Effect of Exogenous Hormone on R-Spondin 2 (Rspo2) and R-Spondin 3 (Rspo3) Gene Expression and Embryo Development in Chinese Soft-Shelled Turtle (Pelodiscus sinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Estradiol Treatment
2.3. RNA Isolation and PCR
2.4. Sequence Analysis and Homology Analyses
2.5. Gene Expression Analysis by Quantitative Real-Time Reverse Transcription–PCR (RT-qPCR)
2.6. Statistical Analysis
3. Results
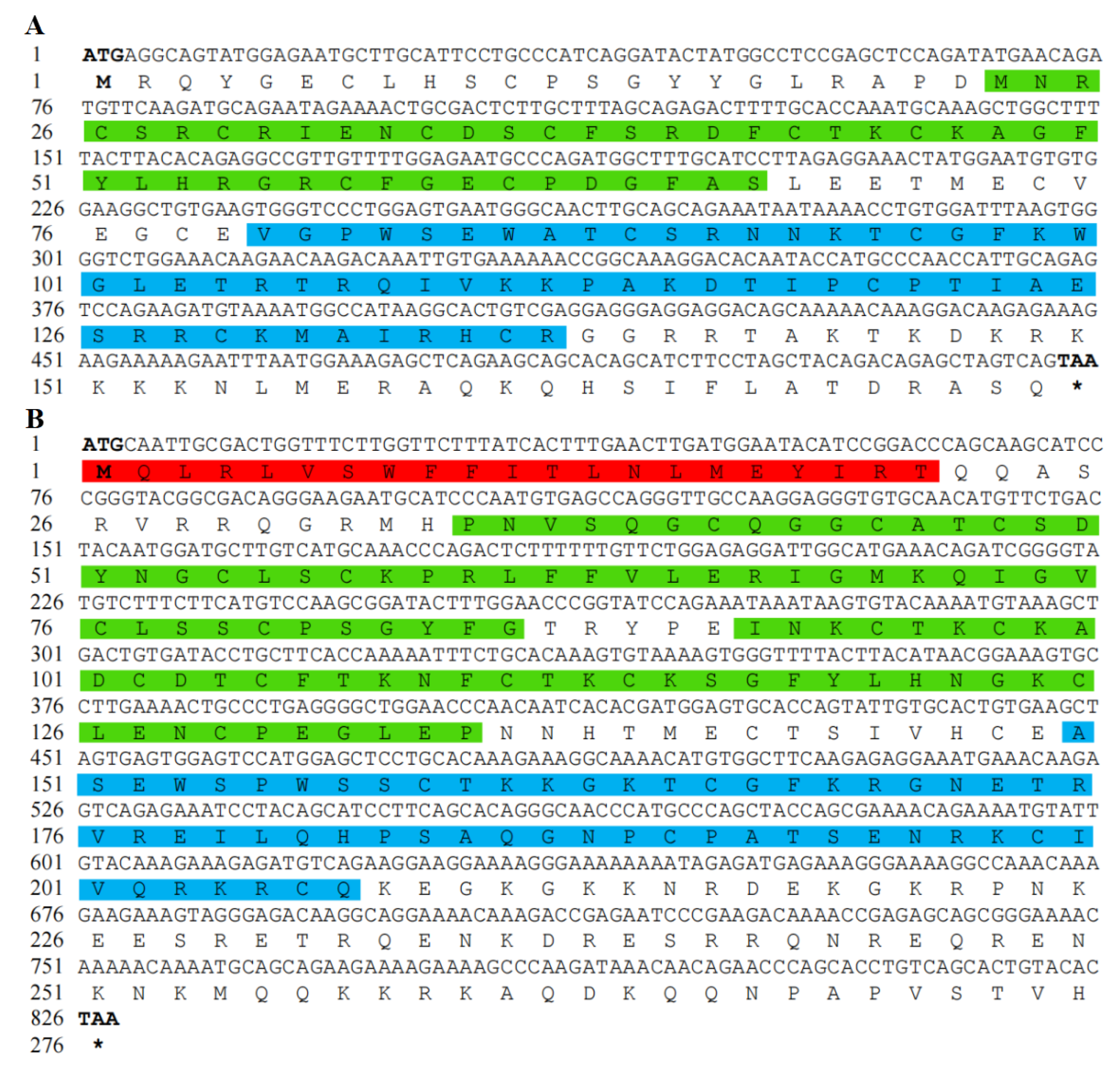
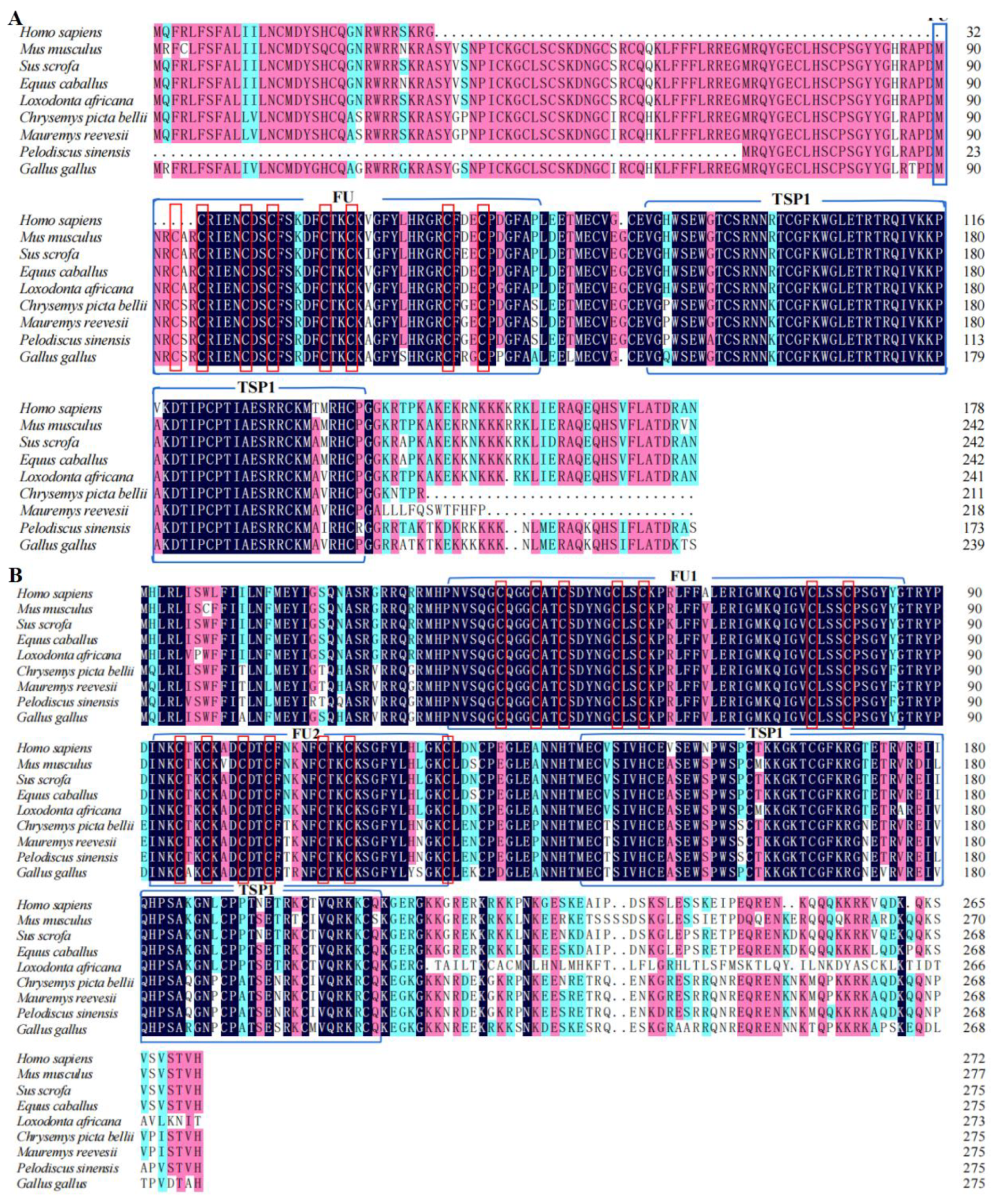
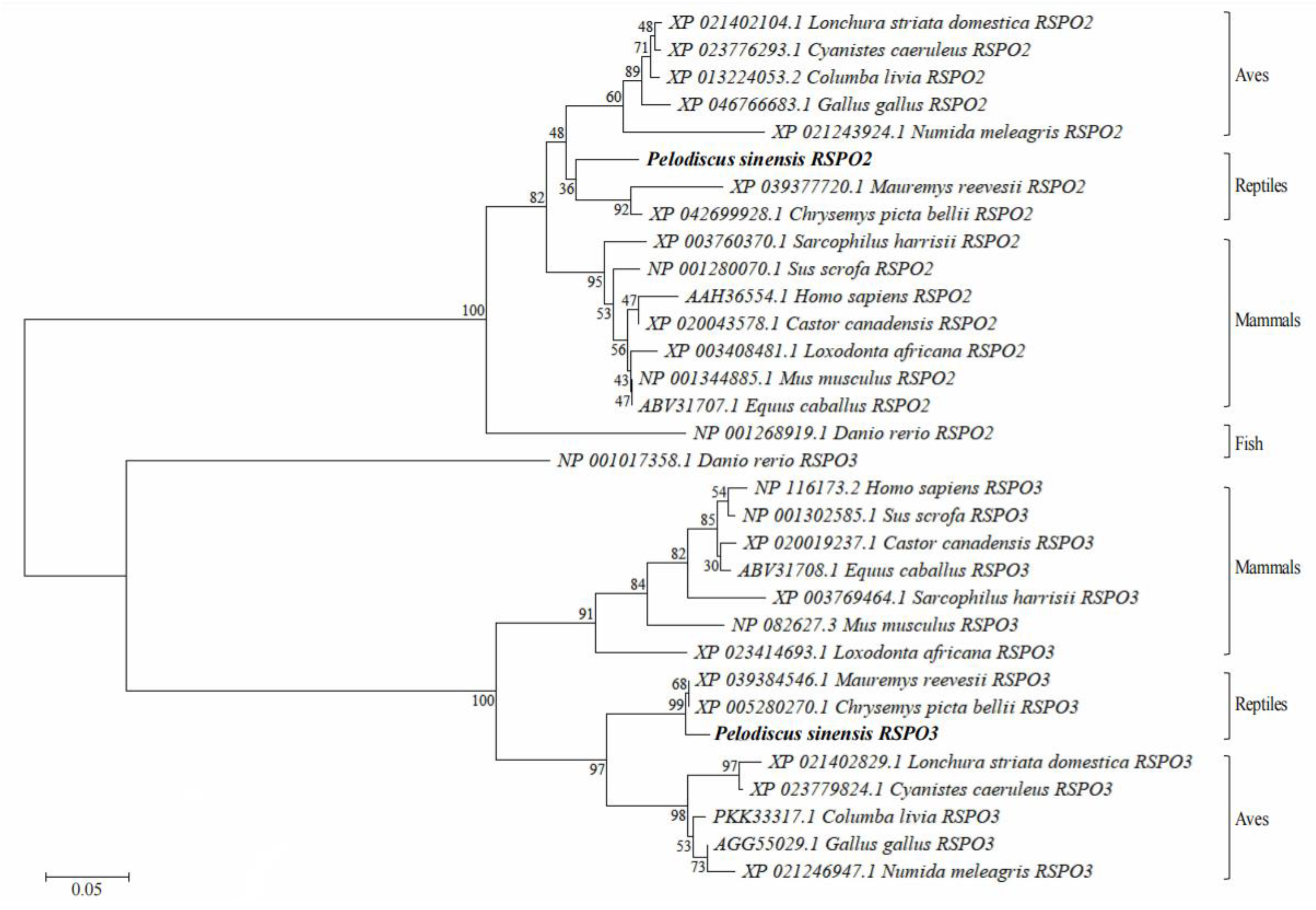
3.1. Analysis of Chinese Soft-Shelled Turtle RSPO2 and RSPO3 cDNA and Protein Sequences
3.2. Expression Patterns of Rspo2 and Rspo3 in Different Tissues of Chinese Soft-Shelled Turtles
3.3. Effects of E2 on the Expression of Rspo2 and Rspo3 during Embryonic Development of Chinese Soft-Shelled Turtles
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, S.P.; Melita, V.; Markus, A.; Peter, P.; Uwe, F. Millennium-old farm breeding of Chinese softshell turtles (Pelodiscus spp.) results in massive erosion of biodiversity. Die Naturwissenschaften 2018, 105, 34. [Google Scholar] [CrossRef]
- Liang, H.W.; Wang, L.H.; Sha, H.; Zou, G.W. Development and Validation of Sex-Specific Markers in Pelodiscus Sinensis Using Restriction Site-Associated DNA Sequencing. Genes 2019, 10, 302. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Tapas, C.; Yu, X.G.; Wu, L.M.; Liu, G.; Sipra, M.; Wang, D.S.; Yoshitaka, N. R-spondins are involved in the ovarian differentiation in a teleost, medaka (Oryzias latipes). BMC Dev. Biol. 2012, 12, 36. [Google Scholar] [CrossRef]
- Kazanskaya, O.; Glinka, A.; Barrantes, I.d.B.; Stannek, P.; Niehrs, C.; Wu, W. R-Spondin2 Is a Secreted Activator of Wnt/β-Catenin Signaling and Is Required for Xenopus Myogenesis. Dev. Cell 2004, 7, 525–534. [Google Scholar] [CrossRef]
- Nam, J.S.; Park, E.; Turcotte, T.J.; Palencia, S.; Zhan, X.M.; Lee, J.; Yun, K.; Funk, W.D.; Yoon, J.K. Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev. Biol. 2007, 311, 124–135. [Google Scholar] [CrossRef]
