Maturity-Associated Polygenic Profiles of under 12–16-Compared to under 17–23-Year-Old Male English Academy Football Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Genetic Procedures
2.2.1. Genotyping
2.2.2. Polymorphism Selection
2.2.3. Polygenic Profiles
2.3. Data Analysis
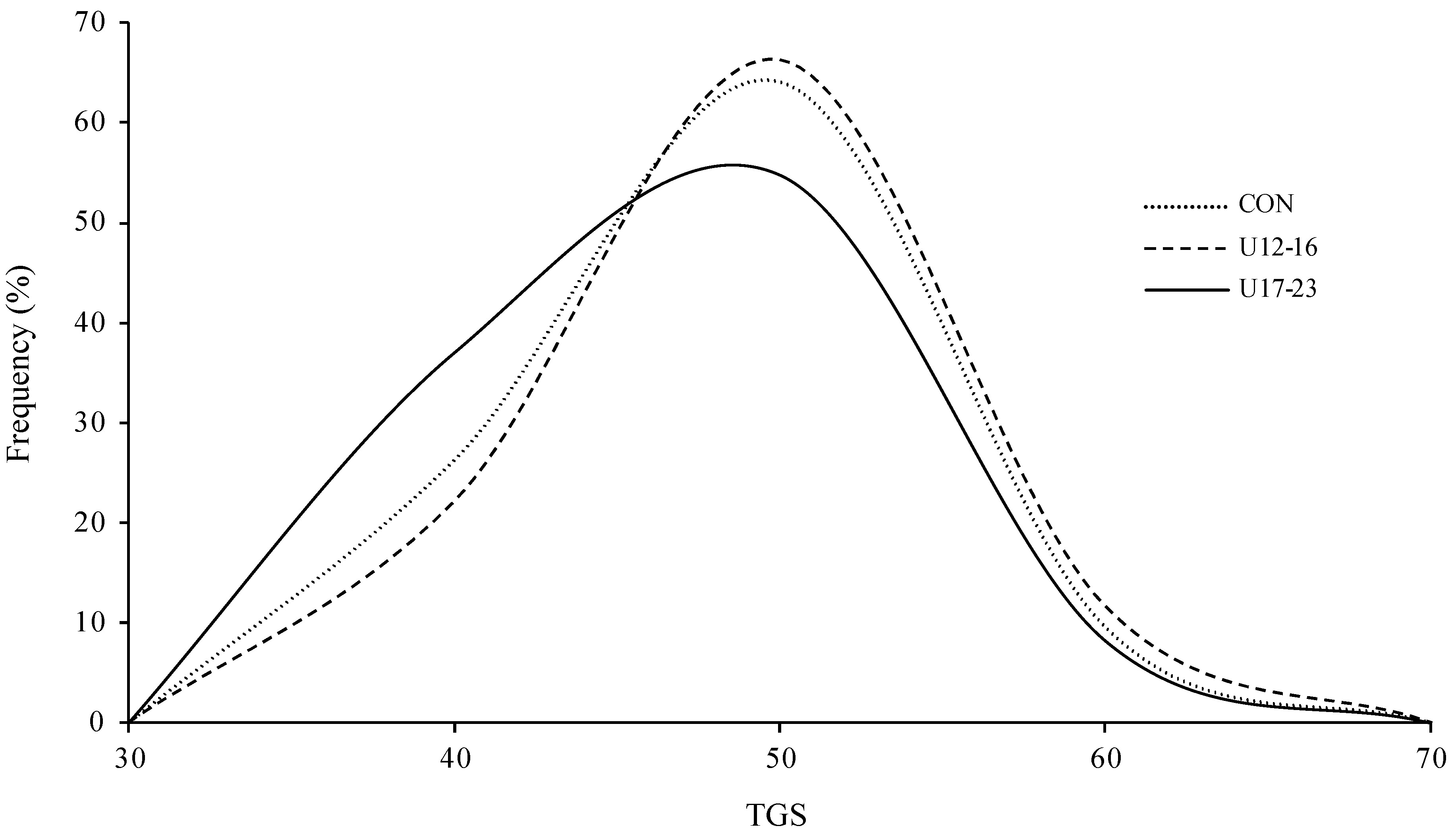
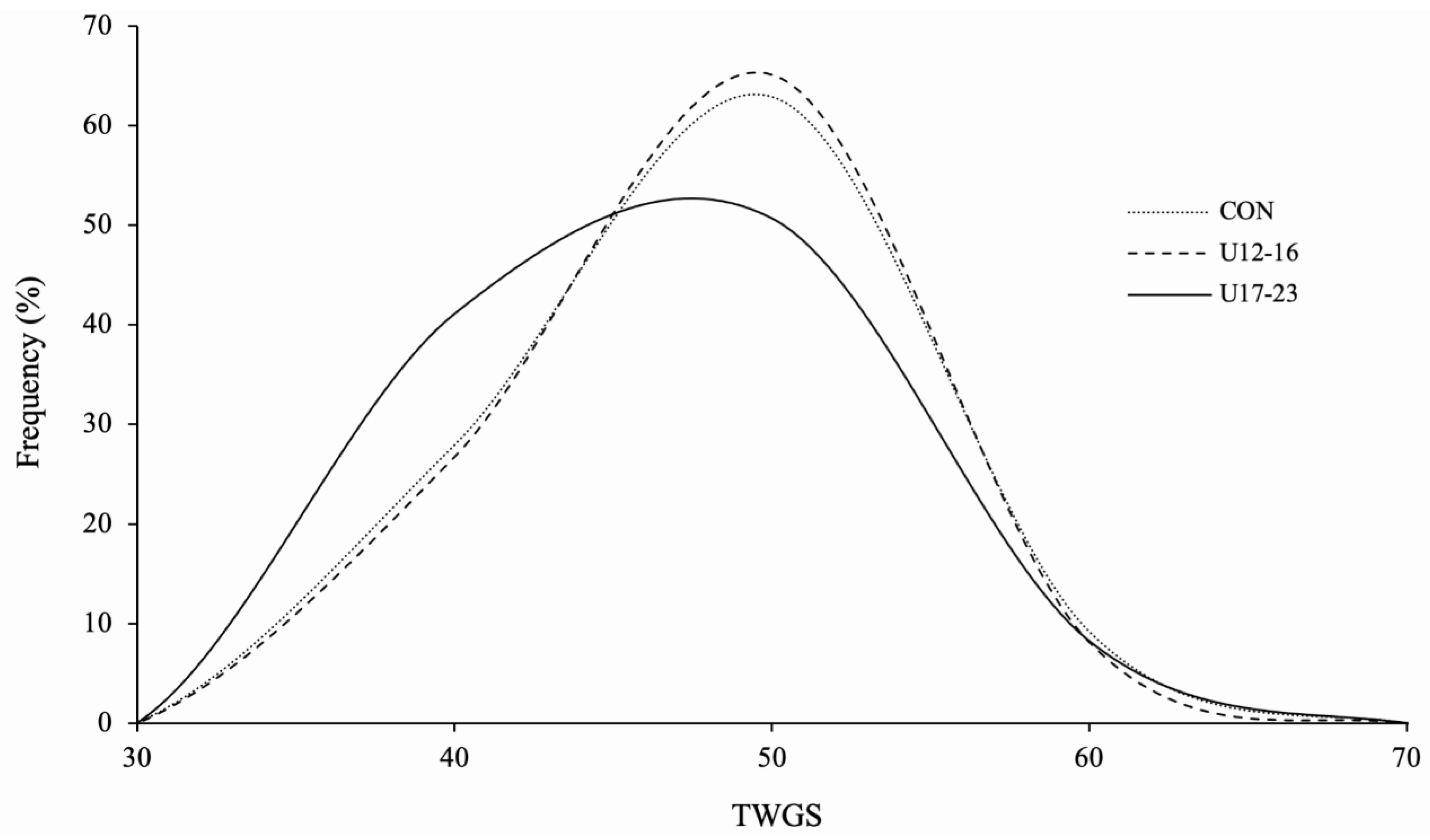
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, A.L.; Williams, C.A. Physical Characteristics and the Talent Identification and Development Processes in Male Youth Soccer: A Narrative Review. Strength Cond. J. 2020, 42, 15–34. [Google Scholar] [CrossRef]
- Sarmento, H.; Anguera, M.T.; Pereira, A.; Araújo, D. Talent Identification and Development in Male Football: A Systematic Review. Sports Med. 2018, 48, 907–931. [Google Scholar] [CrossRef] [PubMed]
- Malina, R.M.; Eisenmann, J.C.; Cumming, S.P.; Ribeiro, B.; Aroso, J. Maturity-associated variation in the growth and functional capacities of youth football (soccer) players 13–15 years. Eur. J. Appl. Physiol. 2004, 91, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Borms, J. The child and exercise: An overview. J. Sports Sci. 1986, 4, 3–20. [Google Scholar] [CrossRef]
- Malina, R.M.; Rogol, A.D.; Cumming, S.P.; Coelho e Silva, M.J.; Figueiredo, A.J. Biological maturation of youth athletes: Assessment and implications. Br. J. Sports Med. 2015, 49, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Philippaerts, R.M.; Vaeyens, R.; Janssens, M.; Van Renterghem, B.; Matthys, D.; Craen, R.; Bourgois, J.; Vrijens, J.; Beunen, G.; Malina, R.M. The relationship between peak height velocity and physical performance in youth soccer players. J. Sports Sci. 2006, 24, 221–230. [Google Scholar] [CrossRef]
- Towlson, C.; Cobley, S.; Parkin, G.; Lovell, R. When does the influence of maturation on anthropometric and physical fitness characteristics increase and subside? Scand. J. Med. Sci. Sports 2018, 28, 1946–1955. [Google Scholar] [CrossRef]
- Figueiredo, A.J.; Gonçalves, C.E.; e Silva, M.J.C.; Malina, R.M. Characteristics of youth soccer players who drop out, persist or move up. J. Sports Sci. 2009, 27, 883–891. [Google Scholar] [CrossRef]
- Meylan, C.; Cronin, J.; Oliver, J.; Hughes, M. Talent Identification in Soccer: The Role of Maturity Status on Physical, Physiological and Technical Characteristics. Int. J. Sports Sci. Coach. 2010, 5, 571–592. [Google Scholar] [CrossRef]
