYT. The genotypes were specifically identified for conditions that correspond to the early-sowing conditions prevalent on India’s >20-million-hectare Indo-Gangetic plain, which are referred to as ME1 and ME4 environments.
A number of yield-contributing factors have been identified as suitable for early planting, which can mitigate yield reductions resulting from increasing seasonal temperatures by achieving maturity well before the onset of terminal heat stress [
1]. Nevertheless, initiating the sowing process at an early stage is accompanied by the potential hazard of inadequate initial establishment and accelerated growth during the initial phases owing to elevated temperatures, which may ultimately lead to diminished yields as a result of decreased dry-matter accumulation.
According to data from the National Informatics Centre under the Government of India in 2016, it has been observed that India’s seasonal air temperature has undergone changes over the past twenty years [
70]. This has resulted in minor heat stress on crops during early planting. In contrast to planting at an optimal time, early planting results in a prolonged exposure of genotypes to a shorter photoperiod during the pre-flowering stage.
The findings indicate that there is no discernible variance from a previous report [
71], which presumes that a decrease in the photoperiod duration is positively correlated with an increase in the duration of the growth stages in wheat. Therefore, if planting is carried out much earlier than the optimal time, then genotypes tend to remain in the booting stage for a longer duration, resulting in the complete manifestation of their genetic potential.
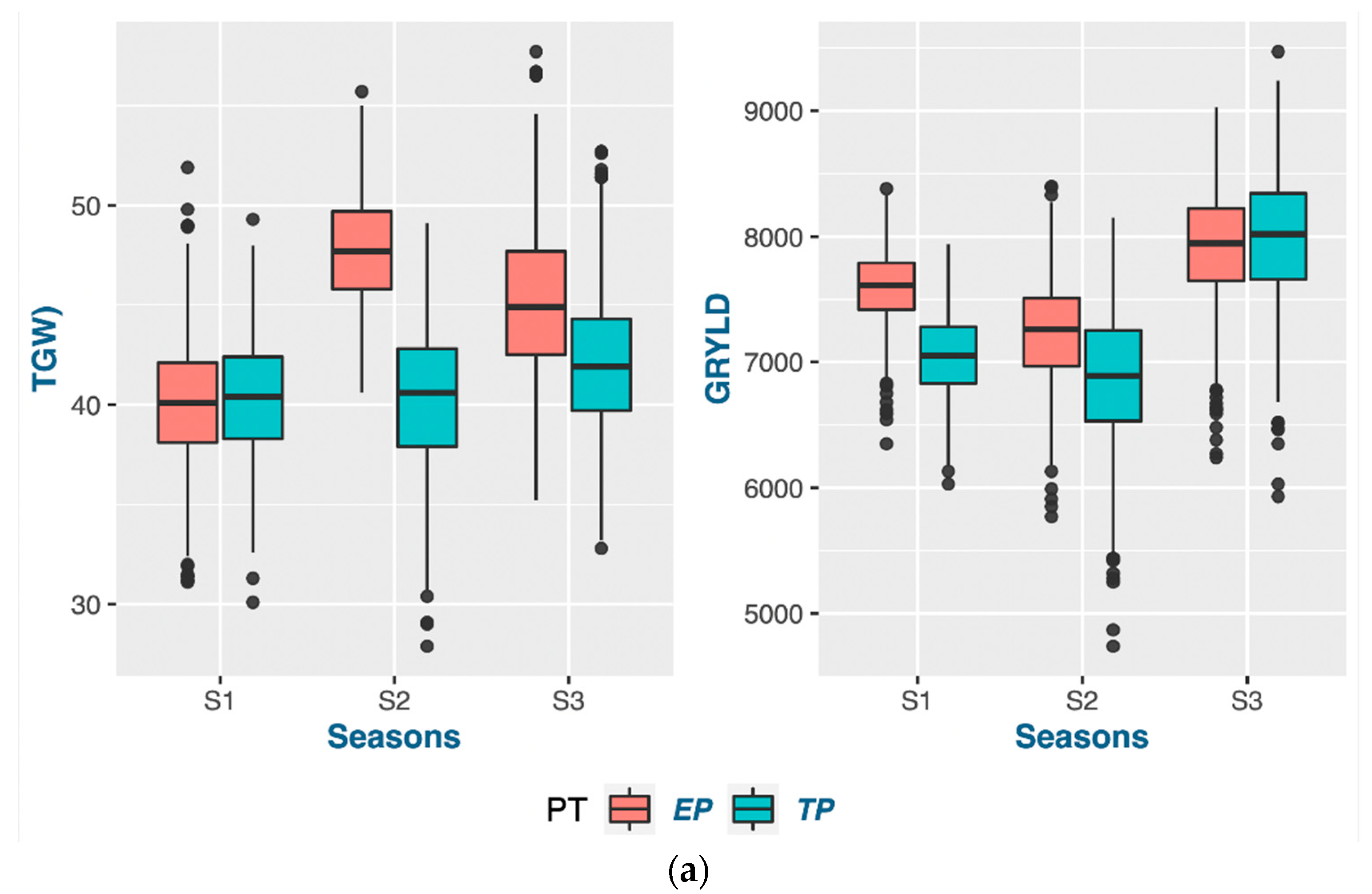
According to the findings of this research, the act of planting crops earlier than usual does not necessarily guarantee an early maturation of the crop. The wheat-growing period was prolonged through early planting, primarily by extending the duration of vegetative growth. The extension of the vegetative phase, as indicated by the PG_DTB and PG_BTH, through early planting, led to a notable boost in crop yields across various genotypes. The findings indicate a noteworthy positive correlation between the TGW and PG_GFD, while exhibiting a negative correlation with other phenological traits in both planting periods. This suggests that the observed increase in the yield during early planting may be attributed to a rise in the number of grains, which is facilitated by a higher count of