- Choi, Y.; Qin, Y.Y.; Berger, M.F.; Ballow, D.J.; Bulyk, M.L.; Rajkovic, A. Microarray analyses of newborn mouse ovaries lacking Nobox. Biol. Reprod. 2007, 77, 312–319. [Google Scholar] [CrossRef]
- Kocer, A.; Pinheiro, I.; Pannetier, M.; Renault, L.; Parma, P.; Radi, O.; Kim, K.A.; Camerino, G.; Pailhoux, E.; Aleksandar, R. R-spondin1 and FOXL2 act into two distinct cellular types during goat ovarian differentiation. BMC Dev. Biol. 2008, 8, 36. [Google Scholar] [CrossRef]
- Motoko, A.; Michihiro, M.; Toshio, I.; Yoshio, H.; Harukazu, N.; Hitoshi, O. R-spondin3 is required for mouse placental development. Dev. Biol. 2007, 301, 218–226. [Google Scholar]
- Hu, Q.M.; Meng, Y.; Tian, H.F.; Zhang, Y.U.; Xiao, H.B. Sexually Dimorphic Expression of Foxl2 and Ftz-F1 in Chinese Giant Salamander Andrias Davidianus. J. Exp. Zool. Part B Mol. Dev. Evol. 2016, 326, 363–374. [Google Scholar] [CrossRef]
- Barske, L.A.; Capel, B. Estrogen represses SOX9 during sex determination in the red-eared slider turtle Trachemys scripta. Dev. Biol. 2010, 341, 305–314. [Google Scholar] [CrossRef]
- Schulz, R.W.; Bogerd, J.; Male, R.; Ball, J.; Fenske, M.; Olsen, L.C.; Tyler, C.R. Estrogen-induced alterations in amh and dmrt1 expression signal for disruption in male sexual development in the zebrafish. Environ. Sci. Technol. 2007, 41, 6305–6310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, G.B.; Chen, M.; Wang, Y.B.; Zou, G.W.; Liang, H.W. Direct Full-Length RNA Sequencing Reveals an Important Role of Epigenetics During Sexual Reversal in Chinese Soft-Shelled Turtle. Front. Cell Dev. Biol. 2022, 10, 876045. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y. Study on the Gender Identity of Trionyx Sinensis and the Impact of Exogenous Hormones. Master’s Thesis, Southwest University, Chongqing, China, 2015. [Google Scholar]