- Radnor, J.M.; Staines, J.; Bevan, J.; Cumming, S.P.; Kelly, A.L.; Lloyd, R.S.; Oliver, J.L. Maturity Has a Greater Association than Relative Age with Physical Performance in English Male Academy Soccer Players. Sports 2021, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Cumming, S.P.; Searle, C.; Hemsley, J.K.; Haswell, F.; Edwards, H.; Scott, S.; Gross, A.; Ryan, D.; Lewis, J.; White, P.; et al. Biological maturation, relative age and self-regulation in male professional academy soccer players: A test of the underdog hypothesis. Psychol. Sport Exerc. 2018, 39, 147–153. [Google Scholar] [CrossRef]
- Hill, M.; Scott, S.; Malina, R.M.; McGee, D.; Cumming, S.P. Relative age and maturation selection biases in academy football. J. Sports Sci. 2020, 38, 1359–1367. [Google Scholar] [CrossRef]
- Johnson, A.; Farooq, A.; Whiteley, R. Skeletal maturation status is more strongly associated with academy selection than birth quarter. Sci. Med. Footb. 2017, 1, 157–163. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Castagna, C.; Gonzalez, J.M.C.; Jukic, I.; Idrizovic, K.; Stojanovic, M. The Biological Age of 14-year-old Boys and Success in Adult Soccer: Do Early Maturers Predominate in the Top-level Game? Res. Sports Med. 2014, 22, 398–407. [Google Scholar] [CrossRef]
- Gibbs, B.G.; Jarvis, J.A.; Dufur, M.J. The rise of the underdog? The relative age effect reversal among Canadian-born NHL hockey players: A reply to Nolan and Howell. Int. Rev. Sociol. Sport 2012, 47, 644–649. [Google Scholar] [CrossRef]
- Beunen, G.P.; Rogol, A.D.; Malina, R.M. Indicators of Biological Maturation and Secular Changes in Biological Maturation. Food Nutr. Bull. 2006, 27, S244–S256. [Google Scholar] [CrossRef] [PubMed]
- McAuley, A.B.T.; Baker, J.; Kelly, A.L. How Nature and Nurture Conspire to Influence Athletic Success. In Birth Advantages and Relative Age Effects in Sport: Exploring Organizational Structures and Creating Appropriate Settings; Kelly, A.L., Côté, J., Jeffreys, M., Turnnidge, J., Eds.; Routledge: London, UK, 2021; pp. 159–183. [Google Scholar]
- Anderson, C.A.; Duffy, D.L.; Martin, N.G.; Visscher, P.M. Estimation of Variance Components for Age at Menarche in Twin Families. Behav. Genet. 2007, 37, 668–677. [Google Scholar] [CrossRef]
- Silventoinen, K.; Haukka, J.; Dunkel, L.; Tynelius, P.; Rasmussen, F. Genetics of Pubertal Timing and Its Associations with Relative Weight in Childhood and Adult Height: The Swedish Young Male Twins Study. Pediatrics 2008, 121, e885–e891. [Google Scholar] [CrossRef]