fertile spikelets in the spike. Increasing the duration of the vegetative stage and expanding the leaf area during early planting can lead to an increase in biomass. This increase in biomass results in the accumulation of more dry matter at the source, which is subsequently transported to the sink. Consequently, it seems to enhance both the quantity and quality of grains. According to a study, a graph estimation model indicated that the most resilient network of traits for enhancing grain yields in all environments comprised the biomass, thousand-grain weight (TGW), and grain number [
69].
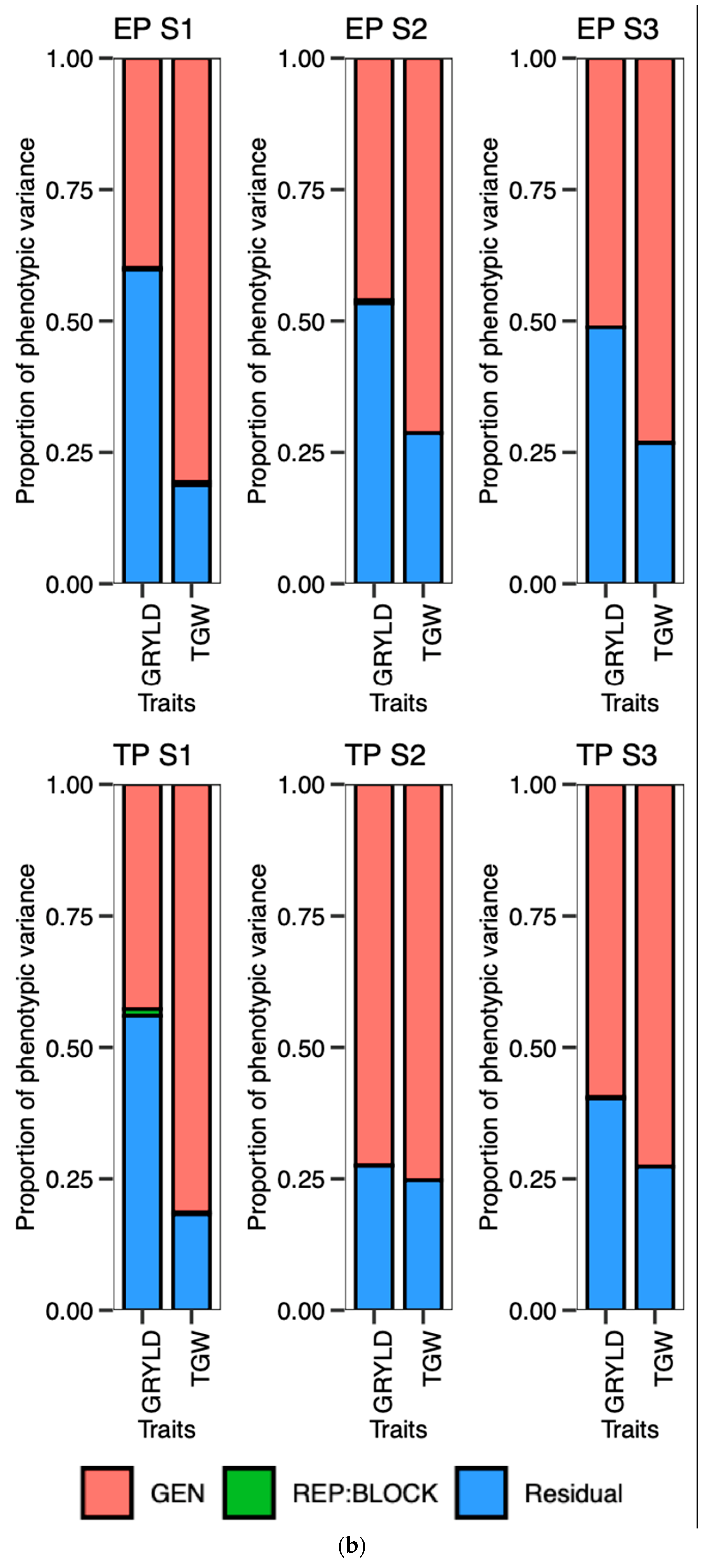
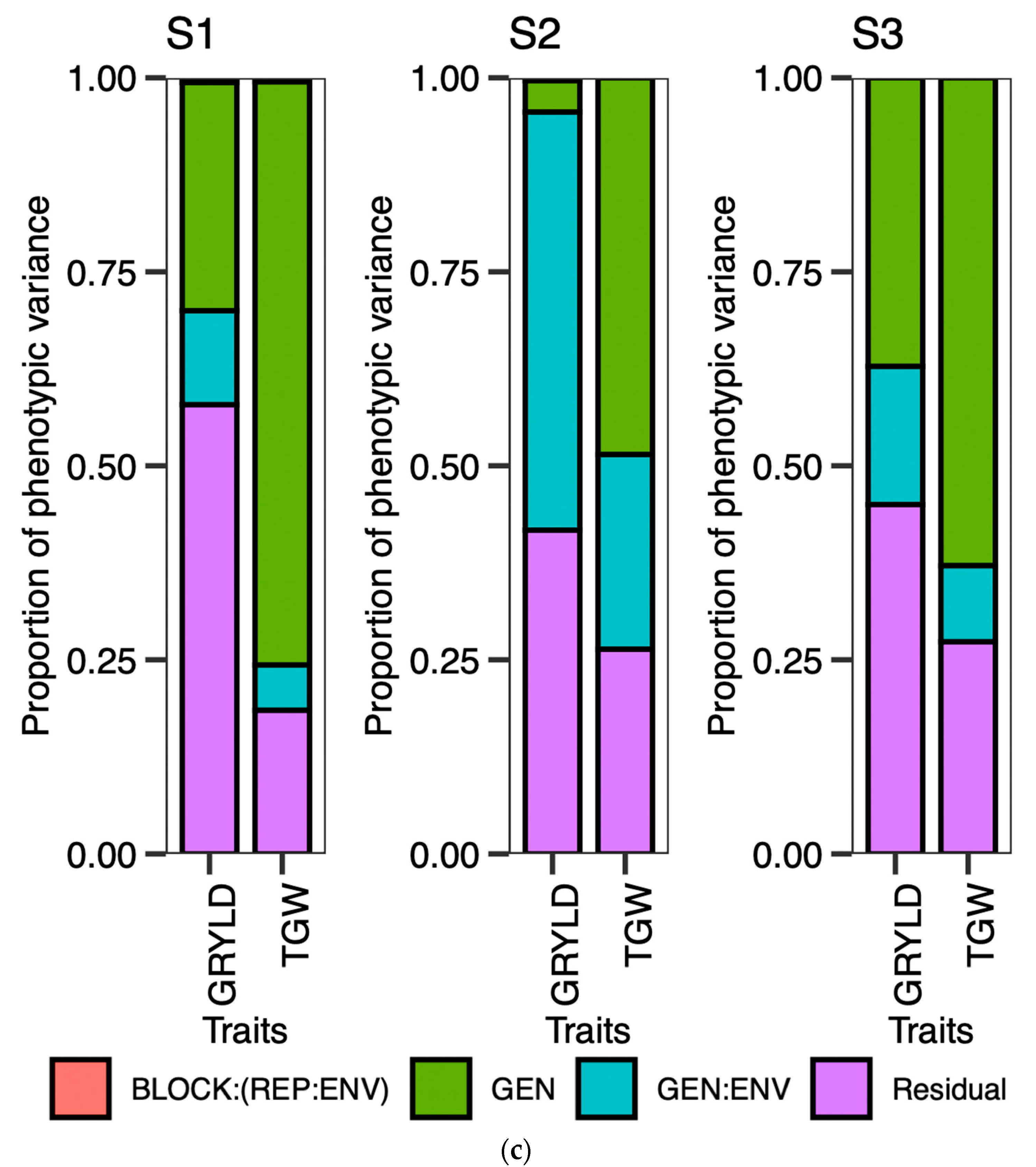
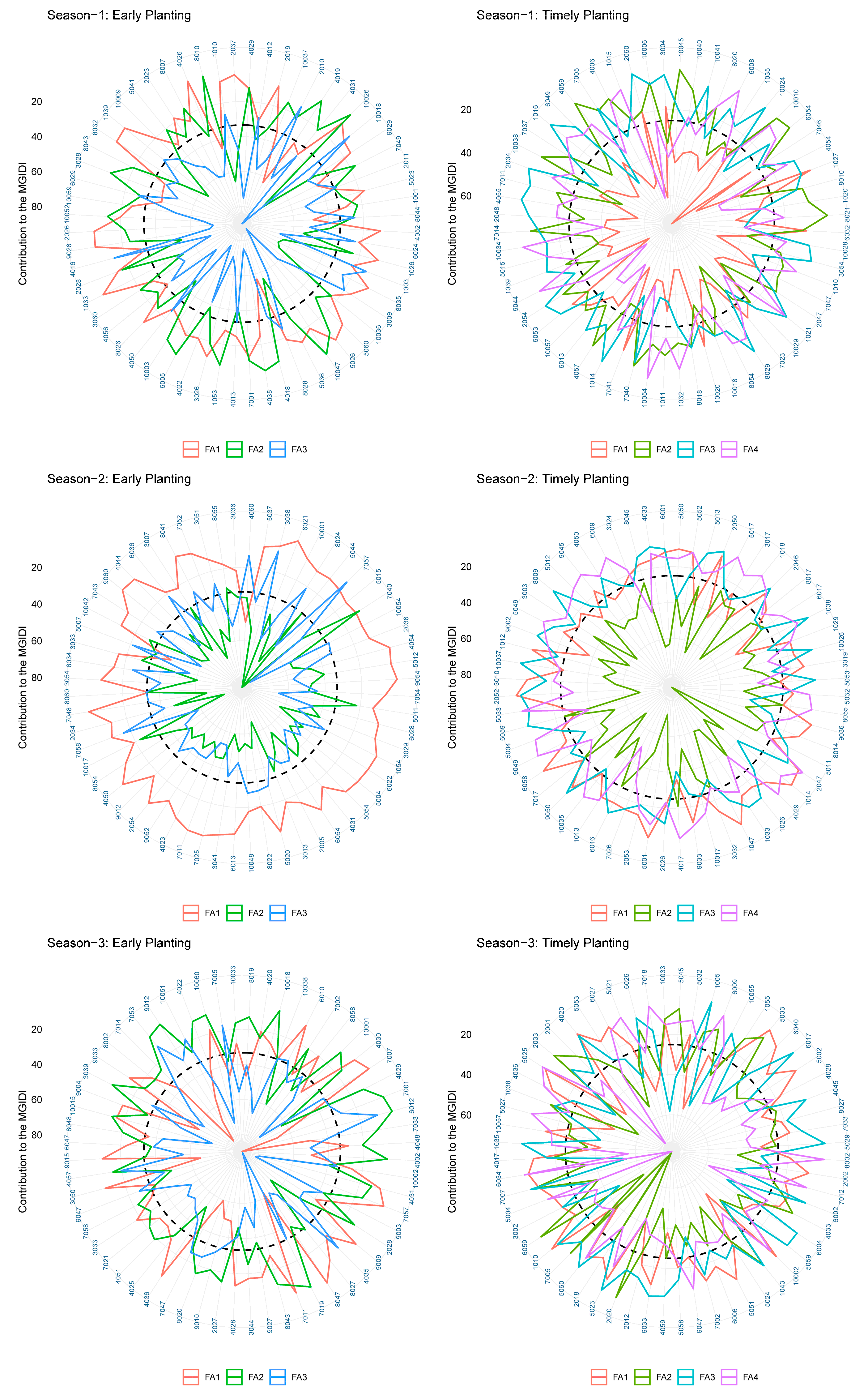
The ideotype design approach aims to enhance crop productivity by concurrently considering the selection of genotypes based on multiple traits. The design of an ideotype, as proposed by Olivoto and Nardino in 2020, involves the favorable selection of multiple traits with satisfactory gains for their application in breeding programs [
40]. The study revealed a satisfactory genetic gain for certain traits, suggesting that the attainment of an ideotype design is feasible through the utilization of the MGIDI, whereby traits are designated