- Liang, H.W.; Meng, Y.; Cao, L.H.; Li, X.; Zou, G.W. Effect of exogenous hormones on R-spondin 1 (RSPO1) gene expression and embryo development in Pelodiscus sinensis. Reprod. Fertil. Dev. 2019, 31, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Zhang, Y.Y.; Ling, C.; Zou, G.W.; Liang, H.W. Chinese Softshelled Turtle Pelodiscus sinensis: Embryonic Development and Embryo Staging. Chin. Agric. Sci. Bull. 2020, 36, 152–158. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Wu, L.M.; Yang, P.; Luo, F.; Wang, D.S.; Zhou, L.Y. R-spondin1 signaling pathway is required for both the ovarian and testicular development in a teleosts, Nile tilapia (Oreochromis niloticus). Gen. Comp. Endocrinol. 2016, 230–231, 177–185. [Google Scholar] [CrossRef]
- De Lau, W.B.; Snel, B.; Clevers, H.C. The R-spondin protein family. Genome Biol. 2012, 13, 242. [Google Scholar] [CrossRef]
- Nam, J.S.; Turcotte, T.J.; Smith, P.F.; Choi, S.; Yoon, J.K. Mouse Cristin/R-spondin Family Proteins Are Novel Ligands for the Frizzled 8 and LRP6 Receptors and Activate β-Catenin-dependent Gene Expression. J. Biol. Chem. 2006, 281, 13247–13257. [Google Scholar] [CrossRef]
- Ter Steege, E.J.; Bakker, E.R.M. The role of R-spondin proteins in cancer biology. Oncogene 2021, 40, 6469–6478. [Google Scholar] [CrossRef]
- Kazanskaya, O.; Ohkawara, B.; Heroult, M.; Wu, W.; Maltry, N.; Augustin, H.G.; Niehrs, C. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development 2008, 135, 3655–3664. [Google Scholar] [CrossRef]
- Cao, L.H. Study on the Effects of Temperature and Hormones on the Sex of the Chinese Soft-Shelled Trutle Pelodiscus sinensis. Master’s Thesis, Huazhong Agruicultural University, Wuhan, China, 2018. [Google Scholar]
- Shoemaker, C.M.; Crews, D. Analyzing the coordinated gene network underlying temperature-dependent sex determination in reptiles. Semin. Cell Dev. Biol. 2008, 20, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Crews, D. Molecular mechanisms of temperature-dependent sex determination in the context of ecological developmental biology. Mol. Cell. Endocrinol. 2012, 354, 103–110. