Shotgun Metagenomic Sequencing Reveals Virome Composition of Mosquitoes from a Transition Ecosystem of North-Northeast Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection Site
2.2. Mosquito Collection and Taxonomic Identification
2.3. RNA Extraction, Library Preparation and Sequencing
2.4. Bioinformatic and Phylogenetic Analysis
3. Results
3.1. Mosquito Collection and Generated Data
3.2. Virome Analysis
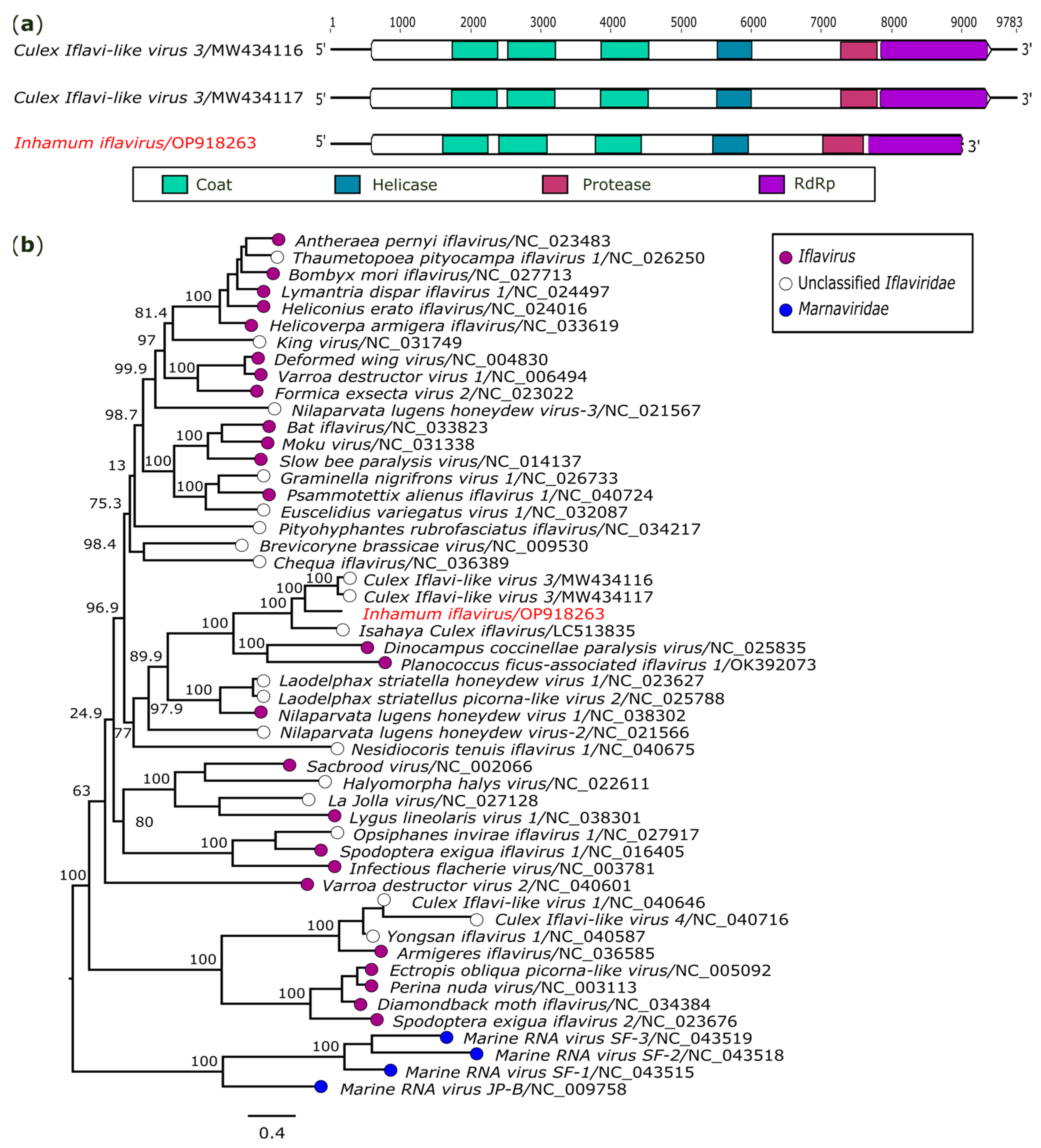
3.2.1. Iflaviridae
3.2.2. Metaviridae
3.2.3. Solemoviridae
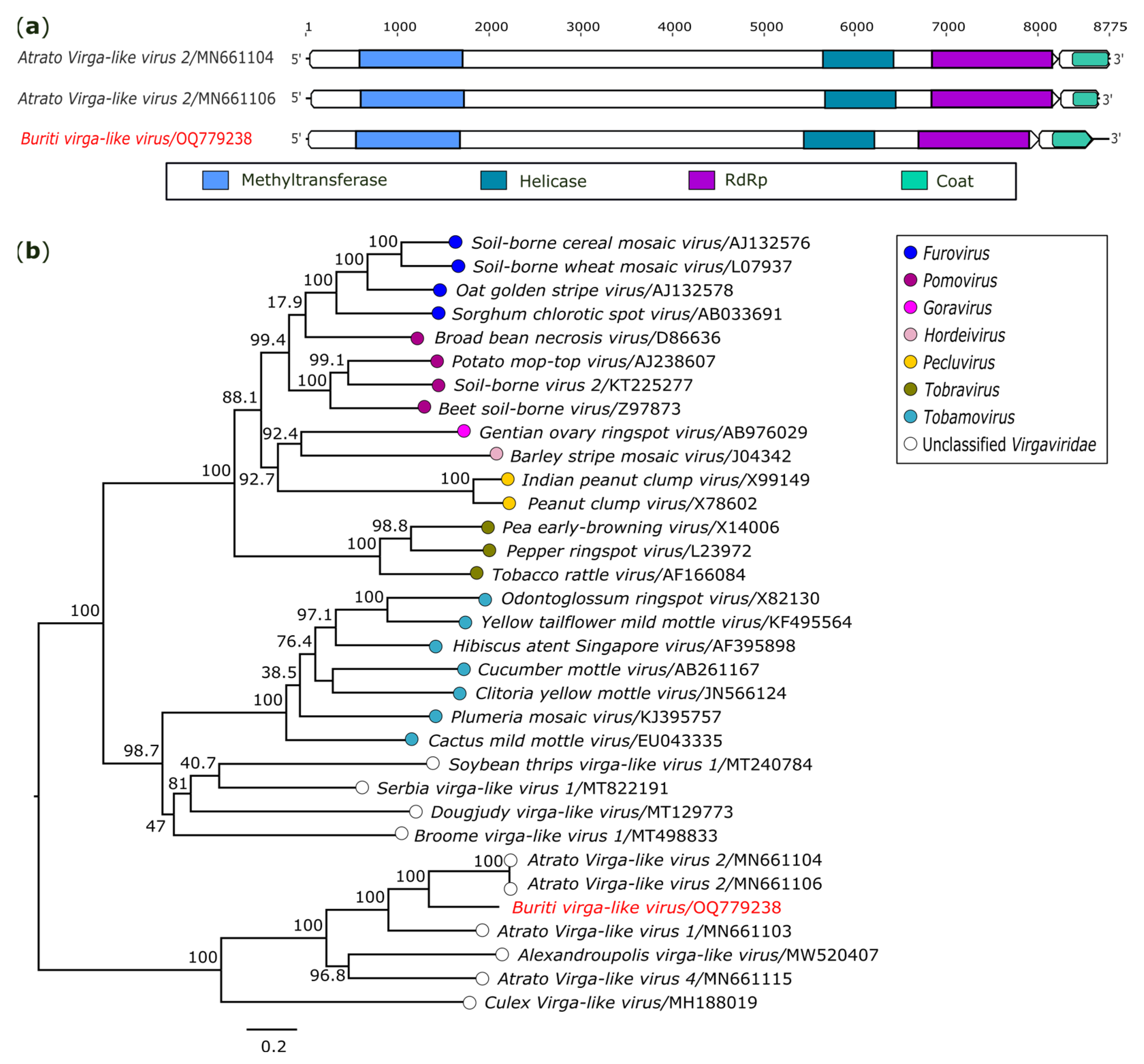
3.2.4. Virgaviridae
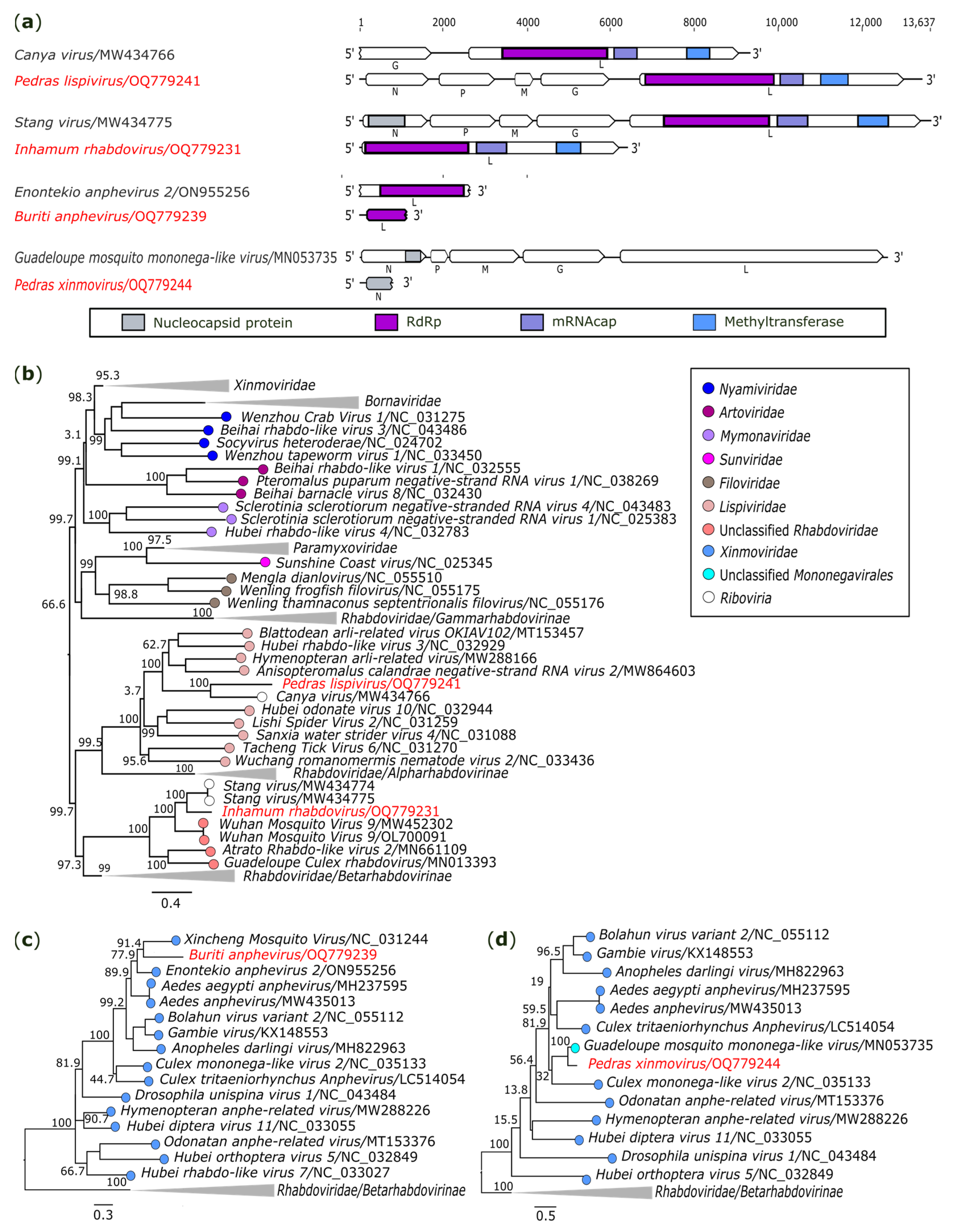
3.2.5. Mononegavirales: Rhabdoviridae, Lispiviridae and Xinmoviridae
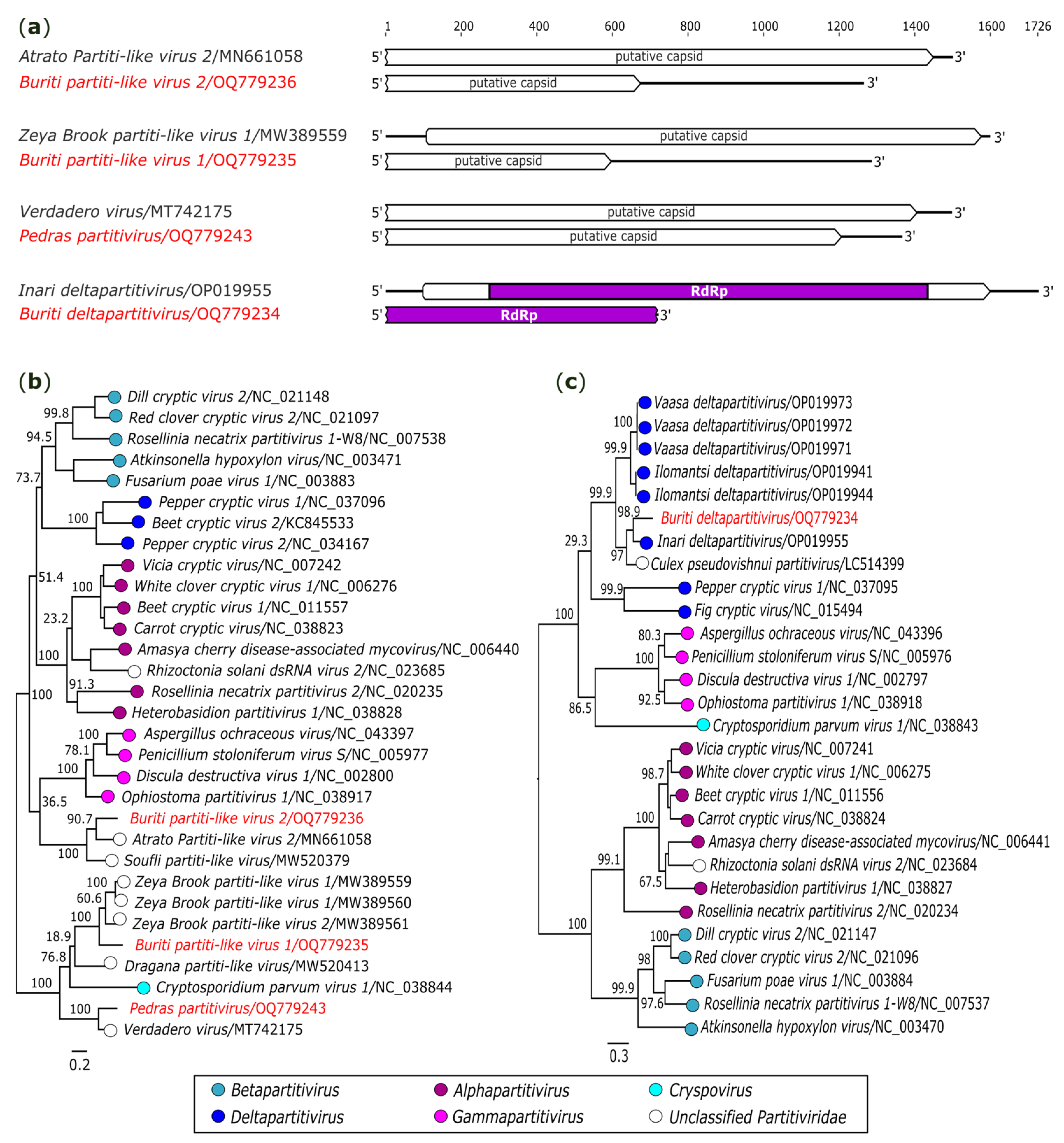
3.2.6. Partitiviridae
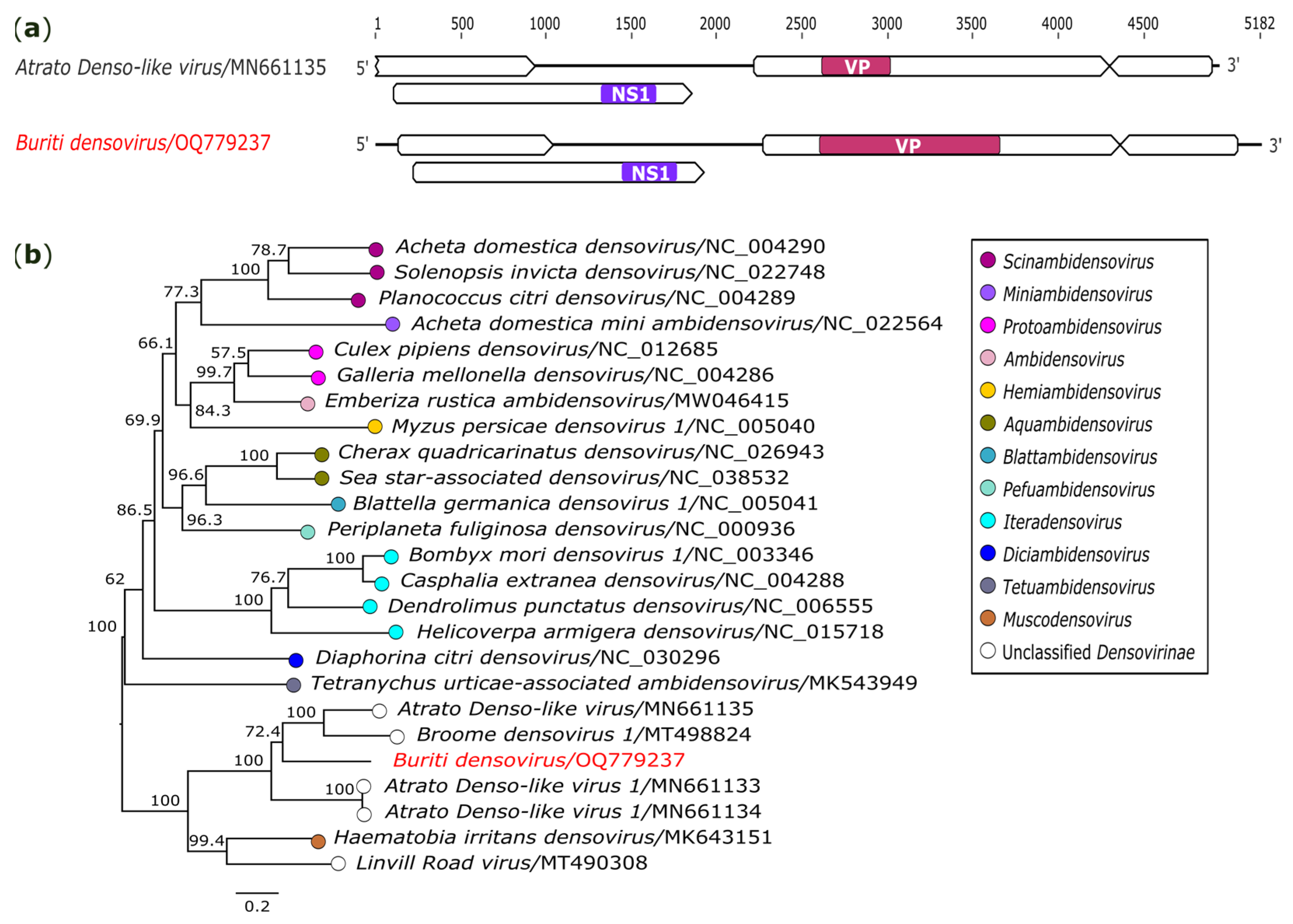
3.2.7. Parvoviridae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caragata, E.P.; Tikhe, C.V.; Dimopoulos, G. Curious Entanglements: Interactions between Mosquitoes, Their Microbiota, and Arboviruses. Curr. Opin. Virol. 2019, 37, 26. [Google Scholar] [CrossRef]
- Patterson, E.I.; Villinger, J.; Muthoni, J.N.; Dobel-Ober, L.; Hughes, G.L. Exploiting Insect-Specific Viruses as a Novel Strategy to Control Vector-Borne Disease. Curr. Opin. Insect Sci. 2020, 39, 50–56. [Google Scholar] [CrossRef]
- Bolling, B.G.; Weaver, S.C.; Tesh, R.B.; Vasilakis, N. Insect-Specific Virus Discovery: Significance for the Arbovirus Community. Viruses 2015, 7, 4911–4928. [Google Scholar] [CrossRef] [PubMed]
- Nouri, S.; Matsumura, E.E.; Kuo, Y.W.; Falk, B.W. Insect-Specific Viruses: From Discovery to Potential Translational Applications. Curr. Opin. Virol. 2018, 33, 33–41. [Google Scholar] [CrossRef]
