A Novel KCNN2 Variant in a Family with Essential Tremor Plus: Clinical Characteristics and In Silico Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Investigation
2.2. FMR1 Analysis
2.3. Exome Investigation
2.4. Sanger Sequencing
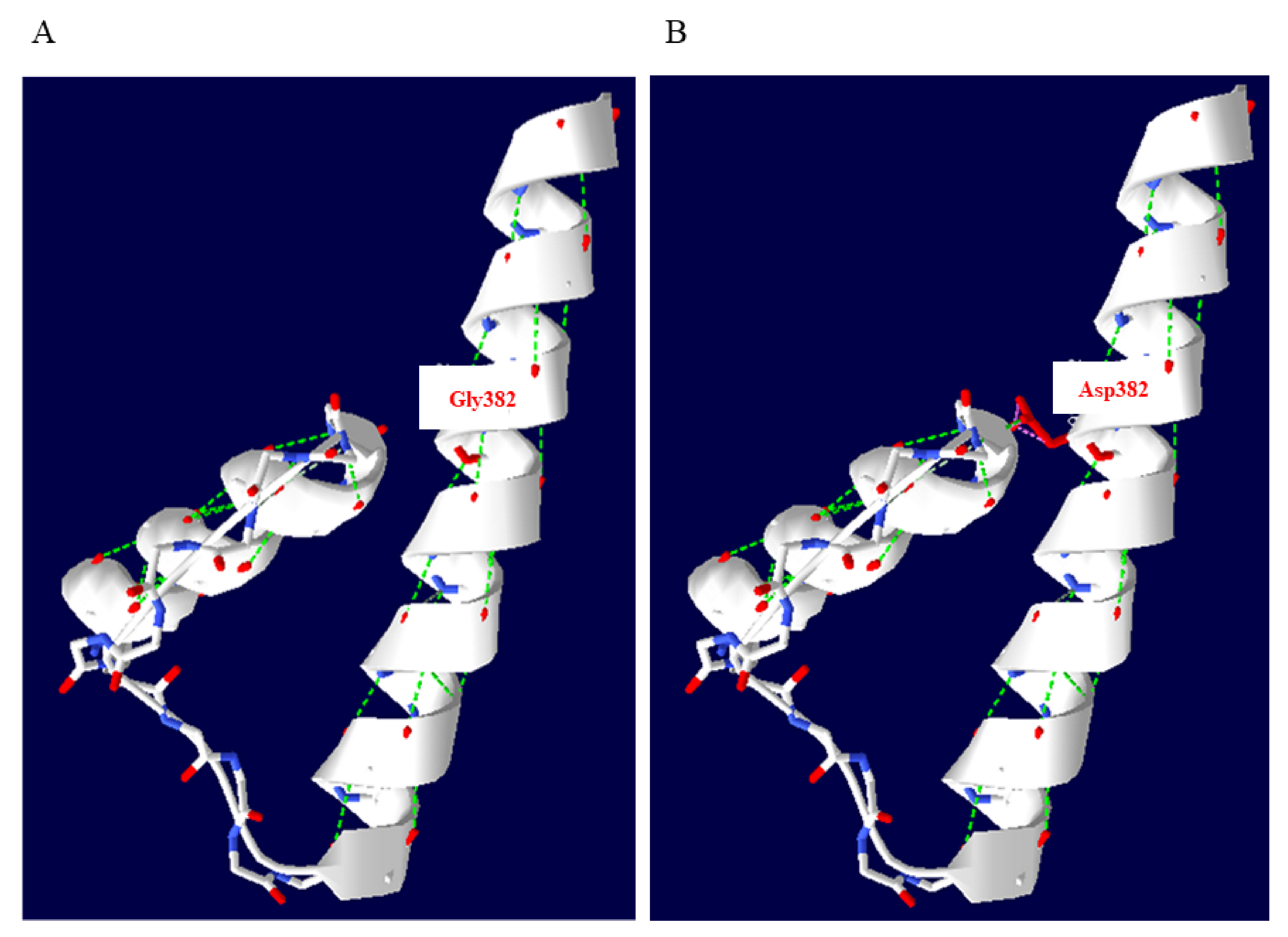
2.5. In Silico Analysis and Protein Structure Modeling
3. Results
3.1. Proband Clinical Characteristics
3.2. Pedigree Investigation
3.3. Genetic Studies
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lou, J.S.; Jankovic, J. Essential tremor, clinical correlates in 350 patients. Neurology 1991, 41, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Tallón-Barranco, A.; Vázquez, A.; Jiménez-Jiménez, F.J.; Ortí-Pareja, M.; Gasalla, T.; Cabrera-Valdivia, F.; Benito-León, J.; Molina, J.A. Clinical features of essential tremor seen in neurology practice, a study of 357 patients. Park. Relat. Disord. 1997, 3, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D. Tremor. Continuum 2019, 25, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; McCreary, M. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Tremor Other Hyperk. Mov. 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Helmich, R.C.; Toni, I.; Deuschl, G.; Bloem, B.R. The pathophysiology of essential tremor and Parkinson’s tremor. Curr. Neurol. Neurosci. 2013, 13, 378. [Google Scholar] [CrossRef]
- Louis, E.D. A new twist for stopping the shakes? Revisiting GABAergic therapy for essential tremor. Arch. Neurol. 1999, 56, 807–808. [Google Scholar] [CrossRef]
- Gironell, A. The GABA Hypothesis in Essential Tremor: Lights and Shadows. Tremor Other Hyperk. Mov. 2014, 4, 254. [Google Scholar] [CrossRef]
- Martin, F.C.; Le, A.T.; Handforth, A. Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov. Disord. 2005, 20, 298–305. [Google Scholar] [CrossRef]
- Park, Y.-G.; Park, H.-Y.; Lee, C.J.; Choi, S.; Jo, S.; Choi, H.; Kim, Y.-H.; Shin, H.-S.; Llinas, R.R.; Kim, D. CaV3.1 is a tremor rhythm pacemaker in the inferior olive. Proc. Natl. Acad. Sci. USA 2010, 107, 10731–10736. [Google Scholar] [CrossRef]
- Higgins, J.J.; Pho, L.T.; Nee, L.E. A gene (ETM) for essential tremor maps to chromosome 2p22–p25. Mov. Disord. 1997, 12, 859–864. [Google Scholar] [CrossRef]