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, G.B.; Chen, M.; Wang, Y.B.; Zou, G.W.; Liang, H.W. Tandem Mass Tag-Based Quantitative Proteomics Analysis of Gonads Reveals New Insight into Sexual Reversal Mechanism in Chinese Soft-Shelled Turtles. Biology 2022, 11, 1081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Sha, H.; Chen, M.; Chen, G.B.; Zou, G.W.; Liang, H.W. MicroRNAs May Play an Important Role in Sexual Reversal Process of Chinese Soft-Shelled Turtle, Pelodiscus sinensis. Genes 2021, 12, 1696. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y. Molecular mechanisms of sex determination and gonadal sex differentiation in fish. Fish Physiol. Biochem. 2005, 31, 105–109. [Google Scholar] [CrossRef]
- Ann, O.C.; Thea, H.B.; Suzanne, C.; Steve, M.; Marius, B.; John, A.M. Developmental Exposure to Anthracenen and Estradiol Alters Reproductive Success in Medaka (Oryzias latipes). Environ. Sci. 2001, 8, 31–45. [Google Scholar]
- Wang, Y.B.; Luo, X.Z.; Qu, C.J.; Xu, T.; Zou, G.W.; Liang, H.W. The Important Role of Sex-Related Sox Family Genes in the Sex Reversal of the Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Biology 2022, 11, 83. [Google Scholar] [CrossRef]
- Flament, S.; Chardard, D.; Chesnel, A.; Dumond, H. Sex Determination and Sexual Differentiation in Amphibians. In Hormones and Reproduction of Vertebrates; Academic Press: Cambridge, MA, USA, 2011; pp. 1–19. [Google Scholar]
- Phuge, S.K.; Gramapurohit, N.P. Sex hormones alter sex ratios in the Indian skipper frog, Euphlyctis cyanophlyctis: Determining sensitive stages for gonadal sex reversal. Gen. Comp. Endocrinol. 2015, 220, 70–77. [Google Scholar] [CrossRef]
- Li, H.J. Study on the Differentiation of Gonad and the Effects of Temperatures on It in Trionyx sinensis. Master’s Thesis, Hebei University, Baoding, China, 2012. [Google Scholar]
- Chen, G.B.; Zhou, T.; Chen, M.; Zou, G.W.; Liang, H.W. Effect of Estradiol on Estrogen Nuclear Receptors Genes Expression on Embryonic Development Stages in Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Fishes 2022, 7, 223. [Google Scholar] [CrossRef]