- Day, F.R.; Bulik-Sullivan, B.; Hinds, D.A.; Finucane, H.K.; Murabito, J.M.; Tung, J.Y.; Ong, K.K.; Perry, J.R. Shared genetic aetiology of puberty timing between sexes and with health-related outcomes. Nat. Commun. 2015, 6, 8842. [Google Scholar] [CrossRef] [PubMed]
- Day, F.R.; The LifeLines Cohort Study; Thompson, D.J.; Helgason, H.; I Chasman, D.; Finucane, H.; Sulem, P.; Ruth, K.S.; Whalen, S.; Sarkar, A.K.; et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 2017, 49, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Sarnowski, C.; Cousminer, D.L.; Franceschini, N.; Raffield, L.M.; Jia, G.; Fernández-Rhodes, L.; A Grant, S.F.; Hakonarson, H.; A Lange, L.; Long, J.; et al. Large trans-ethnic meta-analysis identifies AKR1C4 as a novel gene associated with age at menarche. Hum. Reprod. 2021, 36, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Magnotto, J.C.; Abreu, A.P. Genetics of pubertal timing. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101618. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.; Day, F.R.; Busch, A.S.; Thompson, D.J.; Soares, A.L.G.; Timmers, P.R.H.J.; Kwong, A.; Easton, D.F.; Joshi, P.K.; Timpson, N.J.; et al. Genomic analysis of male puberty timing highlights shared genetic basis with hair colour and lifespan. Nat. Commun. 2020, 11, 1536. [Google Scholar] [CrossRef] [PubMed]
- McAuley, A.B.T.; Hughes, D.C.; Tsaprouni, L.G.; Varley, I.; Suraci, B.; Roos, T.R.; Herbert, A.J.; Kelly, A.L. Genetic association research in football: A systematic review. Eur. J. Sport Sci. 2021, 21, 714–752. [Google Scholar] [CrossRef]
- McAuley, A.B.T.; Hughes, D.C.; Tsaprouni, L.G.; Varley, I.; Suraci, B.; Roos, T.R.; Herbert, A.J.; Kelly, A.L. The association of the ACTN3 R577X and ACE I/D polymorphisms with athlete status in football: A systematic review and meta-analysis. J. Sports Sci. 2021, 39, 200–211. [Google Scholar] [CrossRef]
- McAuley, A.B.T.; Hughes, D.C.; Tsaprouni, L.G.; Varley, I.; Suraci, B.; Roos, T.R.; Herbert, A.J.; Kelly, A.L. Genetic Testing in Professional Football: Perspectives of Key Stakeholders. J. Sci. Sport Exerc. 2022, 4, 49–59. [Google Scholar] [CrossRef]
