Abstract
Asian rice (Oryza sativa L.) has become a model for understanding gene functions and domestication in recent decades; however, its own diversification is still controversial. Although the division of indica and japonica and five subgroups (aus, indica (sensu stricto), japonica (sensu stricto), tropical japonica, and aromatic) are broadly accepted, how they are phylogenetically related is not transparent. To clarify their relationships, a sample of 121 diverse genes was chosen here from 12 Oryza genomes (two parental and ten O. sativa (Os)) in parallel to allow gene genealogy-based mutation (GGM) analysis. From the sample, 361 Os mutations were shared by two or more subgroups (referred to here as trans mutations) from 549 mutations identified at 51 Os loci. The GGM analysis and related tests indicates that aus diverged from indica at a time significantly earlier than when tropical japonica split from japonica. The results also indicate that aromatic was selected from hybrid progeny of aus and tropical japonica and that all five subgroups share a significant number of the early mutations identified previously. The results suggest that aus, tropical japonica, and aromatic emerged sequentially within the most recent 4–5 millennia of rice domestication after the split of indica and japonica.
1. Introduction
Speciation by hybridization is a complex process in nature [1] and can be more difficult to understand when human influence is imposed, as in domesticated plants and animals. Asian rice (Oryza sativa L.) was recently shown to come from hybridization between two closely related species, perennial O. rufipogon Griff. and annual O. nivara Sharma & Shastry, and separate cultivations of the early rice, which took place about 4–5 millennia ago, divided O. sativa L. into indica and japonica subspecies [2]. The boundary of the subspecies corresponds to Hsien and Keng in the traditional cultivation of rice in China [3], but variations extend also to aus, tropical japonica, aromatic, and other types of rice that have been long noted in southeast, south and west Asia [3,4]. Collectively, five subgroups have been broadly recognized in cultivated rice, including indica (sensu stricto), japonica (sensu stricto), tropical japonica, aus, and aromatic [5]; however, their phylogenetic relationships are still obscure due to contentious evidence for origin of subgroups, as shown below. This is one of the issues to be addressed here, since the system of Asian rice provides a good opportunity for understanding how human activities can shape formation of genetic lineages in crops.
Over the long history of rice domestication, which is estimated to be at least eight millennia [6,7], a great number of cultivars and landraces have developed in each subspecies across Asia [8], with the extant members in each of indica (sensu stricto) and japonica (sensu stricto) far outnumbering those in tropical japonica, aus, and aromatic. The five subgroups are supported by genomic variation across a large number of accessions [9,10,11]. Nonetheless, whether or not they share a common early history is unclear, and how aus and aromatic relate to each other and to other subgroups still remains vague.
Relative to indica (sensu stricto) and japonica (sensu stricto) that grow mostly in temperate and subtropical regions of Asia, tropical japonica, aus, and aromatic are mostly cultivated in regions of Asia at a low latitudes. Tropical japonica, once called javanica [8], has long been considered an extension of japonica in tropical regions, which has few morphological differences from temperate japonica but can be differentiated from the latter with molecular markers [12]. In comparison, aus was recognized as a clan limited to south and west Asia in an isozyme survey [3]. Relative to japonica (sensu stricto) and tropical japonica, aus is closer to indica (sensu stricto) in not only nucleotide sequences [5] but also in relation to insertion polymorphism of transposable elements [13]. Though frequently less competitive in yield and quality than other rice varieties, aus has shown tolerance to low soil phosphate [14,15] or resistance to blast [16] and drought [17]. Since aus shows its distinctiveness from both indica (sensu stricto) and japonica in principal component analysis of genomes [10] and profiling of nucleotide diversity along the chromosomes [18], its status as a branch of indica needs more evidence.
Paradoxically, the subgroup aromatic has phenotypic similarities to aus and indica but a SSR similarity to tropical japonica [9]. A genomic analysis on 948 landraces identified DNA regions of japonica, aus, and indica in the circum-Basmati group [19]. Meanwhile, an interrogation of different sets of genomes (nuclear and chloroplastic) suggests a possible origin of aromatic rice from interbreeding of japonica with a local wild rice in the Indian subcontinent [20]. Genomes of two Basmati varieties show signs of gene flow between the circum-Basmati and circum-aus groups [21]. Still, the origin of aromatic has remained speculative.
Rice with aroma is favored by customers worldwide. It contains numerous volatile compounds [22,23], with 2-acetyl-1-pyrroline identified as a major aroma constituent, among others [24,25]. Proline is one of the precursors for acetylpyrroline [26], but the biochemical pathway leading to acetylpyrroline is still elusive. A recessive allele of gene BADH2 that encodes betaine aldehyde dehydrogenase can partly affect production of acetylpyrroline [27,28]. The roles of other genes (e.g., BADH1, [29]) still require more substantiation [30]. Many aromatic cultivars have the same 8 bp deletions in the coding sequence of BADH2 [27,31,32], but other less frequent mutations also show associations with the trait of fragrance [30,33,34]. In addition to genomic components, the aroma of rice can also be influenced by the growth environment, as shading has a positive impact [35], while grain size and salinity have negative impacts on the intensity of aroma of rice [36,37].
With more quality genomes released publicly, it is possible to carry out a genome-wide analysis on all recognized subgroups of Asian rice using the gene genealogy-based mutation (GGM) analysis lately reported [2] and relevant information from various disciplines (e.g., genomes of wild relatives, life-history traits, geographic distributions, gene functions, fossils, records of introgression, etc.). The goal of this study is to use fixed mutations specific to Asian rice to reconstruct phylogenetic relationships among subgroups during domestication. The key differences of this method from a phylogenomic approach are the classification of SNPs temporally and spatially via gene genealogies and the selection of a subset of SNPs and indels that are most relevant to phylogeny to build a reconstruction with minimum restrictions. Instead of evaluating the total biodiversity of Asian rice, the method focuses more on genes of known structures than of unknown structures in order to reduce errors arising from misidentification of mutations in different regions. It assumes that the bias of gene sampling is negligible in the reconstruction of a phylogeny since the phylogenetic relationships have the same impacts on all genes. As the accuracy and precision of mutation identification are vital to the GGM analysis, sampling of genomes was performed in representative lines of the five subgroups which have been well sequenced to allow reliable identifications of mutations. Here, an allelic perspective (instead of SNP or haplotype) is adopted since the known ancestors of Asian rice permit direct identifications of mutations at the genic level, and gene genealogies can be easily built. The footprints left in the genomes will be examined in situ to infer historical events. It is shown that an extensive analysis of mutational distributions on gene genealogies including all five subgroups can provide a clear interpretation for the origin of aromatic rice while clarifying phylogenetic relationships among the current cultivars of aus, tropical japonica, aromatic, indica (sensu stricto) and japonica (sensu stricto). A more complete image of rice domestication outside the Yangtze River begins to emerge, showing how the crop has been changed over millennia of ceaseless cultivation by human populations.
2. Materials and Methods
2.1. Study Samples
A total of 12 nuclear genomes (Table S1) were consulted for the following analysis, which were chosen based on the standards of a high coverage (>100×, except N22 of aus (65×)) and quality methods of sequencing and assembly. Genomes of indica (sensu stricto) were sampled from three varieties (9311, Shuhui498, and Minghui63) to confirm mutations in this lineage, as all three genomes were sequenced only by the PacBio technique (though at a far deeper coverage than those of other genomes) that has a higher error rate than the second-generation techniques. The genomes, representing five subgroups of O. sativa (Os) and two progenitors, O. rufipogon (Or) and O. nivara (On), were downloaded from the NCBI data base (www.ncbi.nlm.nih.org) prior to 2 May 2021. The genes sampled for gene genealogies were mainly from the list of Lu et al. [2], as well as some additional genes, which were selected regardless of their location, function, or size.
2.2. Gene Genealogy-Based Reconstruction of Intra-Specific Phylogeny
2.2.1. Classification of Mutations Based on Gene Genealogies
Since a conventional SNP- or haplotype-based phylogenetic (or phylogenomic) reconstruction typically requires no selection or recombination, it is inappropriate for Asian rice, as the assumptions are clearly violated given its hybrid origin. With no such restrictions, a gene genealogy-based mutation analysis [2] was adopted here in a phylogeny reconstruction. It builds on the known relationship of Asian rice with its parental species (O. nivara and O. rufipogon). Both 5′ and the coding regions were used but treated separately to show regional patterns of genes. Mutations (including both indel and substitution) in O. sativa were identified by aligning orthologous sequences across 12 genomes including the parental species (Figure S1). All the Os mutations were classified as trans-subgroup (trans) or subgroup-specific. This step is essential in our analysis, since trans mutations occurred earlier and entirely during traditional breeding. They are, therefore, closely associated with the past history of rice domestication. Another significant feature of trans mutations is that they are represented at least twice in an alignment, which suggest that they tend to be mutations of historically high frequencies. Fixed mutations are preferred over transient ones, because they contribute more steadily to the divergence between subgroups. The trans mutations across all five subgroups are presented in a table format (Figure S1), with the sites polymorphic between the ancestral lineages but involving no new mutations in O. sativa omitted to simplify the presentation. This step excluded many SNPs present between the parental species. When showing the associations of mutations among lineages, however, the figures typically include all polymorphic sites, following the previous format of gene genealogy [2], to give the context for new changes. Additional details on the counting of trans mutations are given in Figure S1.