- Chikae, M.; Ikeda, R.; Hasan, Q.; Morita, Y.; Tamiya, E. Effects of tamoxifen, 17α-ethynylestradiol, flutamide, and methyltestosterone on plasma vitellogenin levels of male and female Japanese medaka (Oryzias latipes). Environ. Toxicol. Pharmacol. 2004, 17, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.I.; Teodósio, H.R.; Galay-Burgos, M.; Power, D.M.; Sweeney, G.E.; Canário, A.V. Identification of estrogen-responsive genes in the testis of sea bream (Sparus auratus) using suppression subtractive hybridization. Mol. Reprod. Dev. 2006, 73, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Q.; Zhang, S.Y.; Duan, Y.Q.; Zhang, W.P. 17β-estradiol induced channel catfish females. Acta Hydrobiol. Sin. 2022, 46, 1668–1674. [Google Scholar]
- Wang, C.L.; Guan, W.Z.; Li, Y.Q.; Liu, F. Study on 17β-estradiol induced feminization of Pelteobagrus fulvidrac. South China Fish. Sci. 2020, 16, 25–30. [Google Scholar]
- Teal, C.N.; Schill, D.J.; Fogelson, S.B.; Roberts, C.M.; Fitzsimmons, K.; Bauder, J.M.; Stewart, W.T.; Bonar, S.A. The effects of estradiol-17β on the sex reversal, survival, and growth of green sunfish Lepomis cyanellus. Aquaculture 2023, 562, 738853. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence (5′-3′) | Insert Size | Application |
|---|---|---|---|
| Rspo2-F | ACGAGAAGGAATGAGGCAGTATG | 757 | CDS amplification |
| Rspo2-R | AACTGCCACACCACTTCCACT | ||
| Rspo3-F1 | TTATCCCCCTTCAACGCC | 372 | |
| Rspo3-R1 | ATTCTTCCCTGTCGCCGT | ||
| Rspo3-F2 | ATTGCGACTGGTTTCTTGGTT | 415 | |
| Rspo3-R2 | CATCGTGTGATTGTTGGGTTC | ||
| Rspo3-F3 | TTTTTGTTCTGGAGAGGATTGG | 408 | |
| Rspo3-R3 | TTCTGTTTTCGCTGGTAGCTG | ||
| Rspo3-F4 | CAAGAGTCAGAGAAATCCTACAGCA | 333 | |
| Rspo3-R4 | GCAGTGTCCATTTGTGGTTTGA | ||
| Rspo2-qF | ATGGAATGTGTGGAAGGCTG | 164 | qPCR |
| Rspo2-qR | GACTCTGCAATGGTTGGGCA | ||
| Rspo3-qF | CCTACAGCATCCTTCAGCACA | 213 | |
| Rspo3-qR | GTCTTCGGGATTCTCGGTCT | ||