- Patterson, E.I.; Kautz, T.F.; Contreras-Gutierrez, M.A.; Guzman, H.; Tesh, R.B.; Hughes, G.L.; Forrester, N.L. Negeviruses Reduce Replication of Alphaviruses during Coinfection VIRUS-CELL INTERACTIONS. J. Virol. 2021, 95, 433–454. [Google Scholar] [CrossRef] [PubMed]
- Baidaliuk, A.; Miot, E.F.; Lequime, S.; Moltini-Conclois, I.; Delaigue, F.; Dabo, S.; Dickson, L.B.; Aubry, F.; Merkling, S.H.; Cao-Lormeau, V.-M.; et al. Cell-Fusing Agent Virus Reduces Arbovirus Dissemination in Aedes Aegypti Mosquitoes In Vivo. J. Virol. 2019, 93, e00705-19. [Google Scholar] [CrossRef] [PubMed]
- Romo, H.; Kenney, J.L.; Blitvich, B.J.; Brault, A.C. Restriction of Zika Virus Infection and Transmission in Aedes Aegypti Mediated by an Insect-Specific Flavivirus. Emerg. Microbes Infect. 2018, 7, 1–13. [Google Scholar] [CrossRef]
- Öhlund, P.; Lundén, H.; Blomström, A.-L. Insect-Specific Virus Evolution and Potential Effects on Vector Competence. Virus Genes 2019, 55, 127–137. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.d.C.; Azevedo, R.; Rodrigues, S.G.; Martins, L.C.; Chiang, J.O.; Travassos-da-Rosa, A.P.A. Arboviroses. In Medicina Tropical e Infectologia na Amazônia; Leão, R.N.Q., Bichara, C.N.C., Fraiha Neto, H., Vasconcelos, P.F.d.C., Eds.; Samauma Editorial: Belém, PA, Brasil, 2013; pp. 481–503. [Google Scholar]