2.2.2. Gene Genealogies Sampled for Phylogenetic Reconstruction
A gene genealogy is not to be confused with a phylogeny simply by their resemblance in topology. Many samples can be taken from relevant genomes to build gene genealogies, but the phylogeny to be reconstructed is the same. While the former is a ‘gene tree’ and the latter a ‘tree of organisms’, the GGM method uses a sample of ‘gene trees’ to infer a phylogeny. It does so by stratifying Os mutations on gene genealogies (or its simplified table format) to separate mutations in time/space. The method is particularly suitable for a recently diverged group of taxa since their genomes can be easily sampled to allow reliable identifications of mutations without worrying about repeated substitutions across sites. The organized data enable various analyses, statistical tests, and inferences on a phylogeny. It is specified here that on a phylogeny, all branches are divided into terminal branches and inner branches. Terminal branches directly connect subgroups and are mostly influenced by subgroup-specific mutations. An inner branch here refers to either an internal branch (a branch between two adjacent nodes (branching points)) or a branch between any connecting nodes on a phylogeny, which in either case is mainly defined by trans mutations, according to the GGM method. Since the minimum number of gene genealogies needed for a phylogenetic reconstruction varies with each biological system, it is not atypical to sample hundreds of loci for gene genealogies.
2.2.3. Inference of Phylogeny from Distributions of Mutations among Subgroup Combinations
To start the analysis, all the genes sampled were screened for distributions of trans mutations. These mutations were further classified into bins of permuted subgroup combinations (two-subgroup combinations, three-subgroup combinations, etc.). Phylogenetically informative combinations are expected to gather more trans mutations than random combinations. The bins having more mutations or loci than those compatible to randomness are the top candidates for inference of phylogenetic associations. These candidates can be further tested, if needed, with additional gene genealogies, or examined for succession of their emergences via comparisons of inner branches prior to the divergence of associated subgroups. The relative lengths between inner branches of a phylogeny can be compared via distributions of trans mutations by a measure called mutation density (md), which is defined here as number of trans mutations per nucleotide of a gene region (e.g., 5′, or coding) per branch (period of comparison). It is analogous to nucleotide divergence (π) but includes both indels and substitutions from 5′ or coding regions as an indel mutation and a substitution mutation are treated non-differentially. The mean of mutation densities across the sampled gene genealogies for a given branch is used here to infer the branch length of a phylogeny since it measures the relative divergence of the relevant genomes over the period. Factors that are expected to influence the length of a branch on a phylogeny can be modeled as below.
Let a period associated with an inner branch be T on a phylogeny, the mutation rate per individual be v per generation or u per nucleotide site per generation, and the selection intensity be s. Then, the expected number of fixed mutations (m) on the branch is 2vNePT under a deterministic model [2] that assumes a large population and steady environment [38], where Ne is the effective population size, T is the unit of the total generations associated with the branch, and P is the probability for a mutation to be fixed in Asian rice. Here we consider the number of fixed mutations on an inner branch (mi) with a relatively small sample size of genes, and variation in v due to gene size can be ignored by replacing v with u. Let L be the nucleotide number of a gene region under consideration. Then, the expected number of mutations on an inner branch becomes 2LuNePT. From the definition above, md = mi/L = 2uNePT.
Let N be the size of a breeding population; unlike sizes of cultivated populations, it can be more or less steady under traditional breeding of rice. Ne is half of N [39]. The observed mutations (md) on an inner branch can be approximated by uNPT, following the argument above. Since P is primarily a function of s, and s is relatively steady over time under the traditional cultivation, P can be approximated by a constant in Asian rice. The observed mutations (md) on an inner branch can therefore be further approximated simply by T in an arbitrary unit of uNP (assumed collectively a constant during the domestication of rice). Under these conditions, an observed md, which is proportional to T, can be compared between inner branches of the same phylogeny via a statistical test. The results of the test allow us to infer the order of emergence of the inner branches. For instance, under the null hypothesis of no difference in T between branches A and B, the observed values of md for A and B, averaged over sampled genes, can be compared by Student’s t-test or other appropriate tests. If B > A, given that a larger md is expected to be associated with a longer time period before a given branching point, rejection of the hypothesis can lead to an inference of later divergence of the branch B. Here, t-tests of md were carried out between the branch leading to aus and indica (sensu stricto) (A) and one to tropical japonica and japonica (sensu stricto) (B) on the phylogeny connecting the subgroups of O. sativa to its progenitors.
In sampling of genes, those known to be under the influence of pleiotropy or epistasis should be used with caution as mutation patterns may vary due to their impacts on the number and independence of mutations. When the information is not available, a large sample size of genes may help as pleiotropy tends to be restricted rather than universal in genomes [40].
3. Results
3.1. All Five Subgroups Share the Same Early Mutations
A total of 121 genes were surveyed from the sample of 12 nuclear genomes of Asian rice and its wild relatives (Table S1), which yielded 42 phylogenetically informative loci in O. sativa from those previously listed (Table S1 of Lu et al. [2]) and 9 more phylogenetically informative loci from 20 additional genomic regions (Table S2) due to the presence of trans mutations at the loci. Since genes of various functions and sizes, both known and unknown, were sampled across 12 chromosomes of O. sativa (Table S3), the patterns observed can be taken as a genome-wide phenomenon.
The 51 loci of O. sativa (Table 1) had 549 identified mutations, 361 of which were trans mutations shared by at least two subgroups (Figure S1a–l). Significantly, 116 of the trans mutations were present in subgroups of at least indica (sensu stricto) and japonica (sensu stricto) at 29 loci, including 42 at 18 loci present in all five subgroups (Table 1). The proportions of the mutations commonly shared among the five subgroups (35% for sampled loci and 12% of all trans mutations) cannot be explained by random sorting of trans mutations or other chance events. For instance, random sorting of the sampled 361 mutations under a uniform distribution could only explain, on average, about 14 mutations at two loci for mutations in the class of five subgroups, among 26 possible combinations. Yet, the observed pattern of trans mutations in the five subgroups is in line with the early history of rice domestication previously identified [2]. Many of the 19 loci (e.g., SH4, Rc, RAE2, TCP19, GL3.2, DFR, and DAHPS2) were previously tested under positive selection [2]. The 42 trans mutations largely came from fixed early mutations that are expected to pass down constantly to later generations.

Table 1.
A list of 51 loci showing mutations informative to relationships of the subgroups of Asian rice.
3.2. Subgroup Aus Differentiated in Indica and Tropical Japonica in Japonica
Of the 361 trans mutations above, 126 are specific to two subgroups. A majority (59%) of the 126 trans mutations were concentrated in two combinations, japonica (sensu stricto)–tropical japonica and indica (sensu stricto)–aus (Table 1 and Figure S1). A total of 49 trans mutations from 11 loci at 5′ and/or coding regions were specifically shared between japonica (sensu stricto) and tropical japonica, as shown at six representative loci (Figure 1). This suggests that tropical japonica and japonica (sensu stricto) had a common history prior to their differentiation. Meanwhile, 26 trans mutations at 13 loci specifically occurred in indica (sensu stricto) and aus, which also indicates their joint history before the split of aus and indica (sensu stricto). Five representative loci exhibit how these mutations unite the subgroups within the indica subspecies (Figure 2). In both cases, the extensive sharing of specific mutations have little to do with random events or natural introgressions since natural introgression is rare in rice and outcrossing rates are typically less than 1% in a natural field [41]. Phylogenetic associations are the major reason for patterns of enriched trans mutations in the specific combinations of subgroups.

Figure 1.
Gene genealogies showing the association of tropical japonica (tro) with japonica (J, sensu stricto). Gene genealogies are shown for six loci, with relevant mutations indicated in the highlighted circle (orange), following the format of Lu et al. (2022). Nonsynonymous substitutions are in thick bars (black for nucleotide G, green for A, red for T, and blue for C), and indels in flattened circles or arrows. Repeated changes are indicated by the number after x. An early mutation is shown in *. An ortholog from indica (I, sensu lato) is included, along with those from ancestral species (O. nivara (On) and O. rufipogon (Or)) as outgroups. Genealogies are based on coding sequences of Hd3a (a) and AGO2 (b), or 5′ regions of TCP19 (c), PK1 (d), TAC3 (e), and Pi-ta (f).