| Gapdh-qF | AGAACATCATTCCAGCATCCA | 179 | Internal control |
| Gapdh-qR | CTTCATCACCTTCTTAATGTCGTC |
| RSPO2 | RSPO3 | |||
|---|---|---|---|---|
| Species | Accession Number | Identity (%) | Accession Number | Identity (%) |
| M. reevesii | XP_039377720.1 | 97.70 | XP_039384546.1 | 97.09 |
| C. picta bellii | XP_042699928.1 | 95.14 | XP_005280270.1 | 96.73 |
| G. gallus | XP_046766683.1 | 90.23 | AGG55029.1 | 84.73 |
| Equus caballus | ABV31707.1 | 86.21 | ABV31708.1 | 79.64 |
| Mus musculus | NP_001344885.1 | 86.21 | NP_082627.3 | 73.48 |
| Loxodonta africana | XP_003408481.1 | 85.63 | XP_023414693.1 | 78.79 |
| Sus scrofa | NP_001280070.1 | 85.63 | NP_001302585.1 | 78.55 |
| Homo sapiens | AAH36554.1 | 71.10 | NP_116173.2 | 76.84 |
| Danio rerio | NP_001268919.1 | 67.82 | NP_001017358.1 | 51.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Zhou, T.; Chen, G.; Zou, G.; Liang, H. Effect of Exogenous Hormone on R-Spondin 2 (Rspo2) and R-Spondin 3 (Rspo3) Gene Expression and Embryo Development in Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Genes 2023, 14, 1466. https://doi.org/10.3390/genes14071466
Cao J, Zhou T, Chen G, Zou G, Liang H. Effect of Exogenous Hormone on R-Spondin 2 (Rspo2) and R-Spondin 3 (Rspo3) Gene Expression and Embryo Development in Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Genes. 2023; 14(7):1466. https://doi.org/10.3390/genes14071466
Chicago/Turabian StyleCao, Jizeng, Tong Zhou, Guobin Chen, Guiwei Zou, and Hongwei Liang. 2023. "Effect of Exogenous Hormone on R-Spondin 2 (Rspo2) and R-Spondin 3 (Rspo3) Gene Expression and Embryo Development in Chinese Soft-Shelled Turtle (Pelodiscus sinensis)" Genes 14, no. 7: 1466. https://doi.org/10.3390/genes14071466
APA StyleCao, J., Zhou, T., Chen, G., Zou, G., & Liang, H. (2023). Effect of Exogenous Hormone on R-Spondin 2 (Rspo2) and R-Spondin 3 (Rspo3) Gene Expression and Embryo Development in Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Genes, 14(7), 1466. https://doi.org/10.3390/genes14071466