- Ramos, B.A.; Das Chagas, L.L.; de Arruda e Silva, F.; dos Santos, E.B.; Chiang, J.O.; Neto, J.P.N.; Vieira, D.B.R.; Junior, J.W.R.; da Silva, E.V.P.; Freitas, M.N.O.; et al. Arboviruses in Free-Ranging Birds and Hematophagous Arthropods (Diptera, Nematocera) from Forest Remnants and Urbanized Areas of an Environmental Protection Area in the Amazon Biome. Viruses 2022, 14, 2101. [Google Scholar] [CrossRef]
- Araújo, P.A.; Freitas, M.O.; Chiang, J.O.; Silva, F.A.; Chagas, L.L.; Casseb, S.M.; Silva, S.P.; Nunes-Neto, J.P.; Rosa-Júnior, J.W.; Nascimento, B.S.; et al. Investigation about the Occurrence of Transmission Cycles of Arbovirus in the Tropical Forest, Amazon Region. Viruses 2019, 11, 774. [Google Scholar] [CrossRef]
- Silva, F.A.; Ferreira, M.S.; Araújo, P.A.; Casseb, S.M.M.; Silva, S.P.; Nunes Neto, J.P.; Chiang, J.O.; Rosa Junior, J.W.; Chagas, L.L.; Freitas, M.N.O.; et al. Serological and Molecular Evidence of the Circulation of the Venezuelan Equine Encephalitis Virus Subtype IIIA in Humans, Wild Vertebrates and Mosquitos in the Brazilian Amazon. Viruses 2022, 14, 2391. [Google Scholar] [CrossRef]
- Nunes-Neto, J.P.; Reis, L.A.M.; Freitas, M.N.O.; Nascimento, B.L.S.; Chagas, L.L.; Costa, H.H.M.; Rodrigues, J.C.P.; Braga, C.M.; Silva, E.V.P.; Silva, S.P.; et al. First Isolation and Genome Sequence Analysis of West Nile Virus in Mosquitoes in Brazil. Trop. Med. Infect. Dis. 2023, 8, 237. [Google Scholar] [CrossRef]
- Lowe, R.; Lee, S.; Martins Lana, R.; Torres Codeço, C.; Castro, M.C.; Pascual, M. Emerging Arboviruses in the Urbanized Amazon Rainforest. BMJ 2020, 371, m4385. [Google Scholar] [CrossRef]
