Riboflavin 1 Transporter Deficiency: Novel SLC52A1 Variants and Expansion of the Phenotypic Spectrum
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetic Testing
2.2. Protein Modelling
3. Results
3.1. Case Presentations
3.1.1. Case 1
3.1.2. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin Deficiency-Implications for General Human Health and Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef] [PubMed]
- Chiong, M.A.; Sim, K.G.; Carpenter, K.; Rhead, W.; Ho, G.; Olsen, R.K.J.; Christodoulou, J. Transient multiple acyl-CoA dehydrogenation deficiency in a newborn female caused by maternal riboflavin deficiency. Mol. Genet. Metab. 2007, 92, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Prasun, P. Multiple Acyl-CoA Dehydrogenase Deficiency. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Grünert, S.C. Clinical and genetical heterogeneity of late-onset multiple acyl-coenzyme A dehydrogenase deficiency. Orphanet J. Rare Dis. 2014, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Mereis, M.; Wanders, R.J.A.; Schoonen, M.; Dercksen, M.; Smuts, I.; van der Westhuizen, F.H. Disorders of flavin adenine dinucleotide metabolism: MADD and related deficiencies. Int. J. Biochem. Cell Biol. 2021, 132, 105899. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, A.; Masuda, S.; Katsura, T.; Inui, K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am. J. Physiol. Cell Physiol. 2008, 295, C632–C641. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, B.; Bosch, A.M.; Houlden, H. An update on the genetics, clinical presentation, and pathomechanisms of human riboflavin transporter deficiency. J. Inherit. Metab. Dis. 2019, 42, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Mosegaard, S.; Bruun, G.H.; Flyvbjerg, K.F.; Bliksrud, Y.T.; Gregersen, N.; Dembic, M.; Annexstad, E.; Tangeraas, T.; Olsen, R.K.J.; Andresen, B.S. An intronic variation in SLC52A1 causes exon skipping and transient riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Mol. Genet. Metab. 2017, 122, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Yonezawa, A.; Masuda, S.; Inui, K.; Sim, K.G.; Carpenter, K.; Olsen, R.K.; Mitchell, J.J.; Rhead, W.J.; Peters, G.; et al. Maternal riboflavin deficiency, resulting in transient neonatal-onset glutaric aciduria Type 2, is caused by a microdeletion in the riboflavin transporter gene GPR172B. Human Mutat. 2011, 32, E1976–E1984. [Google Scholar] [CrossRef] [PubMed]
- Kang, U.; Yang, D.H.; Nam, S.O.; Lee, Y.; Yeon, G.M. Riboflavin Transporter 1 Deficiency Caused by a Homozygous Single Exonal Deletion of SLC52A1. Ann. Child Neurol. 2020, 28, 160–163. [Google Scholar] [CrossRef]