for increase or decrease. A study on the selection of stress-resistant maize hybrids generated a comparable and evident conclusion to assure long-term gains in primary traits while maintaining genetic gains in secondary traits [
72]. The MGIDI exhibits a superior performance in comparison to other linear selection indices, thereby aiding breeders in the identification of superior genotypes. The utilization of multiple traits for ideotype design holds the potential to mitigate the adverse effects of multicollinearity. As a result, it leads to enhanced conditioned matrices and unbiased index coefficients, which facilitate the estimation of the genetic gain [
40]. The utilization of a graphical and simplified method for evaluating the strengths and weaknesses of genotypes and traits facilitated the identification of suitable candidates for continued implementation in the current breeding program.
Certain traits that do not align with the ideotype and may hinder the optimal performance of genotypes should be taken into account when planning future breeding programs. Crop breeders are required to select genotypes from a pool of advanced lines that exhibit the desired level of strength for the trait of interest in a crop-breeding program. The present investigation revealed a distinct trend of strengths and weaknesses for the traits in question, as evidenced by the prompt and punctual plantings observed across the three-year period. Phenological traits such as the PG_DTB and PG_GFD demonstrated significant selection gain throughout all seasons in early planting. However, weaker support for the selected genotypes in early planting for two seasons was observed in the TGW and GRYLD. Nevertheless, the superior results achieved by well-executed genotypes on both dates of planting exhibited greater stability compared to other genotypes. The possibility exists for their selection in future variety releases within a broader spectrum of the 20 MHA Indo-Gangetic regions, which encompass a range of climatic conditions, from cooler and drier (NWPZ) to warmer and more humid (NEPZ). The vast expanse of wheat cultivation in India constitutes a unique geographical region characterized by predominantly small–marginal farmers who seek a cultivar capable of adapting to diverse sowing periods. The cultivation of the said genotypes can be carried out either during the early sowing period or within the appropriate time frame, contingent upon the cropping system, while maintaining optimal performance.