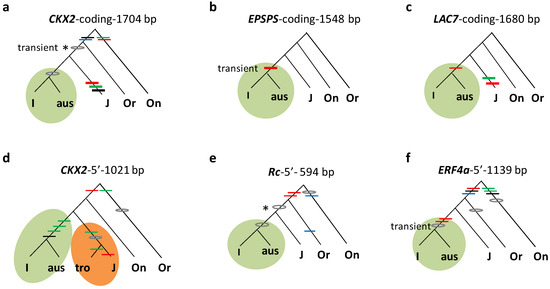
Figure 2.
Specific mutations shared between subgroups aus and indica (I, sensu stricto). Gene genealogies are given at five loci (a–f). The format follows Figure 1. Genealogies are based on the coding sequences of CKX2 (a), EPSPS (b), and LAC7 (c) or 5′ regions in CKX2 (d), Rc (e), and ERF4a (f). An early mutation is shown in *. The colored branches highlight specific trans mutations within subspecies (indica in green, japonica in orange).
Besides the two combinations above, six of the ten possible combinations of two subgroups have fewer than nine trans mutations (Figure S1). Specifically, only one gene shows one trans mutation at GS5 between aromatic and indica (sensu stricto), one trans mutations at Hd1 between aus and tropical japonica, three trans mutations at an unknown locus (tentatively ACS3) between aromatic and japonica (sensu stricto), and four trans mutations at DFR between aus and japonica (sensu stricto); two genes show three trans mutations at GL3.2 and SPL13 between tropical japonica and indica (sensu stricto) and eight trans mutations in ACS3 and Hd1 between japonica (sensu stricto) and indica (sensu stricto). These sporadic distributions are more or less compatible with random events and provide little information for phylogeny. Two combinations, aromatic–aus and aromatic–tropical japonica, however, show more mutations at more loci and are examined below.
3.3. Subgroup Aromatic Was Derived from Hybrid Progeny between Aus and Tropical Japonica
The top two subgroups that aromatic shared trans mutations with were tropical japonica and aus. Table 2 lists 13 loci in aromatic that contain 36 mutations specifically shared with either aus (14 at 5 loci) or tropical japonica (22 at 8 loci). Given the origins of tropical japonica and aus in two subspecies, their relationships with aromatic are more consistent with gene flow than with sharing of a common ancestor as it is difficult for aromatic to derive from two different lineages unless it is a hybrid. There are at least two cases from the sampled genomes that clearly indicate gene flows from aus and tropical japonica to aromatic and not vice versa. One case is the coding region of MYB3 on chromosome 3, which displays an aromatic-specific sequence that appears to be a recombinant of aus and tropical japonica alleles after three crossing-over events identified at the regions (Figure 3a). Another case is the coding region of Hd1 on chromosome 6, which shows that the aromatic allele came from two recombination events between aus and tropical japonica, encoding a significantly altered Hd1 (Figure S2). The scenario of gene flows from aus and tropical japonica to aromatic is also consistent with patterns of loci having new mutations across terminal branches. It is expected that the earlier the emergence time of a subgroup, the more loci are likely under human selection when other conditions are similar. In comparison with the number of loci having mutations specific to tropical japonica (14) or aus (21), aromatic has nine loci that contain at least one aromatic-specific mutation at either 5′ and/or coding regions (Figure S1). This lowest number among the three subgroups indicates that aromatic is a relatively young subgroup, which is consistent with its emergence as hybrid progeny of aus and tropical japonica.

Table 2.
Gene regions showing specific mutations shared by aromatic and aus or tropical japonica.
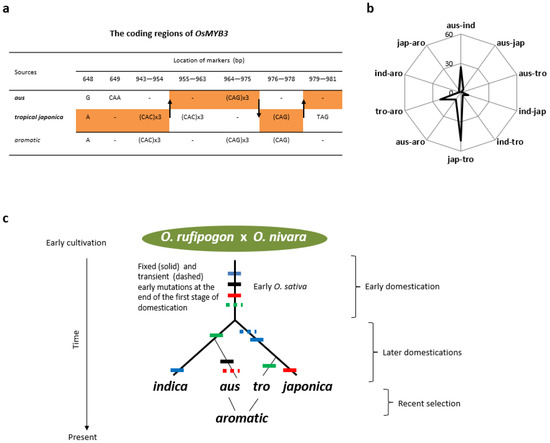
Figure 3.
Events leading to phylogenetic relationships among subgroups of Asian rice. (a) Recombination events detected at MYB3. The sequences from three representative genomes were aligned over 1053 sites of the coding regions. The arrows indicate three likely events of crossing-over between chromatids of aus and tropical japonica (in bold) in early aromatic, which led to the aromatic allele via reconnecting the gene regions in yellow. Nucleotide positions are labeled partly around the recombination events. (b) A radar graph on the distribution of 126 trans mutations among ten classes of two-subgroup pairings. The five subgroups are ind (indica sensu stricto), jap (japonica sensu stricto), tro (tropical japonica), aus (aus), and aro (aromatic). (c) The phylogeny of Asian rice. The subgroups (sensu Garris et al., 2005) are in italic and bold. Mutations are featured by the colored bars in each lineage (dashed for transient and solid for fixed mutations). Major stages of domestication (after parenthesis) follow the evidence presented here for the later domestication and in Lu et al., 2022 [2] for the early domestication. The branches leading to aus, tropical japonica, and aromatic are not proportional.
To collect more evidence of subgroup-specific selection, BADH2 in aromatic was examined and only one deletion in its 5′ region was seen; by contrast, the gene in aus contains multiple mutations (Figure S3). In addition to MYB3 shown above, aromatic-specific alleles were also observed at GL3.2 (Figure S1c), TCP19 (Figure S1f), and MYC2 (Figure S1j).
3.4. Subgroup Aus Differentiated from Indica Earlier than Tropical Japonica from Japonica
To understand why trans mutations are more abundant between tropical japonica and japonica (sensu stricto) than between aus and indica (sensu stricto), the likelihood that the two bifurcating (selective) events that caused the split of the subgroups happened at different times of rice domestication was tested. Given that aromatic inherited specific mutations from aus and tropical japonica, the trans mutations it shared with aus or tropical japonica were also included in the tests. These mutations came from the same period before the emergence of aus and indica (sensu stricto) or tropical japonica and japonica (sensu stricto) and happened to be sorted into aromatic during the hybridization detected above. This led to a total of 156 trans mutations (37 in coding and 119 in 5′ regions) from 33 loci at 5′ and/or coding regions allocated to the period immediately before the split of tropical japonica and japonica (sensu stricto). Meanwhile, 29 trans mutations (12 in coding and 17 in 5′ regions) from 16 loci at 5′ and/or coding regions are specific to the branch leading to the split of indica (sensu stricto) and aus (Table 1). The average mutation densities on the internal branch leading to the split of aus and indica (sensu stricto) were 0.0017 (s.e. 0.0004, n = 10) for 5′ regions and 0.0010 (s.e. 0.0001, n = 10) for coding regions. Those on the branch prior to tropical japonica and japonica (sensu stricto) were 0.0035 (s.e. 0.0007, n = 32) and 0.0019 (s.e. 0.0004, n = 16), respectively (Table 1). The comparisons of mutation densities were made over the longer time of the two internal branches, with the assumption that mutations are similarly distributed over the time on the branches. Under the null hypothesis of no difference in mutation density between branches, results of one-sided t-tests indicate that the internal branch before tropical japonica (and japonica (sensu stricto)) was significantly longer (about two times) than the one preceding aus (and indica (sensu stricto)), and the pattern held at both 5′ (p = 0.010, naus = 10, ntro = 32) and coding regions (p = 0.017, naus = 10, ntro = 16). In other words, aus branched off significantly earlier in indica to form its own lineage, whereas tropical japonica separated from japonica (sensu stricto) significantly later.
Additional evidence is in line with the statistical result above. First, alleles in aus inherited more O. nivara (or sometimes O. rufipogon) type at various loci (MYB3, Hd6, EPSPS, SPL16, PGI, ACS3, and WG7) than alleles of other subgroups (Figure S1). This may stem from its early divergence in indica since indica inherited more alleles from O. nivara than from O. rufipogon during its initial selection [2]. Secondly, when transient mutations segregated with ancestral nucleotides, aus frequently kept the ancestral ones, as shown by several 5′ mutations at Hd6 and GL3.2 (Figure S1c) and three transient mutations (two in 5′ and one in the coding regions) at the Rc locus (Figure S1g). Obviously, early divergence of aus could directly lead to its low sharing of mutations with indica (sensu stricto).