- McAuley, A.B.; Hughes, D.C.; Tsaprouni, L.G.; Varley, I.; Suraci, B.; Baker, J.; Herbert, A.J.; Kelly, A.L. Genetic associations with personality and mental toughness profiles of English academy football players: An exploratory study. Psychol. Sport Exerc. 2022, 61, 102209. [Google Scholar] [CrossRef]
- Mcauley, A.B.; Hughes, D.C.; Tsaprouni, L.G.; Varley, I.; Suraci, B.; Baker, J.; Herbert, A.J.; Kelly, A.L. Genetic associations with technical capabilities in English academy football players: A preliminary study. J. Sports Med. Phys. Fit. 2023, 63, 230–240. [Google Scholar] [CrossRef]
- McAuley, A.B.T.; Hughes, D.C.; Tsaprouni, L.G.; Varley, I.; Suraci, B.; Roos, T.R.; Herbert, A.J.; Jackson, D.T.; Kelly, A.L. A Systematic Review of the Genetic Predisposition to Injury in Football. J. Sci. Sport Exerc. 2022, 5, 97–115. [Google Scholar] [CrossRef]
- Hall, E.C.R.; Baumert, P.; Larruskain, J.; Gil, S.M.; Lekue, J.A.; Rienzi, E.; Moreno, S.; Tannure, M.; Murtagh, C.F.; Ade, J.D.; et al. The genetic association with injury risk in male academy soccer players depends on maturity status. Scand. J. Med. Sci. Sports 2022, 32, 338–350. [Google Scholar] [CrossRef] [PubMed]
- McAuley, A.B.; Hughes, D.C.; Tsaprouni, L.G.; Varley, I.; Suraci, B.; Baker, J.; Herbert, A.J.; Kelly, A.L. Genetic Variations between Youth and Professional Development Phase English Academy Football Players. Genes 2022, 13, 2001. [Google Scholar] [CrossRef]
- Murtagh, C.F.; Brownlee, T.E.; Rienzi, E.; Roquero, S.; Moreno, S.; Huertas, G.; Lugioratto, G.; Baumert, P.; Turner, D.C.; Lee, D.; et al. The genetic profile of elite youth soccer players and its association with power and speed depends on maturity status. PLoS ONE 2020, 15, e0234458. [Google Scholar] [CrossRef]
- Williams, A.G.; Folland, J.P. Similarity of polygenic profiles limits the potential for elite human physical performance. J. Physiol. 2008, 586, 113–121. [Google Scholar] [CrossRef]
- McCabe, K.; Collins, C. Can Genetics Predict Sports Injury? The Association of the Genes GDF5, AMPD1, COL5A1 and IGF2 on Soccer Player Injury Occurrence. Sports 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Varley, I.; Hughes, D.C.; Greeves, J.P.; Stellingwerff, T.; Ranson, C.; Fraser, W.D.; Sale, C. RANK/RANKL/OPG pathway: Genetic associations with stress fracture period prevalence in elite athletes. Bone 2015, 71, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Varley, I.; Hughes, D.C.; Greeves, J.P.; Stellingwerff, T.; Ranson, C.; Fraser, W.D.; Sale, C. The association of novel polymorphisms with stress fracture injury in Elite Athletes: Further insights from the SFEA cohort. J. Sci. Med. Sport 2018, 21, 564–568. [Google Scholar] [CrossRef]