- Barredo, E.; DeGennaro, M. Not Just from Blood: Mosquito Nutrient Acquisition from Nectar Sources. Trends Parasitol. 2020, 36, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Nouzova, M.; Clifton, M.E.; Noriega, F.G. Mosquito Adaptations to Hematophagia Impact Pathogen Transmission. Curr. Opin. Insect Sci. 2019, 34, 21–26. [Google Scholar] [CrossRef]
- Bonning, B.C. The Insect Virome: Opportunities and Challenges. Curr. Issues Mol. Biol. 2020, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yin, Q.; Zhou, L.; Meng, L.; Hu, W.; Li, F.; Li, Y.; Han, K.; Zhang, S.; Fu, S.; et al. Metagenomic Sequencing Reveals Viral Abundance and Diversity in Mosquitoes from the Shaanxi-Gansu-Ningxia Region, China. PLoS Negl. Trop. Dis. 2021, 15, e0009381. [Google Scholar] [CrossRef]
- Beuchle, R.; Grecchi, R.C.; Shimabukuro, Y.E.; Seliger, R.; Eva, H.D.; Sano, E.; Achard, F. Land Cover Changes in the Brazilian Cerrado and Caatinga Biomes from 1990 to 2010 Based on a Systematic Remote Sensing Sampling Approach. Appl. Geogr. 2015, 58, 116–127. [Google Scholar] [CrossRef]
- Lane, J. Neotropical Culicidae; Edusp: São Paulo, SP, Brasil, 1953; Volume 2. [Google Scholar]
- Lane, J. Neotropical Culicidae; Edusp: São Paulo, SP, Brasil, 1953; Volume 1. [Google Scholar]
- Forattini, O.P. Entomologia Médica. Culicini: Culex, Aedes e Psorophora; Editora da Universidade de São Paulo: São Paulo, SP, Brasil, 1965; Volume 2. [Google Scholar]
- Forattini, O.P. Entomologia Médica. Parte Geral, Diptera, Anophelini; Faculdade de Higiene e Saúde Pública: São Paulo, SP, Brasil, 1962; Volume 1. [Google Scholar]