3.5. Phylogeny Reconstructed from Collective Evidence
Together, four pairs of subgroups (tropical japonica–japonica (sensu stricto), aus–indica (sensu stricto), aromatic–tropical japonica, and aromatic–aus) accounted for 84% (106 out of 126) of the trans mutations specific to two subgroups (Figure 3b). The distribution gives the basic structure of the phylogeny of Asian rice, along with the analysis above showing that the subgroups aus, tropical japonica, and aromatic emerged sequentially during domestication, after the split of the indica and japonica subspecies (Figure 3c). The early branch leading to indica and japonica was addressed previously [2] and is further corroborated here by 42 trans mutations shared among five subgroups. Further support for the phylogeny comes from the distributions of trans mutations allocated to certain bins of ten possible combinations of three subgroups and five possible combinations of four subgroups. Of 160 trans mutations shared by three subgroups, 107 at 24 loci are in the combinations of japonica (sensu stricto), tropical japonica, and aromatic, and three at three loci are shared among indica (sensu stricto), aus, and aromatic. One combination, indica (sensu stricto), japonica (sensu stricto), and tropical japonica, collected 40 trans mutations at 11 loci. This pattern is largely a continuation of the early mutations. Of the 32 trans mutations shared by four subgroups, 30 are seen in the combination of indica (sensu stricto), japonica (sensu stricto), tropical japonica, and aromatic, with none or one mutation in the other four combinations.
The phylogeny (Figure 3c) indicates that aus, tropical japonica, and aromatic are subgroups that emerged at the later stage of domestication of Asian rice. Given the previous estimate on the duration of the later-stage domestication [2], it is inferred that selection events leading to aus, tropical japonica, and aromatic took place within the last 4–5 millennia in the order shown.
3.6. Validity of Mutation Identification
To check the error rate of the mutations identified above, independent data of 82 sequences were sampled from the NCBI database (accessed during the period 14–18 April 2023) for seven genes (SH4, Hd1, DFR, Hd3a, CHS, Rc, and SPL16). Alignments of homologous sequences indicate that 26 Os mutations fall in the overlapping regions between the two data sets (the data used for identification and those for testing). The test data are consistent with 24 of the 26 mutations (Table S4), with one at the 5′ region of SH4 being dubious and one at the 5′ region of Rc not supported. This result supports that at least 92% of mutations were identified correctly.
4. Discussion
4.1. GGM-Based Analysis and Specific Test for Asian Rice
Phylogeny reconstruction is mostly dependent on similarity matrices. While valid for evaluating genetic diversity, the prevailing methods (such as SNP-based analyses) have their limitations in handling a complex phylogeny when hybridization and recombination occur. Unlike SNPs-based methods that exclude indels, the GGM-based analysis uses all types of variations and stratifies them to extract phylogenetic relationships among taxa. It does not depend on a matrix of similarity, avoids restrictions on life-history traits, and thus is suitable for many organisms for its enhanced statistical power in inference making in the era of genomics.
The example here shows that although trans mutations are present at less than half of the genes surveyed, they are sufficient for phylogenetic inference in Asian rice. The sensitivity of the analysis may vary with other organisms with different selection and/or evolutionary history. It requires an incremental sampling of genes across genomes or increasing genomes in order to attain a sufficient power for phylogeny reconstruction. Identification of recombinant alleles at different loci can further validate the hybridized origin of a relevant taxon. In comparison with these steps generally applicable to other organisms, model-based statistical tests on inner branches, as used here for Asian rice, require some conditions including a relatively large N and a steady environment and selection intensity. When these conditions are not closely met, simulation studies can be conducted to evaluate the bias of using the specific test. Alternatively, different models can be engaged to test the relative lengths of inner branches. The GGM-based method, as shown here, is able to solve issues of complex phylogeny that includes hybridization and recombination. These two processes occur hand-in-hand and are frequently observed in domesticated plants and animals but are typically recalcitrant to the conventional methods of phylogeny reconstruction.
4.2. The Early Rice Laid the Foundation for All Subgroups of Asian Rice
Rice domestication is a process of constant fixation of mutations and recombinants favored by past breeders. Passing down of early mutants to later subgroups is more than random sorting but carries phylogenetic information associated with selection (natural and artificial). For O. sativa, trans mutations shared in all five subgroups are most likely from early mutations that were fixed or occurred at high frequencies, and their distributions can be little altered by later practices of breeding (excluding gene editing). For early mutations that were not of high frequency, the tendency to retain the ancestral alleles in aus and/or the random (nonrandom) loss of mutations from the other two subgroups (tropical japonica and aromatic) could lead to a smaller number of shared mutations among the five subgroups (42 at 18 loci) than the number of trans mutations (91 at 30 loci) cited earlier between indica and japonica [2].
Compared with the early mutations, later mutations which emerged after the split of indica and japonica, may have a different fate. Those specific to the subgroup can be still transient, thus easily subject to random (or nonrandom) loss. Meanwhile, a later differentiated group generally shows fewer loci altered by additional human selection and also fewer varieties than those of an earlier group due to its short history. Since the varietal pattern itself can be easily influenced by the size of cultivation areas and/or replacements by modern hybrids, mutations from recent selection are most vulnerable in modern agriculture, which features mass production of a small number of varieties.
Geographically, the early rice that existed 4–5 millennia ago was found mainly in regions of the lower and central Yangtze River [42]. The later stage of rice domestication clearly involved much broader regions, starting from the split of indica (sensu lato) and japonica (sensu lato) in eastern Asia to more southern and northern areas to local selections mostly in southern Asia, some of which have been revealed lately [43]. Archeological evidence indicates that rice cultivation possibly reached south/south east parts of Asia about 4–5 millennia ago [44,45]. Signals of recombination here suggest that a specific member of tropical japonica (Chao-Meo type) was likely one of the immediate ancestors of aromatic. Consistent with the scenarios above, Chinese germplasm of rice consists of varieties primarily of indica and japonica, lacks aus and aromatic and includes tropical japonica at a frequency lower than germplasm in the tropical regions [46].
4.3. Subgroup Aus Branched off Early in Indica
Since aus possesses not only the early mutations of rice but also trans mutations specifically shared with indica (sensu stricto), its root in indica is well supported. Its earlier exposure to selection as an independent population made it possible for some transient mutations from early rice to be excluded from its lineage by chance and/or selection. Meanwhile, a significant number of its loci have been under lineage-specific selection. Both processes can cause its distinctiveness, as shown previously [10,18]. The statistical tests on mutation density, which confirm that the branch leading to aus and indica (sensu stricto) is shorter than that leading to tropical japonica and japonica (sensu stricto), simply recapture the pattern of early divergence of aus. Although transient trans mutations, which were expected to be in a minority, could exist across inner branches, their effect on the statistical tests is benign or negligible when the two branches compared have a recent shared history, as in the cases here with aus and indica (sensu stricto) or tropical japonica and japonica (sensu stricto). This is because the common history (the early rice period) provided the same genetic background, which consequently led to similar proportions of transient mutations in the branches.
Since aus is distributed mainly in the subcontinent of India and is hardly seen in Chinese germplasm, its emergence most likely occurred after indica reached south and west Asia. The early breeders of aus were possibly local to the Himalaya hills [47], whose ancestors or traders could carry early indica to the region. Because O. nivara, one of the wild progenitors of O. sativa, is tolerant of dry environments, many of its alleles could be passed to aus via early indica during local selection. Rice strains selected to be capable of growing in harsh environments gave aus its characteristic features, i.e., some genomic regions of aus are closer to those of O. nivara than to those of the present day indica (sensu stricto). This can lead to an erroneous result on its origin under a similarity-based analysis.
At a much later time (about twice longer than the time leading to the separation of aus from indica), japonica was introduced to southeast/south Asia, possibly by different groups of people, leading to the formation of tropical japonica. The selection on early tropical japonica most likely occurred in a tropical environment on the existing variation in japonica. The specifics of these local selections need more investigation.