Effective breeding programs require excellent genetic resources with a diversified gene pool in the breeding material [
73]. A huge genetic variation exists across the wheat genome, and it was noticeably high for the A and B genomes in CIMMYT germplasms [
56]. It was also noted in the same article that CIMMYT’s synthetic wheat germplasm has lower gene diversity in the D genome compared to the A and B genomes. The materials used in the current study also had less genomic diversity in the D genome. Again, the proper understanding of and acquaintance with the linkage disequilibrium extent are necessary to determine the adequate requirement of the marker density for proper MTA study, where it is notably true that the LD declines swiftly with distance [
74]. It was observed in a recent study [
65] that the marker number is no longer a critical limiting factor for prediction accuracies in wheat in which the LD is high. Moreover, counterfeit MTA might cause less diverse population structures in GWASs [
75,
76,
77]. In this study, the population was highly stratified, giving a broadened general overview and knowledge of the biological background of the mapping panel.
The GWAS revealed several significant marker–trait associations. The marker diversity in the D genome was lower compared to the A and B genomes. A similar result was found in the genetic analysis of the spring wheat association-mapping panel [
78]. The GWAS for the trait of interest in early adaptation was found to be valuable in the genetic architecture study and co-localization of loci for the traits. Several captivating co-localizations were identified in a study for phenology, plant stature, disease resistance, and even yield, contributing traits with potential associations with the GRYLD and stability, and representing its possible significance in global wheat-breeding strategies [
65]. Our current study also found several genomic regions with associated significant markers in several genomes for multiple traits. LD analysis removed all the false-positive markers in the GWAS and, finally, 96 unique
QTLs were identified. Several of these
QTLs were co-localized with multiple traits at different planting times. The co-localization of SNPs and
QTLs for different traits in different environments has been reported in several studies [
62,
79,
80,
81,
82,
83,
84].
The early heat tolerance can be examined by applying selection pressure to a diverse genetic materials cultivated under October sowing conditions [
85]. This selection intensity is influenced mainly by several phenology-affecting QTLs or genes. In our study, we found QTLs not only associated with phenology, but also with other groups of traits, such as plant stature and physiological traits. Several heat-tolerant QTLs have been receiving increased attention over the last few years [