- Forattini, O.P. Entomologia Médica. Culicini: Haemagogus, Mansonia, Culiseta, Sabethini, Toxorhynchitini, Arboviroses, Filariose Bancroftiana, Genética; Editora da Universidade de São Paulo: São Paulo, SP, Brasil, 1965; Volume 3. [Google Scholar]
- Forattini, O.P. Culicidologia Médica; Universidade de São Paulo: São Paulo, SP, Brasil, 2002; Volume 2, ISBN 85-314-0699-4. [Google Scholar]
- Consoli, R.A.G.B.; Lourenço-de-Oliveira, R. Principais Mosquitos de Importância Sanitária No Brasil; Editora Fiocruz: Rio de Janeiro, RJ, Brasil, 1994; ISBN 85-85676-03-5. [Google Scholar]
- Tesh, R.B. A Method for the Isolation and Identification of Dengue Viruses, Using Mosquito Cell Cultures. Am. J. Trop. Med. Hyg. 1979, 28, 1053–1059. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Huson, D.H.; Auch, A.F.; Qi, J.; Schuster, S.C. MEGAN Analysis of Metagenomic Data. Genome Res. 2007, 17, 377–386. [Google Scholar] [CrossRef]
- Myung, I.J. Tutorial on Maximum Likelihood Estimation. J. Math. Psychol. 2003, 47, 90–100. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Valles, S.M.; Chen, Y.; Firth, A.E.; Guérin, D.M.A.; Hashimoto, Y.; Herrero, S.; De Miranda, J.R.; Ryabov, E. ICTV Virus Taxonomy Profile: Iflaviridae. J. Gen. Virol. 2017, 98, 527–528. [Google Scholar] [CrossRef]
- Sõmera, M.; Fargette, D.; Hébrard, E.; Sarmiento, C. ICTV Virus Taxonomy Profile: Solemoviridae 2021. J. Gen. Virol. 2021, 102, 001707. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Valles, S.; Firth, A.; Miranda, J.; Ryabov, E.; Echeverría, M.; Schroeder, D.; Jan, E.; Zheng, H.; Parry, R. Family: Iflaviridae. Available online: https://ictv.global/report/chapter/iflaviridae/iflaviridae (accessed on 19 December 2022).