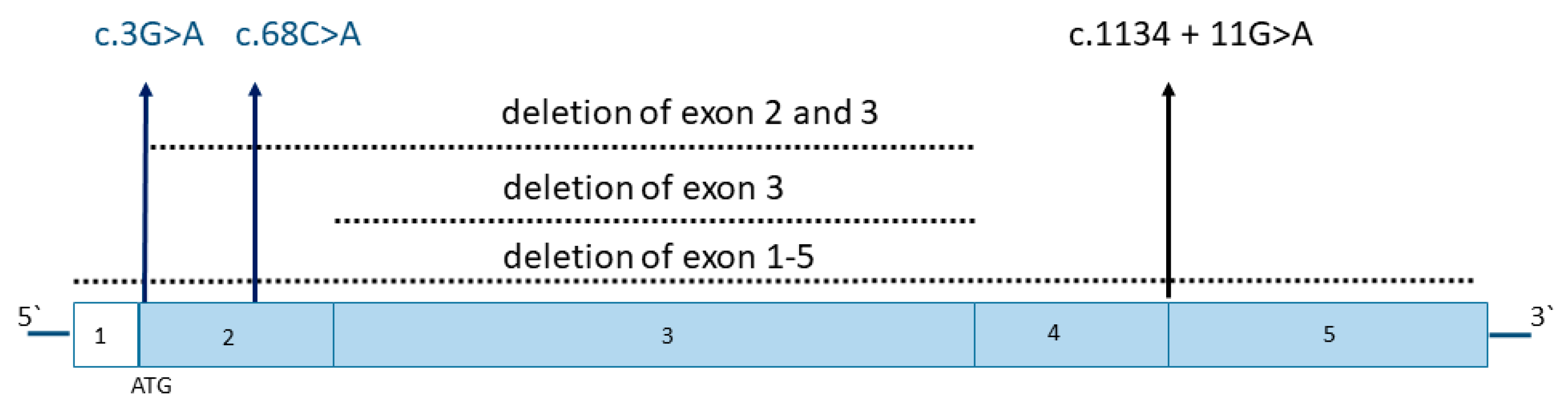

| Patient | Genetic Variant | Symptoms | References |
|---|---|---|---|
| #1 | heterozygous variant c.68C > A, p. Ser23Tyr | episode of mild hyperammonemia following gastroenteritis | this report |
| #2 | heterozygous variant c.3G > A, p. Met1Ile | infantile seizures (hypsarrhythmia) at 7 months, mild motor delay | this report |
| #3 | heterozygous de novo deletion spanning exons 2 and 3 | mild MADD profile, but no clinical symptoms, transient MADD-like clinical and biochemical picture in newborn child of this woman (genetic variant not present in the child) | Chiong et al. [2], Ho et al. [9] |
| #4 (mother of patient #5) | heterozygous variant c.1134 + 11G > A, causing exon 4 skipping | neither clinical nor biochemical symptoms, borderline low riboflavin levels after parturition | Mosegaard et al. [8] |
| #5 (child of patient #4) | heterozygous variant c.1134 + 11G > A, causing exon 4 skipping | MADD-like clinical and biochemical picture in the newborn child, corrected with riboflavin supplementation | Mosegaard et al. [8] |
| #6 (child of #7) | homozygous exon 3 deletion and heterozygous deletions in exons 1, 2, 4, and 5 | recurrent seizures at 4 months, normal development under riboflavin treatment | Kang et al. [10] |
| #7 (father of #6) | heterozygous deletions in exons 1–5 | no clinical symptoms | Kang et al. [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grünert, S.C.; Ziagaki, A.; Heinen, A.; Schumann, A.; Tucci, S.; Spiekerkoetter, U.; Schmidts, M. Riboflavin 1 Transporter Deficiency: Novel SLC52A1 Variants and Expansion of the Phenotypic Spectrum. Genes 2023, 14, 1408. https://doi.org/10.3390/genes14071408
Grünert SC, Ziagaki A, Heinen A, Schumann A, Tucci S, Spiekerkoetter U, Schmidts M. Riboflavin 1 Transporter Deficiency: Novel SLC52A1 Variants and Expansion of the Phenotypic Spectrum. Genes. 2023; 14(7):1408. https://doi.org/10.3390/genes14071408
Chicago/Turabian StyleGrünert, Sarah C., Athanasia Ziagaki, André Heinen, Anke Schumann, Sara Tucci, Ute Spiekerkoetter, and Miriam Schmidts. 2023. "Riboflavin 1 Transporter Deficiency: Novel SLC52A1 Variants and Expansion of the Phenotypic Spectrum" Genes 14, no. 7: 1408. https://doi.org/10.3390/genes14071408
APA StyleGrünert, S. C., Ziagaki, A., Heinen, A., Schumann, A., Tucci, S., Spiekerkoetter, U., & Schmidts, M. (2023). Riboflavin 1 Transporter Deficiency: Novel SLC52A1 Variants and Expansion of the Phenotypic Spectrum. Genes, 14(7), 1408. https://doi.org/10.3390/genes14071408