- Kelly, A.L.; Wilson, M.R.; Gough, L.A.; Knapman, H.; Morgan, P.; Cole, M.; Jackson, D.T.; Williams, C.A. A longitudinal investigation into the relative age effect in an English professional football club: Exploring the ‘underdog hypothesis’. Sci. Med. Footb. 2020, 4, 111–118. [Google Scholar] [CrossRef]
- Johnston, K.; Baker, J. Waste Reduction Strategies: Factors Affecting Talent Wastage and the Efficacy of Talent Selection in Sport. Front. Psychol. 2019, 10, 2925. [Google Scholar] [CrossRef]
- Baker, J.; Johnston, K.; Wojtowicz, M.; Wattie, N. What do we really know about elite athlete development? Limitations and gaps in current understanding. Br. J. Sports Med. 2022, 56, 1331–1332. [Google Scholar] [CrossRef]
- Abdellaoui, A.; Yengo, L.; Verweij, K.J.; Visscher, P.M. 15 years of GWAS discovery: Realizing the promise. Am. J. Hum. Genet. 2023, 110, 179–194. [Google Scholar] [CrossRef]
- Yengo, L.; Vedantam, S.; Marouli, E.; Sidorenko, J.; Bartell, E.; Sakaue, S.; Graff, M.; Eliasen, A.U.; Jiang, Y.; Raghavan, S.; et al. A saturated map of common genetic variants associated with human height. Nature 2022, 610, 704–712. [Google Scholar] [CrossRef] [PubMed]
- McAuley, A.B.T.; Baker, J.; Kelly, A.L. Defining “elite” status in sport: From chaos to clarity. Ger. J. Exerc. Sport Res. 2022, 52, 193–197. [Google Scholar] [CrossRef]
- Johnston, K.; McAuley, A.B.T.; Kelly, A.L.; Baker, J. Language games and blurry terminology: Can clarity enhance athlete development? Front. Sports Act. Living 2023, 5, 1150047. [Google Scholar] [CrossRef] [PubMed]
| Gene | SNP | β | Minor Allele Frequency % | ||
|---|---|---|---|---|---|
| Controls | U12–16 | U17–23 | |||
| ZNF483 | rs10980922 | 0.055 | 7 | 9 | 7 |
| HERC2 | rs7402990 | 0.051 | 10 | 8 | 11 |
| C16orf55 | rs35063026 | 0.051 | 7 | 9 | 10 |
| MIR193B | rs1659127 | 0.050 | 34 | 30 | 25 |
| LIN28B | rs11156429 | 0.049 | 45 | 26 | 26 |
| TMEM38B | rs9408817 | 0.041 | 32 | 33 | 33 |
| SOX2OT | rs73182377 | 0.040 | 28 | 26 | 32 |
| C11orf63 | rs6589961 | 0.038 | 43 | 41 | 39 |
| PHF15 | rs62379978 | 0.036 | 16 | 14 | 17 |
| C1orf127 | rs77578010 | 0.036 | 21 | 26 | 23 |
| MKLN1 | rs71578952 | 0.035 | 48 | 41 | 48 |
| IRF4 | rs12203592 | 0.035 | 12 | 19 | 12 |
| NCOA6 | rs4911442 | 0.029 | 10 | 17 | 13 |