- Schultz, M.J.; Frydman, H.M.; Connor, J.H. Dual Insect Specific Virus Infection Limits Arbovirus Replication in Aedes Mosquito Cells. Virology 2018, 518, 406–413. [Google Scholar] [CrossRef] [PubMed]
- White, A.V.; Fan, M.; Mazzara, J.M.; Roper, R.L.; Richards, S.L. Mosquito-Infecting Virus Espirito Santo Virus Inhibits Replication and Spread of Dengue Virus. J. Med. Virol. 2021, 93, 3362–3373. [Google Scholar] [CrossRef]
- Urakova, N.; Brustolin, M.; Joseph, R.E.; Johnson, R.M.; Pujhari, S.; Rasgon, J.L. Anopheles Gambiae Densovirus (AgDNV) Negatively Affects Mayaro Virus Infection in Anopheles Gambiae Cells and Mosquitoes. Parasit. Vectors 2020, 13, 210. [Google Scholar] [CrossRef]
- Ponnuvel, K.M.; de Miranda, J.R.; Terenius, O.; Li, W.; Ito, K.; Khajje, D.; Shamitha, G.; Jagadish, A.; Dubey, H.; Mishra, R.K. Genetic Characterisation of an Iflavirus Associated with a Vomiting Disease in the Indian Tropical Tasar Silkworm, Antheraea Mylitta. Virus Res. 2022, 311, 198703. [Google Scholar] [CrossRef]
- Carballo, A.; Williams, T.; Murillo, R.; Caballero, P. Iflavirus Covert Infection Increases Susceptibility to Nucleopolyhedrovirus Disease in Spodoptera Exigua. Viruses 2020, 12, 509. [Google Scholar] [CrossRef]
- Linscheid, Y.; Kessel, T.; Vilcinskas, A.; Lee, K.Z. Pathogenicity of La Jolla Virus in Drosophila Suzukii Following Oral Administration. Viruses 2022, 14, 2158. [Google Scholar] [CrossRef]
- Faizah, A.N.; Kobayashi, D.; Isawa, H.; Amoa-Bosompem, M.; Murota, K.; Higa, Y.; Futami, K.; Shimada, S.; Kim, K.S.; Itokawa, K.; et al. Deciphering the Virome of Culex Vishnui Subgroup Mosquitoes, the Major Vectors of Japanese Encephalitis, in Japan. Viruses 2020, 12, 264. [Google Scholar] [CrossRef]
- Cholleti, H.; Hayer, J.; Fafetine, J.; Berg, M.; Blomström, A.L. Genetic Characterization of a Novel Picorna-like Virus in Culex spp. Mosquitoes from Mozambique. Virol. J. 2018, 15, 71. [Google Scholar] [CrossRef]
- Atoni, E.; Wang, Y.; Karungu, S.; Waruhiu, C.; Zohaib, A.; Obanda, V.; Agwanda, B.; Mutua, M.; Xia, H.; Yuan, Z. Metagenomic Virome Analysis of Culex Mosquitoes from Kenya and China. Viruses 2018, 10, 30. [Google Scholar] [CrossRef]
- Batson, J.; Dudas, G.; Haas-Stapleton, E.; Kistler, A.L.; Li, L.M.; Logan, P.; Ratnasiri, K.; Retallack, H. Single Mosquito Metatranscriptomics Identifies Vectors, Emerging Pathogens and Reservoirs in One Assay. eLife 2021, 10, e68353. [Google Scholar] [CrossRef]
- de Oliveira Ribeiro, G.; Morais, V.S.; Monteiro, F.J.C.; Ribeiro, E.S.D.A.; da Rego, M.O.S.; Souto, R.N.P.; Villanova, F.; Tahmasebi, R.; Hefford, P.M.; Deng, X.; et al. Aedes Aegypti from Amazon Basin Harbor High Diversity of Novel Viral Species. Viruses 2020, 12, 866. [Google Scholar] [CrossRef]
- Parry, R.; Naccache, F.; Ndiaye, E.H.; Fall, G.; Castelli, I.; Lühken, R.; Medlock, J.; Cull, B.; Hesson, J.C.; Montarsi, F.; et al. Identification and RNAi Profile of a Novel Iflavirus Infecting Senegalese Aedes Vexans Arabiensis Mosquitoes. Viruses 2020, 12, 440. [Google Scholar] [CrossRef]
- Stefanov, Y.; Salenko, V.; Glukhov, I. Drosophila Errantiviruses. Mob. Genet. Elem. 2012, 2, 36–45. [Google Scholar] [CrossRef]
- Llorens, C.; Soriano, B.; Krupovic, M. ICTV Virus Taxonomy Profile: Metaviridae. J. Gen. Virol. 2020, 101, 1131–1132. [Google Scholar] [CrossRef]
- Duarte, M.A.; Campos, F.S.; Araújo Neto, O.F.; Silva, L.A.; Silva, A.B.; Aguiar, T.C.; Santos, R.N.; Souza, U.J.B.; Alves, G.B.; Melo, F.L.; et al. Identification of Potential New Mosquito-Associated Viruses of Adult Aedes Aegypti Mosquitoes from Tocantins State, Brazil. Braz. J. Microbiol. 2022, 53, 51. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef]
- ICTV Subfamily: Densovirinae. Available online: https://ictv.global/report/chapter/parvoviridae/parvoviridae/densovirinae (accessed on 7 May 2023).