4.4. Aromatic Rice as Hybrid Progeny of Aus and Tropical japonica
Though a principal component analysis [10] already indicated that features of aromatic were somewhat between aus and japonica and a subsequent analysis suggested that aromatic could be a hybrid between aus and japonica [18], the phylogenetic relationship between aromatic and tropical japonica was unclear until this study. While mutations specific to tropical japonica or aus are found in aromatic genomes, few traces of japonica (sensu stricto) are seen in aromatic here, which diminishes its direct role in the formation of aromatic rice. The recombinants of MYB3 or Hd1 in the aromatic genome clearly support the event of hybridization between aus and tropical japonica, and more recombinants will be found in future. The shared genomic regions between aromatic and aus [21] are clearly a consequence of a hybridization event. Incidentally, the previously suspected factor for the aroma, a nonfunctional allele (badh2.1) of BADH2, is absent in the two aus genomes here. Its presence in some accessions of both aus and tropical japonica [32] is congruent with the hybrid origin of aromatic, indicating that the allele is older than the lineage of aromatic itself. Since human selection on early aromatic could involve more than a specific allele/locus, for instance, gene combinations or recombinants, the lack of selection signal on BADH2 is not all surprising; instead, other loci with aromatic-specific alleles (e.g., MYB3, TCP19, MYC2) should be explored, not only for understanding of selection on aromatic but also for their influence on the trait of rice aroma.
Geographically, the selection on early aromatic must have occurred in an area where both cultivations of aus and tropical japonica co-existed at the time. As traditional aromatic varieties were mainly from the Himalayan foothills of the Indian subcontinent [48,49], an area which largely overlaps with the current distribution of aus [50], early aromatic was likely confined to the Indian subcontinent at its incipient stage. As for aus and tropical japonica, the human history associated with lineage-specific selection on aromatic requires more studies.
4.5. Applications and Error Rates
Since conventional reconstruction of phylogeny requires data without the effects of selection and/or recombination, only small fragments of genomes (e.g., intergenic regions) are considered suitable. When other regions are used, they need to be tested to confirm they are free of selection or recombination prior to being used. A modern phylogenomic approach can use entire genomic sequences or whole gene sets to leverage regional signals but ignore the heterogeneity of SNPs and indels. The method presented here has none of the above restrictions; thus, it is applicable to all regions of a nuclear genome in theory. The key requirement of the GGM-based method is being able to identify mutations reliably. This requirement may not be met for cases when genomes of immediate ancestor(s) are not available or can be approximated as in rice, which is common. With increasing genomic data becoming available in the future, this limitation can be gradually removed.
For rice, the original parents are no longer extant, but the species (Oryza rufipogon and O. nivara) that contributed the parental individuals [2] about 8–10 kyr ago [6] still exist. Genomes of the wild species can serve as proxies of the original parents with only a small number of errors introduced. This is because the new mutations emerging in the wild relatives over the last 8–10 kyr are in a minority compared with the historical mutations that accumulated in and separated O. rufipogon and O. nivara in the period estimated to be from ~160 kyr [51] to about 340–380 kyr [52]. Even when mutations did occur in a gene in the wild relatives during the past 8–10 kyr, the likelihood of the orthologous gene in rice having the same mutations by chance is minimal. Part of the reason is that the different evolutionary histories of parental species and rice would have impacts on different loci, causing varied distributions of mutations across the genomes. This pattern can significantly lower the error rate of misidentification of a mutation.
Two additional errors, however, may emerge. One is that a mutation identified is not new in rice but inherited from the ancestors and happens to be absent in the current genomes referenced. This error may inflate the number of mutations specific to rice. The probability of such errors is, however, low since the probability for a site to remain unchanged is high for closely related O. rufipogon and O. nivara. For instance, if the substitution rate is about 5 × 10−8 per site per year in Oryza, the probability of two orthologs being identical at a site is 0.98 after 160 kyr or 0.95 after 380 kyr under the Jukes–Cantor model when no selection is involved. Positive selection reduces the probability, while negative selection increases it. The generally low levels of genetic polymorphism between closely related taxa hardly require a specific model of substitutions since similar results are given by the known substitution models during their early divergence.
Another error is taking transient mutations as fixed ones, with the result that comparisons of numbers of presumably fixed mutations between branches can be less accurate. For phylogenetic reconstruction, fixed (or frequent) mutations are preferred over transient (rare) mutations because they contribute more steadily to the physical divergence than the latter. The probability of such errors is, however, smaller for mutations on inner branches than those on the terminal ones. Because of high frequencies, fixed mutations are highly represented in inner branches and also in comparisons between species when each species is represented by one genome. Depending on the level of genetic polymorphism of a taxon, this error is not necessarily reduced by including a few more genomes. In all cases, the error rate of misidentification of mutations can be evaluated with additional sequences, particularly from parental species.
Lastly, the short history of rice implies that repeated mutations at the same nucleotide site of rice are most unlikely, which suggests that nearly all mutations are original, rather than secondary, in rice. This feature permits the simple permutation analysis on trans mutations among subgroups here.
Because phylogenetic relationships have a genome-wide influence on all taxa, sampling of loci regardless of chromosome, function, location, or size of a gene permits the phylogenetic relationships to be revealed from a manageable sample size. Here, genes showing epistasis should be sampled only once to reduce bias from correlated mutations. Genes from the same biochemical pathway, however, can be sampled more than once to reflect possibly diverse selection, as seen at the loci of Asr1 and Asr2 of the abscisic acid pathway in the common bean [53]. Larger sample sizes can possibly reduce errors due to experiments or complex gene–phenotype relationships but are unlikely to overturn the branching pattern already shown at the phylogenetically informative loci.
4.6. Implications of the Phylogeny
With clarified phylogeny, the classification of five subgroups of Asian rice [5] can easily accept new additions. For instance, Madagascar was the latest area in the Old World to grow Asian rice, with subgroups aus and tropical japonica introduced as the initial major types. Selection on interbred progeny has contributed to the local diversity of rice in the region [54], which can be better understood with the phylogeny. In Africa and America, selection on rice tends to be more environmentally oriented [55,56]. In China, breeders have focused on taking advantage of hybrid vigor. Knowing how rice domestication has shaped its biodiversity over the past millennia helps future planning of healthy agriculture, as much can be learnt from early breeders.
The GGM-based method can be integrated into a broad application that targets uncovering key alleles/mutations that affect traits of interest such as tolerance to stress [57]. When a package that can reliably delineate gene regions on a selected number of genes and integrate all mutations is provided in a phylogenomic program, the MMG method can readily stratify mutations and use their temporal distributions to build a phylogeny or test for or against candidate phylogenetic trees inferred from other methods. For complex phylogenies, the MMG method can rebuild the past history alone when the data have a sufficient power, providing a valid solution for phylogeny reconstruction, as shown here. In future, an MMG-based phylogeny could be statistically tested by a program using approximate Bayesian computation when all mutations are used by machine-learning to generate a valid distribution of random mutations among branches.
5. Conclusions
Knowing the domestication history of rice can benefit targeted research on food shortages and nutrition, as well as provide temporal coordinates in understanding past human migration and development. The phylogenetic relationships among the five subgroups were clarified in this study; evidently, after much selection during the phase I domestication of rice [2], significant events of human selection led to the emergence of aus, tropical japonica, and aromatic in the phase-II domestication of rice. Cultivation of Asian rice should be continued in the direction of improved biodiversity, with the purpose of ensuring that the crop remains healthy for generations to come.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14071412/s1. Figure S1: Identification of rice mutations across 51 genes; Figure S2: The coding regions of OsHd1 compared among three subgroups; Figure S3: Mutation distributions at the locus BADH2 (Os08g32870.1); Table S1: Genomes analyzed in this study; Table S2: List of 121 Oryza genes surveyed in this study; Table S3: Information on the 51 Os loci with trans mutations; Table S4: An examination of Os mutations with additional 82 NCBI entries. References [58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103] are cited in the supplementary materials.
Funding
This work was supported in part by National Natural Science Foundation of China (91331116), the Chinese Academy of Sciences (XDA08020204), and the State Key Laboratory of Systematic Botany and Evolution at the Institute of Botany, Chinese Academy of Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this publication.
Acknowledgments
I thank members of the EEG group at IBCAS for their encouragements in moving the research forward and researchers at other institutes (particularly the University of Arizona) for making their sequences publicly available.
Conflicts of Interest
The author declares no conflict of interest.