| PRDM2 | rs2473234 | 0.029 | 15 | 11 | 18 |
| DET1 | rs17190166 | 0.028 | 42 | 47 | 43 |
| AKAP1 | rs17833789 | 0.028 | 44 | 40 | 46 |
| MIR548A1 | rs2842385 | 0.027 | 16 | 16 | 22 |
| BDNF | rs2049045 | 0.027 | 21 | 20 | 16 |
| ZNF536 | rs11671893 | 0.026 | 18 | 12 | 14 |
| TMEM18 | rs10188334 | 0.025 | 19 | 17 | 22 |
| SEC16B | rs6670873 | 0.025 | 20 | 17 | 16 |
| PDGFA | rs9690350 | 0.025 | 41 | 42 | 49 |
| CYFIP2 | rs438830 | 0.025 | 21 | 24 | 14 |
| RORA | rs3743266 | 0.024 | 36 | 32 | 36 |
| ODZ2 | rs2923177 | 0.023 | 45 | 48 | 48 |
| SATB2 | rs1598656 | 0.022 | 27 | 31 | 23 |
| PRICKLE4 | rs6925777 | 0.022 | 49 | 49 | 47 |
| RMI1 | rs7853970 | 0.022 | 47 | 42 | 45 |
| HPGDS | rs767657 | 0.022 | 40 | 40 | 35 |
| FTO | rs1121980 | 0.022 | 47 | 45 | 46 |
| FPGT-TNNI3K | rs1514177 | 0.022 | 44 | 42 | 49 |
| IGSF11 | rs10934420 | 0.020 | 49 | 47 | 47 |
| ADARB2 | rs7896371 | 0.020 | 43 | 47 | 41 |
| GCKR | rs780094 | 0.020 | 41 | 32 | 35 |
| HLF | rs12940636 | 0.020 | 29 | 33 | 35 |
| ZNF324B | rs4801593 | 0.020 | 27 | 26 | 28 |
| DLGAP1 | rs11873906 | 0.020 | 27 | 31 | 28 |
| FAM178A | rs11190751 | 0.020 | 44 | 42 | 49 |
| KDM4C | rs913588 | 0.019 | 44 | 42 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAuley, A.B.T.; Varley, I.; Herbert, A.J.; Suraci, B.; Baker, J.; Johnston, K.; Kelly, A.L. Maturity-Associated Polygenic Profiles of under 12–16-Compared to under 17–23-Year-Old Male English Academy Football Players. Genes 2023, 14, 1431. https://doi.org/10.3390/genes14071431
McAuley ABT, Varley I, Herbert AJ, Suraci B, Baker J, Johnston K, Kelly AL. Maturity-Associated Polygenic Profiles of under 12–16-Compared to under 17–23-Year-Old Male English Academy Football Players. Genes. 2023; 14(7):1431. https://doi.org/10.3390/genes14071431
Chicago/Turabian StyleMcAuley, Alexander B. T., Ian Varley, Adam J. Herbert, Bruce Suraci, Joseph Baker, Kathryn Johnston, and Adam L. Kelly. 2023. "Maturity-Associated Polygenic Profiles of under 12–16-Compared to under 17–23-Year-Old Male English Academy Football Players" Genes 14, no. 7: 1431. https://doi.org/10.3390/genes14071431
APA StyleMcAuley, A. B. T., Varley, I., Herbert, A. J., Suraci, B., Baker, J., Johnston, K., & Kelly, A. L. (2023). Maturity-Associated Polygenic Profiles of under 12–16-Compared to under 17–23-Year-Old Male English Academy Football Players. Genes, 14(7), 1431. https://doi.org/10.3390/genes14071431