- Caicedo, E.Y.; Charniga, K.; Rueda, A.; Dorigatti, I.; Mendez, Y.; Hamlet, A.; Carrera, J.P.; Cucunubá, Z.M. The Epidemiology of Mayaro Virus in the Americas: A Systematic Review and Key Parameter Estimates for Outbreak Modelling. PLoS Negl. Trop. Dis. 2021, 15, e0009418. [Google Scholar] [CrossRef] [PubMed]
- González-González, A.; de Stefano, N.T.; Rosenbaum, D.A.; Wayne, M.L. Rhabdoviruses of Insects (Rhabdoviridae). Encycl. Virol. 2021, 4, 883–887. [Google Scholar] [CrossRef]
- Li, C.X.; Shi, M.; Tian, J.H.; Lin, X.D.; Kang, Y.J.; Chen, L.J.; Qin, X.C.; Xu, J.; Holmes, E.C.; Zhang, Y.Z. Unprecedented Genomic Diversity of RNA Viruses in Arthropods Reveals the Ancestry of Negative-Sense RNA Viruses. eLife 2015, 4, e05378. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.-D.; Vasilakis, N.; Tian, J.-H.; Li, C.-X.; Chen, L.-J.; Eastwood, G.; Diao, X.-N.; Chen, M.-H.; Chen, X.; et al. Divergent Viruses Discovered in Arthropods and Vertebrates Revise the Evolutionary History of the Flaviviridae and Related Viruses. J. Virol. 2016, 90, 659. [Google Scholar] [CrossRef]
- Maes, P.; Amarasinghe, G.K.; Ayllón, M.A.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the Order Mononegavirales: Second Update 2018. Arch. Virol. 2019, 164, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.; Asgari, S. Aedes Anphevirus: An Insect-Specific Virus Distributed Worldwide in Aedes Aegypti Mosquitoes That Has Complex Interplays with Wolbachia and Dengue Virus Infection in Cells. J. Virol. 2018, 92, 10-1128. [Google Scholar] [CrossRef]
- Silva Ferreira, R.; Toni Aquino da Cruz, L.C.; Souza, V.J.; Silva Neves, N.A.; Souza, V.C.; Franco Filho, L.C.; Silva Lemos, P.; Lima, C.P.S.; Naveca, F.G.; Atanaka, M.; et al. Insect-Specific Viruses and Arboviruses in Adult Male Culicids from Midwestern Brazil. Infect. Genet. Evol. 2020, 85, 104561. [Google Scholar] [CrossRef]
- Chen, Q.; Wei, T. Cell Biology during Infection of Plant Viruses in Insect Vectors and Plant Hosts. Mol. Plant-Microbe Interact. 2020, 33, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Vainio, E.J.; Chiba, S.; Ghabrial, S.A.; Maiss, E.; Roossinck, M.; Sabanadzovic, S.; Suzuki, N.; Xie, J.; Nibert, M. ICTV Virus Taxonomy Profile: Partitiviridae. J. Gen. Virol. 2018, 99, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Adams, M.J.; Benk, M.; Breitbart, M.; Brister, J.R.; Carstens, E.B.; Davison, A.J.; Delwart, E.; Gorbalenya, A.E.; Harrach, B.; et al. Virus Taxonomy in the Age of Metagenomics. Nat. Rev. Microbiol. 2017, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]



| Sample Name | Species | Number of Individuals | Locations | Collection Date | Raw Reads | Reads after Fastp Treatment | Reads after SortMeRNA Treatment |
|---|---|---|---|---|---|---|---|
| AR872456 | Culex (Culex) spp. 1 | 13 | CX 2 | 19 October 2021 | 35,747,762 | 33,641,832 | 17,581,122 |
| AR872459 | Aedes serratus | 4 | CX 2 | 20 October 2021 | 23,234,440 | 21,562,940 | 14,833,379 |
| AR872460 | Aedes albopictus | 1 | CX 2 | 21 October 2021 | 38,297,936 | 34,832,610 | 14,935,751 |
| AR872461 | Aedes serratus | 20 | CX 2 | 20 October 2021 | 24,493,722 | 23,393,402 | 2,480,108 |
| AR872465 | Sabethes chloropterus | 1 | CX 2 | 21 October 2021 | 22,534,910 | 21,033,654 | 8,606,130 |
| AR872468-76 | Aedes albopictus | 6 | TM 3 | 25 October 2021 | 25,453,046 | 23,883,884 | 11,137,227 |
| AR872471-85 | Sabethes quasicyaneus | 18 | TM 3 | 25 October 2021 | 27,797,262 | 26,406,522 | 26,216,665 |
| AR872474 | Aedes fluviatilis | 2 | TM 3 | 25 October 2021 | 16,882,686 | 15,932,038 | 4,184,885 |
| AR872475 | Sabethes chloropterus | 2 | SJS 4 | 25 October 2021 | 40,165,978 | 37,486,668 | 34,960,981 |
| AR872486 | Sabethes chloropterus | 2 | TM 3 | 25 October 2021 | 22,567,056 | 20,857,106 | 4,593,808 |
| AR872487 | Sabethes glaucodaemon | 2 | TM 3 | 25 October 2021 | 38,840,018 | 36,253,540 | 14,174,187 |
| AR872498 | Culex usquatus | 1 | TM 3 | 25 October 2021 | 65,179,918 | 61,690,026 | 29,604,510 |
| AR872499 | Culex (Melanoconion) spp. 1 | 4 | TM 3 | 25 October 2021 | 52,819,748 | 49,845,674 | 31,194,633 |
| AR872505-09 | Aedes scapularis | 3 | SJS 4 | 26 October 2021 | 31,780,386 | 29,756,330 | 14,765,661 |
| AR872508 | Sabethes quasicyaneus | 1 | SJS 4 | 26 October 2021 | 7,430,430 | 7,020,402 | 1,460,524 |
| AR872510 | Aedes serratus | 9 | SJS 4 | 26 October 2021 | 31,152,824 | 29,451,912 | 6,142,182 |
| AR872511 | Aedes albopictus | 2 | SJS 4 | 26 October 2021 | 39,201,792 | 36,788,368 | 9,662,953 |