References
- Baack, E.J.; Rieseberg, L.H. A genomic view of introgression and hybrid speciation. Curr. Opin. Genet. Dev. 2007, 17, 513–518. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Xu, Y.Z.; Li, N. Early domestication history of Asian rice revealed by mutations and genome-wide analysis of gene genealogies. Rice 2022, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Glaszmann, J.C. Isozymes and classification of Asian rice varieties. Theor. Appl. Genet. 1987, 74, 21–30. [Google Scholar] [CrossRef]
- Parsons, B.J.; Newbury, H.J.; Jackson, M.T.; Ford-Lloyd, B.V. The genetic structure and conservation of aus, aman and boro rices from Bangladesh. Genet. Resour. Crop Evol. 1999, 46, 587–598. [Google Scholar] [CrossRef]
- Garris, A.J.; Tai, T.H.; Coburn, J.; Kresovich, S.; McCouch, S. Genetic structure and diversity in Oryza sativa L. Genetics 2005, 169, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J. The Middle Yangtze region in China is one place where rice was domesticated: Phytolith evidence from the Diaotonghuan Cave, Northern Jiangxi. Antiquity 1998, 72, 885–897. [Google Scholar]
- Gross, B.L.; Zhao, Z.J. Archaeological and genetic insights into the origins of domesticated rice. Proc. Natl. Acad. Sci. USA 2014, 111, 6190–6197. [Google Scholar] [CrossRef] [PubMed]
- Khush, G.S. Origin, dispersal, cultivation and variation of rice. Plant Mol.Biol. 1997, 35, 25–34. [Google Scholar] [CrossRef]
- Ali, M.L.; McClung, A.M.; Jia, M.H.; Kimball, J.A.; McCouch, S.R.; Eizenga, G.C. A rice diversity panel evaluated for genetic and agro-morphological diversity between subpopulations and its geographic distribution. Crop Sci. 2011, 51, 2021–2035. [Google Scholar]
- Schatz, M.C.; Maron, L.G.; Stein, J.C.; Hernandez Wences, A.; Gurtowski, J.; Biggers, E.; Lee, H.; Kramer, M.; Antoniou, E.; Ghiban, E.; et al. Whole genome de novo assemblies of three divergent strains of rice, Oryza sativa, document novel gene space of aus and indica. Genome Biol. 2014, 15, 506. [Google Scholar]
- Wang, W.S.; Mauleon, R.; Hu, Z.Q.; Chebotarov, D.; Tai, S.S.; Wu, Z.C.; Li, M.; Zheng, T.Q.; Fuentes, R.R.; Zhang, F.; et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 2018, 557, 43–49. [Google Scholar] [CrossRef]
- Mackill, D.J.; Lei, X.M. Genetic variation for traits related to temperate adaptation of rice cultivars. Crop Sci. 1997, 37, 1340–1346. [Google Scholar] [CrossRef]
- Carpentier, M.C.; Manfroi, E.; Wei, F.J.; Wu, H.P.; Lasserre, E.; Llauro, C.; Debladis, E.; Akakpo, R.; Hsing, Y.I.; Panaud, O. Retrotranspositional landscape of Asian rice revealed by 3000 genomes. Nat. Commun. 2019, 10, 12. [Google Scholar] [CrossRef]
- Chin, J.H.; Gamuyao, R.; Dalid, C.; Bustamam, M.; Prasetiyono, J.; Moeljopawiro, S.; Wissuwa, M.; Heuer, S. Developing rice with high yield under phosphorus deficiency: Pup1 sequence to application. Plant Physiol. 2011, 156, 1202–1216. [Google Scholar] [CrossRef]
- Gamuyao, R.; Chin, J.H.; Pariasca-Tanaka, J.; Pesaresi, P.; Catausan, S.; Dalid, C.; Slamet-Loedin, I.; Tecson-Mendoza, E.M.; Wissuwa, M.; Heuer, S. The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 2012, 488, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.J.; Wang, L.; Pan, Q.H. A new recessive gene conferring resistance against rice blast. Rice 2016, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Casartelli, A.; Riewe, D. Exploring traditional aus-type rice for metabolites conferring drought tolerance. Rice 2018, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Civan, P.; Craig, H.; Cox, C.J.; Brown, T.A. Three geographically separate domestications of Asian rice. Nat. Plants 2015, 1, 15164. [Google Scholar] [CrossRef]
- Santos, J.D.; Chebotarov, D.; McNally, K.L.; Bartholomé, J.; Droc, G.; Billot, C.; Glaszmann, J.C. Fine scale genomic signals of admixture and alien introgression among Asian rice landraces. Genome Biol. Evol. 2019, 11, 1358–1373. [Google Scholar] [CrossRef]
- Civáň, P.; Ali, S.; Batista-Navarro, R.; Drosou, K.; Ihejieto, C.; Chakraborty, D.; Ray, A.; Gladieux, P.; Brown, T.A. Origin of the aromatic group of cultivated rice (Oryza sativa L.) traced to the Indian subcontinent. Genome Biol. Evol. 2019, 11, 832–843. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lye, Z.N.; Groen, S.C.; Dai, X.G.; Rughani, P.; Zaaijer, S.; Harrington, E.D.; Juul, S.; Purugganan, M.D. Nanopore sequencing-based genome assembly and evolutionary genomics of circum-basmati rice. Genome Biol. 2020, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Maga, J.A. Rice product volatiles—A review. J. Agric. Food Chem. 1984, 32, 964–970. [Google Scholar] [CrossRef]
- Widjaja, R.; Craske, J.D.; Wootton, M. Comparative studies on volatile components of non-fragrant and fragrant rices. J. Sci. Food Agric. 1996, 70, 151–161. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Juliano, B.O.; Turnbaugh, J.G. Cooked rice aroma and 2-acetyl-1-pyrroline. J. Agric. Food Chem. 1983, 31, 823–826. [Google Scholar] [CrossRef]
- Wakte, K.; Zanan, R.; Hinge, V.; Khandagale, K.; Nadaf, A.; Henry, R. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): A status review. J. Sci. Food Agric. 2017, 97, 384–395. [Google Scholar] [CrossRef]
- Yoshihashi, T.; Huong, N.T.T.; Inatomi, H. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J. Agric. Food Chem. 2002, 50, 2001–2004. [Google Scholar] [CrossRef]
- Bradbury, L.M.T.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.S.; Waters, D.L.E. The gene for fragrance in rice. Plant Biotechnol. J. 2007, 3, 363–370. [Google Scholar] [CrossRef]
- Chen, S.H.; Yang, Y.; Shi, W.W.; Ji, Q.; He, F.; Zhang, Z.D.; Cheng, Z.K.; Liu, X.N.; Xu, M.L. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 2008, 20, 1850–1861. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P.K.; Singh, R.; Pandit, A.; Mahato, A.K.; Gupta, D.K.; Tyagi, K.; Singh, A.K.; Singh, N.K.; Sharma, T.R. SNP haplotypes of the BADH1 gene and their association with aroma in rice (Oryza sativa L.). Mol. Breed. 2010, 26, 325–338. [Google Scholar] [CrossRef]
- He, Q.; Yu, J.; Kim, T.S.; Cho, Y.H.; Lee, Y.S.; Park, Y.J. Resequencing reveals different domestication rate for BADH1 and BADH2 in rice (Oryza sativa). PLoS ONE 2015, 10, 12. [Google Scholar] [CrossRef]
- Bourgis, F.; Guyot, R.; Gherbi, H.; Tailliez, E.; Amabile, I.; Salse, J.; Lorieux, M.; Delseny, M.; Ghesquiere, A. Characterization of the major fragance gene from an aromatic japonica rice and analysis of its diversity in Asian cultivated rice. Theor. Appl. Genet. 2008, 117, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.J.; Calingacion, M.N.; Fitzgerald, M.A.; McCouch, S.R. The origin and evolution of fragrance in rice (Oryza sativa L.). Proc. Natl. Acad. Sci. USA 2009, 106, 14444–14449. [Google Scholar] [CrossRef]
- Sakthivel, K.; Sundaram, R.M.; Rani, N.S.; Balachandran, S.M.; Neeraja, C.N. Genetic and molecular basis of fragrance in rice. Biotechnol. Adv. 2009, 27, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Myint, K.M.; Courtois, B.; Risterucci, A.M.; Frouin, J.; Soe, K.; Thet, K.M.; Vanavichit, A.; Glaszmann, J.C. Specific patterns of genetic diversity among aromatic rice varieties in Myanmar. Rice 2012, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.W.; Li, W.; Pan, S.G.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.J.; Wang, Y.L.; Duan, M.Y.; Tian, H.; Tang, X.R. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 10. [Google Scholar] [CrossRef]
- Gay, F.; Maraval, I.; Roques, S.; Gunata, Z.; Boulanger, R.; Audebert, A.; Mestres, C. Effect of salinity on yield and 2-acetyl-1-pyrroline content in the grains of three fragrant rice cultivars (Oryza sativa L.) in Camargue (France). Field Crop. Res. 2010, 117, 154–160. [Google Scholar] [CrossRef]