86,
87,
88,
89]. A review on recent GWASs on wheat over the last decade (from 2010 to 2020) found thousands of MTAs conferring abiotic stresses [
90]. Among these MTAs, seedling heat tolerance was reported by Maulana et al. (2018) [
87], who identified several QTLs containing potential sources of candidate genes of early heat tolerance in winter wheat. In our study, we found 44 unique QTLs associated with early adaptation in spring wheat. Among these QTLs, co-localization for various traits were common. The QTL prefix with “QMpt” in our study refers to the co-localized loci for multiple traits. Again, some QTLs were found to be co-localized even for both planting times. These QTLs are obviously crucial for definitive study, but we considered unique QTLs for different planting times. Several QTLs linked to yield component traits have been detected in the last decades through GWASs [
90]; some of them were validated using bi-parental mapping population analysis [
90,
91] or meta-QTL analysis [
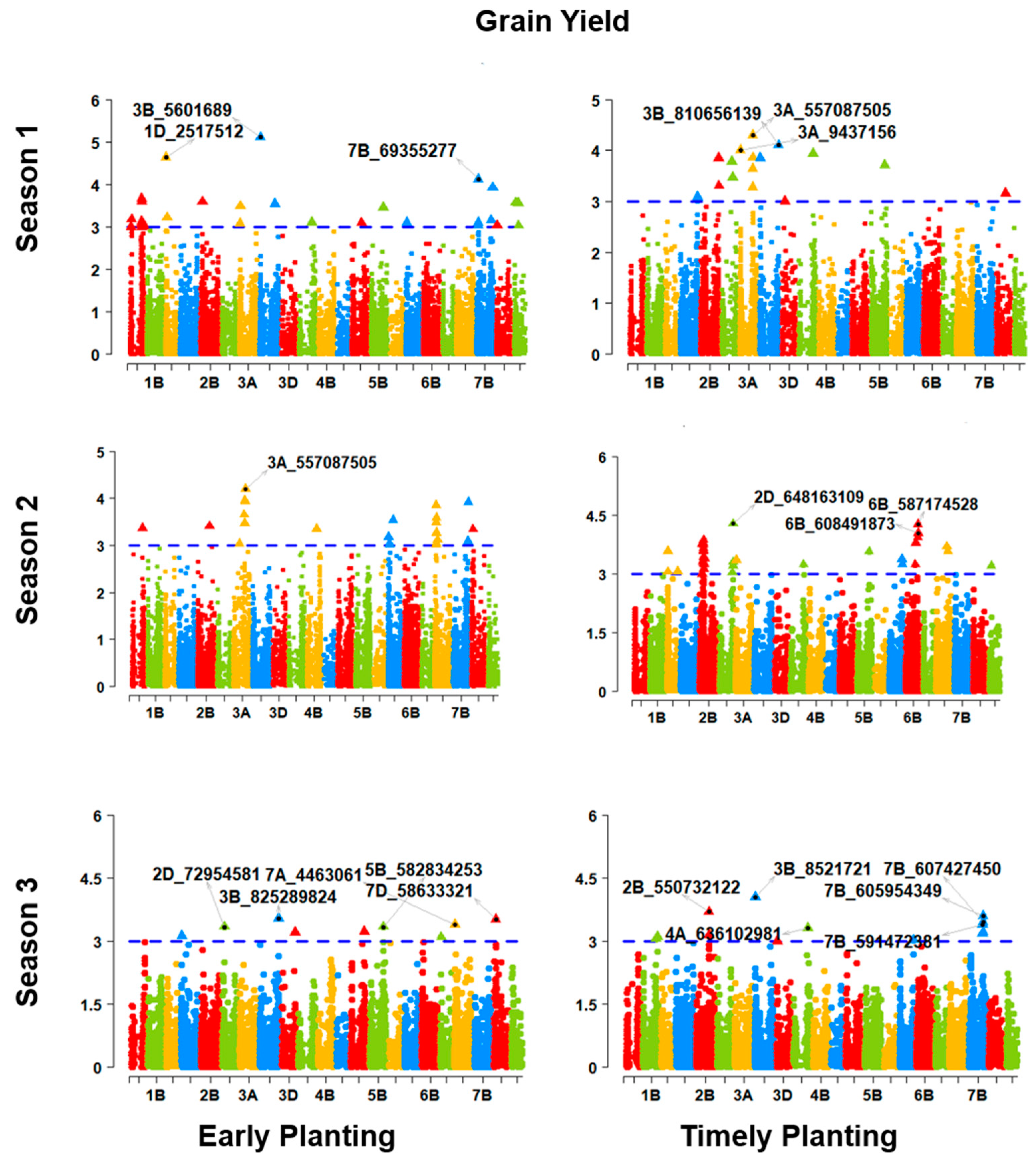
92]. In early planting conditions, two unique QTLs (QMpt.bisa.2B.2 and QMpt.bisa.2D.3) linked to photoperiod-sensitivity genes, such as
Ppd-B1 and
Ppd-D1, controlled the PG_BTH. This phenomenon confirms the importance of photoperiod sensitivity in early adaptation. Again,
Vrn-B1 was found to be linked with two QTLs (QMpt.bisa.5B.3 and QMpt.bisa.5B.4) to control the trait PG_DTB in early planting. The linkage of the days to booting with
Vrn-B1-linked QTLs suggests a vernalization requirement in the early-adapted genotype. We can conclude a mild vernalization requirement for early adaptation, as we worked with spring wheat. Genes for photoperiod sensitivity and the vernalization requirements were also shown to make an effective contribution to early adaptation [
55]. In early planting, a higher number of QTLs responsible for all three phenology-affecting traits reveals that early adaptation influences many putative genes or QTLs to exert their impacts compared to timely planting. Similar cases also were found in physiological traits, except for the CTIR. The highly quantitative traits TGW and GRYLD were also revealed to be associated with a higher number of QTLs for early planting compared to timely planting. In a nutshell, early planting is influenced by more QTLs for phenology, physiology, the TGW, and the GRYLD. The genotype–phenotype map connecting key markers to the RefSeq illustrates the RefSeq’s use as a platform for comparing and confirming GWAS results. Targeted selection for the desired region is now easy using this community resource for wheat breeding. Our study provides an opportunity for accelerating GWAS-assisted wheat breeding for early adaptation in the Indo-Gangetic region.