| AR872515 | Haemagogus janthinomys | 1 | SJS 4 | 27 October 2021 | 6,970,298 | 6,527,944 | 3,606,142 |
| AR872521 | Aedes scapularis | 1 | TM 3 | 26 October 2021 | 27,952,604 | 26,417,466 | 6,581,716 |
| AR872524 | Aedes scapularis | 1 | SJS 4 | 27 October 2021 | 29,945,114 | 27,968,266 | 17,607,240 |
| Total | - | 94 | - | - | 608,447,930 | 570,750,584 | 274,329,804 |
| Molecule Type | Virus Name (GenBank Accession) | Sample/Host | Length (nt) | Mean Coverage | Classification | Closest Virus (GenBank Accession) | BlastX | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Amino Acid Identity (%) | QC 1 (%) | E-Value | |||||||
| +ssRNA | Inhamum iflavirus (OP918263) | AR872456/Cx. (Cux.) spp. | 9020 | 16x | Iflaviridae | Culex Iflavi-like virus 3 (MW434116) | Pol 2 | 58.00 | 99 | 0.0 |
| Inhamum errantivirus (OQ779233) | AR872465/Sa. chloropterus | 5823 | 34.1x | Metaviridae | Chibugado virus (MN661043) | Pol 2 | 64.31 | 99 | 0.0 | |
| Buriti errantivirus (OQ779240) | AR872498/Cx. Usquatus | 5014 | 16.5x | Metaviridae | Chibugado virus (MN661043) | Pol 2 | 63.42 | 98 | 0.0 | |
| Atrato Sobemo-like virus 1 (OQ779232) | AR872461/Ae. serratus | 2726 | 547.7x | Solemoviridae | Atrato Sobemo-like virus 1 (MN661087) | RdRp 3 | 97.37 | 99 | 0.0 | |
| Atrato Sobemo-like virus 1 (OQ779242) | AR872510/Ae. serratus | 1451 | 1861.8x | Solemoviridae | Atrato Sobemo-like virus 1 (MN661087) | RdRp 3 | 98.76 | 99 | 0.0 | |
| Buriti virga-like virus (OQ779238) | AR872486/Sb. Chloropterus | 8757 | 1878.4x | Virgaviridae | Atrato Virga-like virus 2 (MN661104/MN661105) | Pol 2 | 60.86 | 99 | 0.0 | |
| −ssRNA | Pedras lispivirus (OQ779241) | AR872508/Sa. quasicyaneus | 13,424 | 237.8x | Lispiviridae | Canya virus (MW434766) | RdRp 3 | 36.24 | 99 | 0.0 |
| Inhamum rhabdovirus (OQ779231) | AR872456/Cx. (Cux.) spp. | 6397 | 11.5x | Rhabdoviridae | Stang virus (MW434775) | RdRp 3 | 77.36 | 99 | 0.0 | |
| Buriti anphevirus (OQ779239) | AR872487/Sa. glaucodaemon | 1139 | 1441.1x | Xinmoviridae | Enontekio anphevirus 2/ON955256 | RdRp 3 | 53.44 | 99 | 2E-144 | |
| Pedras xinmovirus (OQ779244) | AR872521/Ae. scapularis | 787 | 3.3x | Xinmoviridae | Guadeloupe mosquito mononega-like virus/MN053735 | N 4 | 87.20 | 100 | 2E-132 | |
| dsRNA | Pedras partitivirus (OQ779243) | AR872511/Ae. albopictus | 1364 | 5.5x | Partitiviridae | Verdadero virus (MT742175) | Capsid | 80.81 | 98 | 0.0 |
| Buriti partiti-like virus 1 (OQ779235) | AR872471-85/Sa. quasicyaneus | 1285 | 358.6x | Partitiviridae | Zeya Brook partiti-like virus 1 (MW389559) | Capsid | 66.16 | 99 | 2E-87 | |
| Buriti partiti-like virus 2 (OQ779236) | AR872471-85/Sa. quasicyaneus | 1264 | 4.6x | Partitiviridae | Atrato Partiti-like virus 2 (MN661058) | Capsid | 58.57 | 93 | 3E-69 | |
| Buriti deltapartitivirus (OQ779234) | AR872471-85/Sa. quasicyaneus | 720 | 3.6x | Partitiviridae | Inari deltapartitivirus (OP019955) | RdRp 3 | 84.69 | 99 | 9E-127 | |
| ssDNA | Buriti densovirus (OQ779237) | AR872471-85/Sa. quasicyaneus | 5182 | 1684.9x | Parvoviridae | Atrato Denso-like virus (MN661135) | NS1 5 | 42.42 | 91 | 7E-144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragão, C.F.; da Silva, S.P.; do Nascimento, B.L.S.; da Silva, F.S.; Nunes Neto, J.P.; Pinheiro, V.C.S.; Cruz, A.C.R. Shotgun Metagenomic Sequencing Reveals Virome Composition of Mosquitoes from a Transition Ecosystem of North-Northeast Brazil. Genes 2023, 14, 1443. https://doi.org/10.3390/genes14071443
Aragão CF, da Silva SP, do Nascimento BLS, da Silva FS, Nunes Neto JP, Pinheiro VCS, Cruz ACR. Shotgun Metagenomic Sequencing Reveals Virome Composition of Mosquitoes from a Transition Ecosystem of North-Northeast Brazil. Genes. 2023; 14(7):1443. https://doi.org/10.3390/genes14071443
Chicago/Turabian StyleAragão, Carine Fortes, Sandro Patroca da Silva, Bruna Laís Sena do Nascimento, Fábio Silva da Silva, Joaquim Pinto Nunes Neto, Valéria Cristina Soares Pinheiro, and Ana Cecília Ribeiro Cruz. 2023. "Shotgun Metagenomic Sequencing Reveals Virome Composition of Mosquitoes from a Transition Ecosystem of North-Northeast Brazil" Genes 14, no. 7: 1443. https://doi.org/10.3390/genes14071443
APA StyleAragão, C. F., da Silva, S. P., do Nascimento, B. L. S., da Silva, F. S., Nunes Neto, J. P., Pinheiro, V. C. S., & Cruz, A. C. R. (2023). Shotgun Metagenomic Sequencing Reveals Virome Composition of Mosquitoes from a Transition Ecosystem of North-Northeast Brazil. Genes, 14(7), 1443. https://doi.org/10.3390/genes14071443