- Banerjee, A.; Ghosh, P.; Roychoudhury, A. Salt acclimation differentially regulates the metabolites commonly involved in stress tolerance and aroma synthesis in indica rice cultivars. Plant Growth Regul. 2019, 88, 87–97. [Google Scholar] [CrossRef]
- Li, W.-H. Molecular Evolution; Sinauer Associates: Sunderland, MA, USA, 1997. [Google Scholar]
- Nordborg, M.; Donnelly, P. The coalescent process with selfing. Genetics 1997, 146, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.P.; Zhang, J.Z. The pleiotropic structure of the genotype-phenotype map: The evolvability of complex organisms. Nat. Rev. Genet. 2011, 12, 204–213. [Google Scholar] [CrossRef]
- Shivrain, V.K.; Burgos, N.R.; Anders, M.M.; Rajguru, S.N.; Moore, J.; Sales, M.A. Gene flow between Clearfield (TM) rice and red rice. Crop Prot. 2007, 26, 349–356. [Google Scholar] [CrossRef]
- Long, T.W.; Chen, H.S.; Leipe, C.; Wagner, M.; Tarasov, P.E. Modelling the chronology and dynamics of the spread of Asian rice from ca. 8000 BCE to 1000 CE. Quat. Int. 2022, 623, 101–109. [Google Scholar] [CrossRef]
- Gutaker, R.M.; Groen, S.C.; Bellis, E.S.; Choi, J.Y.; Pires, I.S.; Bocinsky, R.K.; Slayton, E.R.; Wilkins, O.; Castillo, C.C.; Negrao, S.; et al. Genomic history and ecology of the geographic spread of rice. Nat. Plants 2020, 6, 492–502. [Google Scholar] [CrossRef]
- Deng, Z.H.; Huang, B.X.; Zhang, Q.L.; Zhang, M. First farmers in the south China coast: New evidence from the Gancaoling site of Guangdong province. Front. Earth Sci. 2022, 10, 11. [Google Scholar] [CrossRef]
- Wang, W.W.; Nguyen, K.D.; Le, H.D.; Zhao, C.G.; Carson, M.T.; Yang, X.Y.; Hung, H.C. Before rice and the first rice: Archaeobotanical study in Ha Long Bay, Northern Vietnam. Front. Earth Sci. 2022, 10, 15. [Google Scholar]
- Wang, C.H.; Zheng, X.M.; Xu, Q.; Yuan, X.P.; Huang, L.; Zhou, H.F.; Wei, X.H.; Ge, S. Genetic diversity and classification of Oryza sativa with emphasis on Chinese rice germplasm. Heredity 2014, 112, 489–496. [Google Scholar] [CrossRef]
- Chang, T.T. Origin, evolution, cultivation, dissemination, and diversification of Asian and African rices. Euphytica 1976, 25, 425–441. [Google Scholar] [CrossRef]
- Bligh, H.F.J. Detection of adulteration of Basmati rice with non-premium long-grain rice. Int. J. Food Sci. Technol. 2000, 35, 257–265. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Singhal, R.S.; Kulkarni, P.R. Basmati rice: A review. Int. J. Food Sci. Technol. 2002, 37, 1–12. [Google Scholar] [CrossRef]
- Londo, J.P.; Chiang, Y.C.; Hung, K.H.; Chiang, T.Y.; Schaal, B.A. Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proc. Natl. Acad. Sci. USA 2006, 103, 9578–9583. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.M.; Ge, S. Ecological divergence in the presence of gene flow in two closely related Oryza species (Oryza rufipogon and O. nivara). Mol. Ecol. 2010, 19, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Platts, A.E.; Fuller, D.Q.; Hsing, Y.-I.; Wing, R.A.; Purugganan, M.D. The rice paradox: Multiple origins but single domestication in Asian rice. Mol. Biol. Evol. 2017, 34, 969–979. [Google Scholar] [CrossRef]
- Cortés, A.J.; Chavarro, M.C.; Madrinan, S.; This, D.; Blair, M.W. Molecular ecology and selection in the drought-related Asr gene polymorphisms in wild and cultivated common bean (Phaseolus vulgaris L.). BMC Genet. 2012, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Ramanantsoanirina, A.; Santos, J.D.; Frouin, J.; Radanielina, T. Evolutionary processes involved in the emergence and expansion of an atypical O. sativa group in Madagascar. Rice 2021, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Suvi, W.T.; Shimelis, H.; Laing, M. Breeding rice for rice yellow mottle virus resistance in Sub-Saharan Africa: A review. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2019, 69, 181–188. [Google Scholar] [CrossRef]
- Cruz, M.; Arbelaez, J.D.; Loaiza, K.; Cuasquer, J.; Rosas, J.; Graterol, E. Genetic and phenotypic characterization of rice grain quality traits to define research strategies for improving rice milling, appearance, and cooking qualities in Latin America and the Caribbean. Plant Genome 2021, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.J.; López-Hernández, F. Harnessing crop wild diversity for climate change adaptation. Genes 2021, 12, 783. [Google Scholar] [CrossRef]
- Ashikari, M.; Sakakibara, H.; Lin, S.Y.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef]
- Furukawa, T.; Maekawa, M.; Oki, T.; Suda, I.; Iida, S.; Shimada, H.; Takamure, I.; Kadowaki, K.I. The Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant J. 2007, 49, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Wu, S.; Zhu, Z.F.; Liu, F.X.; Fu, Y.C.; Cai, H.W.; Sun, X.Y.; Gu, P.; Xie, D.X.; Tan, L.B.; et al. NOG1 increases grain production in rice. Nat. Commun. 2017, 8, 11. [Google Scholar] [CrossRef]
- Joo, J.; Choi, H.J.; Lee, Y.H.; Kim, Y.K.; Song, S.I. A transcriptional repressor of the ERF family confers drought tolerance to rice and regulates genes preferentially located on chromosome 11. Planta 2013, 238, 155–170. [Google Scholar] [CrossRef]
- Swetha, C.; Basu, D.; Pachamuthu, K.; Tirumalai, V.; Nair, A.; Prasad, M.; Shivaprasad, P.V. Major domestication-related phenotypes in Indica rice are due to loss of miRNA-mediated laccase silencing. Plant Cell 2018, 30, 2649–2662. [Google Scholar] [CrossRef]
- Ashikari, M.; Sasaki, A.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Datta, S.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Loss-of-function of a rice gibberellin biosynthetic gene, GA20 oxidase (GA20ox-2), led to the rice ’green revolution’. Breed. Sci. 2002, 52, 143–150. [Google Scholar] [CrossRef]
- Chen, J.; Gao, H.; Zheng, X.M.; Jin, M.N.; Weng, J.F.; Ma, J.; Ren, Y.L.; Zhou, K.N.; Wang, Q.; Wang, J.; et al. An evolutionarily conserved gene, FUWA, plays a role in determining panicle architecture, grain shape and grain weight in rice. Plant J. 2015, 83, 427–438. [Google Scholar] [CrossRef]
- Song, X.J.; Huang, W.; Shi, M.; Zhu, M.Z.; Lin, H.X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nature Genet. 2007, 39, 623–630. [Google Scholar] [CrossRef]
- Wu, C.Y.; Trieu, A.; Radhakrishnan, P.; Kwok, S.F.; Harris, S.; Zhang, K.; Wang, J.L.; Wan, J.M.; Zhai, H.Q.; Takatsuto, S.; et al. Brassinosteroids regulate grain filling in rice. Plant Cell 2008, 20, 2130–2145. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, H.; Zhao, M.C.; Yang, Z.A.; Zhou, Z.H.; Guo, Y.M.; Lin, Y.J.; Chen, H. OsMYB3 is a R2R3-MYB gene responsible for anthocyanin biosynthesis in black rice. Mol. Breed. 2021, 41, 15. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Fang, J.; Ou, S.J.; Gao, S.P.; Zhang, F.X.; Du, L.; Xiao, Y.H.; Wang, H.R.; Sun, X.H.; Chu, J.F.; et al. Variations in CYP78A13 coding region influence grain size and yield in rice. Plant Cell Environ. 2015, 38, 800–811. [Google Scholar] [CrossRef]
- Dong, H.J.; Zhao, H.; Xie, W.B.; Han, Z.M.; Li, G.W.; Yao, W.; Bai, X.F.; Hu, Y.; Guo, Z.L.; Lu, K.; et al. A novel tiller angle gene, TAC3, together with TAC1 and D2 largely determine the natural variation of tiller angle in rice cultivars. PLoS Genet. 2016, 12. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shomura, A.; Sasaki, T.; Yano, M. Hd6, a rice quantitative trait locus involved in photoperiod sensitivity, encodes the alpha subunit of protein kinase CK2. Proc. Natl. Acad. Sci. USA 2001, 98, 7922–7927. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhao, B.R.; Yuan, D.Y.; Duan, M.J.; Qian, Q.; Tang, L.; Wang, B.; Liu, X.Q.; Zhang, J.; Wang, J.; et al. Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc. Natl. Acad. Sci. USA 2013, 110, 3167–3172. [Google Scholar] [CrossRef]
- Shih, C.H.; Chu, H.; Tang, L.K.; Sakamoto, W.; Maekawa, M.; Chu, I.K.; Wang, M.; Lo, C. Functional characterization of key structural genes in rice flavonoid biosynthesis. Planta 2008, 228, 1043–1054. [Google Scholar] [CrossRef]
- Wang, E.; Wang, J.; Zhu, X.D.; Hao, W.; Wang, L.Y.; Li, Q.; Zhang, L.X.; He, W.; Lu, B.R.; Lin, H.X.; et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nature Genet. 2008, 40, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yun, K.Y.; Ressom, H.W.; Mohanty, B.; Bajic, V.B.; Jia, Y.L.; Yun, S.J.; de los Reyes, B.G. An early response regulatory cluster induced by low temperature and hydrogen peroxide in seedlings of chilling-tolerant japonica rice. BMC Genomics 2007, 8, 18. [Google Scholar] [CrossRef]
- Gu, B.G.; Zhou, T.Y.; Luo, J.H.; Liu, H.; Wang, Y.C.; Shangguan, Y.Y.; Zhu, J.J.; Li, Y.; Sang, T.; Wang, Z.X.; et al. An-2 encodes a cytokinin synthesis enzyme that regulates awn length and grain production in rice. Mol. Plant. 2015, 8, 1635–1650. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.C.; Xiao, Y.H.; Niu, M.; Meng, W.J.; Li, L.L.; Zhang, X.X.; Liu, D.P.; Zhang, G.X.; Qian, Y.W.; Sun, Z.T.; et al. ARGONAUTE2 enhances grain length and salt tolerance by activating BIG GRAIN3 to modulate cytokinin distribution in rice. Plant Cell 2020, 32, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tanaka, K.; Kuwano, M.; Yoshida, K.T. Expression pattern of inositol phosphate-related enzymes in rice (Oryza sativa L.): Implications for the phytic acid biosynthetic pathway. Gene 2007, 405, 55–64. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.J.; Kim, B.G.; Lim, Y.; Ahn, J.H. Flavanone 3 beta-hydroxylases from rice: Key enzymes for favonol and anthocyanin biosynthesis. Mol. Cells 2008, 25, 312–316. [Google Scholar]
- Li, C.B.; Zhou, A.L.; Sang, T. Rice domestication by reducing shattering. Science 2006, 311, 1936–1939. [Google Scholar] [CrossRef]
- Li, Y.B.; Fan, C.C.; Xing, Y.Z.; Jiang, Y.H.; Luo, L.J.; Sun, L.; Shao, D.; Xu, C.J.; Li, X.H.; Xiao, J.H.; et al. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nature Genet. 2011, 43, 1266–1269. [Google Scholar] [CrossRef]
- Iwai, T.; Miyasaka, A.; Seo, S.; Ohashi, Y. Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiol. 2006, 142, 1202–1215. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Kim, S.L.; Choi, H.; Koh, H.J.; An, G. The BEL1-type homeobox gene SH5 induces seed shattering by enhancing abscission-zone development and inhibiting lignin biosynthesis. Plant J. 2014, 79, 717–728. [Google Scholar] [CrossRef]
- Li, H.W.; Zang, B.S.; Deng, X.W.; Wang, X.P. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011, 234, 1007–1018. [Google Scholar] [CrossRef]
- Xu, J.W.; Feng, D.J.; Li, X.G.; Chang, T.J.; Zhu, Z. Cloning of genomic DNA of rice 5-enolpyruvylshikimate 3-phosphate synthase gene and chromosomal localization of the gene. Sci. China Ser. C-Life Sci. 2002, 45, 251–259. [Google Scholar] [CrossRef]
- Kojima, S.; Takahashi, Y.; Kobayashi, Y.; Monna, L.; Sasaki, T.; Araki, T.; Yano, M. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 2002, 43, 1096–1105. [Google Scholar] [CrossRef]
- Reddy, V.S.; Scheffler, B.E.; Wienand, U.; Wessler, S.R.; Reddy, A.R. EMBL accession #: Y15219. Plant Mol.Biol. 1998, 36, 497–498. [Google Scholar]
- Liu, Y.; Wang, H.; Jiang, Z.; Wang, W.; Xu, R.; Wang, Q.; Zhang, Z.; Li, A.; Liang, Y.; Ou, S.; et al. Genomic basis of geographical adaptation to soil nitrogen in rice. Nature 2021, 590, 600–605. [Google Scholar] [CrossRef]
- Yano, M.; Katayose, Y.; Ashikari, M.; Yamanouchi, U.; Monna, L.; Fuse, T.; Baba, T.; Yamamoto, K.; Umehara, Y.; Nagamura, Y.; et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 2000, 12, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Huang, W.; Gao, J.P.; Yang, J.; Shi, M.; Zhu, M.Z.; Luo, D.; Lin, H.X. Genetic control of rice plant architecture under domestication. Nature Genet. 2008, 40, 1365–1369. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Thomson, M.J.; Pfeil, B.E.; McCouch, S. Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. Plant Cell 2006, 18, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Si, L.Z.; Chen, J.Y.; Huang, X.H.; Gong, H.; Luo, J.H.; Hou, Q.Q.; Zhou, T.Y.; Lu, T.T.; Zhu, J.J.; Shangguan, Y.Y.; et al. OsSPL13 controls grain size in cultivated rice. Nature Genet. 2016, 48, 447–456. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, X.F.; Cheng, N.N.; Xiao, J.H.; Li, X.H.; Xing, Y.Z. Wide Grain 7 increases grain width by enhancing H3K4me3 enrichment in the OsMADS1 promoter in rice (Oryza sativa L.). Plant J. 2020, 102, 517–528. [Google Scholar] [CrossRef]
- Bessho-Uehara, K.; Wang, D.R.; Furuta, T.; Minami, A.; Nagai, K.; Gamuyao, R.; Asano, K.; Angeles-Shim, R.B.; Shimizu, Y.; Ayano, M.; et al. Loss of function at RAE2, a previously unidentified EPFL, is required for awnlessness in cultivated Asian rice. Proc. Natl. Acad. Sci. USA 2016, 113, 8969–8974. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.Q.; Wang, S.S.; Wang, Y.; Chen, X.B.; Zhang, Y.; Gao, C.X.; et al. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nature Genet. 2015, 47, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Qian, Q.; Liu, Z.B.; Sun, H.Y.; He, S.Y.; Luo, D.; Xia, G.M.; Chu, C.C.; Li, J.Y.; Fu, X.D. Natural variation at the DEP1 locus enhances grain yield in rice. Nature Genet. 2009, 41, 494–497. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, J.; Dardick, C.; Seo, Y.S.; Cao, P.; Canlas, P.; Phetsom, J.; Xu, X.; Ouyang, S.; An, K.; et al. Identification and functional analysis of light-responsive unique genes and gene family members in rice. PLoS Genet. 2008, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ashikari, M.; Miura, K.; Yamashino, T.; Mizuno, T. The evolutionarily conserved OsPRR quintet: Rice pseudo-response regulators implicated in circadian rhythm. Plant Cell Physiol. 2003, 44, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A.R. Shikimate and phenylalanine biosynthesis in the green lineage. Front. Plant Sci. 2013, 4, 13. [Google Scholar] [CrossRef]
- Cai, Q.; Yuan, Z.; Chen, M.J.; Yin, C.S.; Luo, Z.J.; Zhao, X.X.; Liang, W.Q.; Hu, J.P.; Zhang, D.B. Jasmonic acid regulates spikelet development in rice. Nat. Commun. 2014, 5, 13. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Gao, P.; Xing, Z.; Jin, S.M.; Chen, Z.D.; Liu, L.T.; Constantino, N.; Wang, X.W.; Shi, W.B.; Yuan, J.S.; et al. Application of an improved proteomics method for abundant protein cleanup: Molecular and genomic mechanisms study in plant defense. Mol. Cell. Proteom. 2013, 12, 3431–3442. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Taylor, J.M.; Spriggs, A.; Zhang, H.Y.; Wu, X.J.; Russell, S.; Singh, M.; Koltunow, A. A genome-wide survey of imprinted genes in rice seeds reveals imprinting primarily occurs in the endosperm. PLoS Genet. 2011, 7, 14. [Google Scholar] [CrossRef]
- Reddy, A.R.; Scheffler, B.; Madhuri, G.; Srivastava, M.N.; Kumar, A.; Sathyanarayanan, P.V.; Nair, S.; Mohan, M. Chalcone synthase in rice (Oryza sativa L.): Detection of the CHS protein in seedlings and molecular mapping of the chs locus. Plant Mol. Biol. 1996, 32, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Bryan, G.T.; Wu, K.S.; Farrall, L.; Jia, Y.L.; Hershey, H.P.; McAdams, S.A.; Faulk, K.N.; Donaldson, G.K.; Tarchini, R.; Valent, B. A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 2000, 12, 2033–2045. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).