- Gulcher, J.R.; Jonson, P.; Kong, A.; Kristjánsson, K.; Frigge, M.L.; Kárason, A.; Einarsdóttir, I.E.; Stefánsson, H.; Einarsdóttir, A.S.; Sigurthoardóttir, S.; et al. Mapping of a familial essential tremor gene, FET1, to chromosome 3q13. Nat. Genet. 1997, 17, 84–87. [Google Scholar] [CrossRef]
- Hicks, J.E.; Konidari, I.; Scott, B.L.; Stajich, J.M.; Ashley-Koch, A.E.; Gilbert, J.R.; Scott, W.K. Linkage of familial essential tremor to chromosome 5q35. Mov. Disord. 2016, 31, 1059–1062. [Google Scholar] [CrossRef]
- Shatunov, A.; Sambuughin, N.; Jankovic, J.; Elble, R.; Lee, H.S.; Singleton, A.B.; Dagvadorj, A.; Ji, J.; Zhang, Y.; Kimonis, V.E.; et al. Genomewide scans in North American families reveal genetic linkage of essential tremor to a region on chromosome 6p23. Brain 2006, 129, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, H.; Steinberg, S.; Petursson, H.; Gustafsson, O.; Gudjonsdottir, I.H.; Jonsdottir, G.A.; Palsson, S.T.; Jonsson, T.; Saemundsdottir, J.; Bjornsdottir, G.; et al. Variant in the sequence of the LINGO1 gene confers risk of essential tremor. Nat. Genet. 2009, 41, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Their, S.; Lorenz, D.; Nothnagel, M.; Poremba, C.; Papengut, F.; Appenzeller, S.; Paschen, S.; Hofschulte, F.; Hussl, A.C.; Hering, S.; et al. Polymorphisms in the glial glutamate transporter SLC1A2 are associated with essential tremor. Neurology 2012, 79, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.H.; Girard, S.L.; Hopfner, F.; Merner, N.D.; Bourassa, C.V.; Lorenz, D.; Clark, L.N.; Tittmann, L.; Soto-Ortolaza, A.I.; Klebe, S.; et al. Genome-wide association study in essential tremor identifies three new loci. Brain 2016, 139, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Merner, N.D.; Girard, S.L.; Catoire, H.; Bourassa, C.V.; Belzil, V.V.; Rivière, J.B.; Hince, P.; Levert, A.; Dionne-Laporte, A.; Spiegelman, D.; et al. Exome sequencing identifies FUS mutations as a cause of essential tremor. Am. J. Hum. Genet. 2012, 91, 313–319. [Google Scholar] [CrossRef]
- Unal Gulsuner, H.; Gulsuner, S.; Mercan, F.N.; Onat, O.E.; Walsh, T.; Shahin, H.; Lee, M.K.; Dogu, O.; Kansu, T.; Topaloglu, H.; et al. Mitochondrial serine protease HTRA2 p.G399S in a kindred with essential tremor and Parkinson disease. Proc. Natl. Acad. Sci. USA 2014, 111, 18285–18290. [Google Scholar] [CrossRef]
- Hor, H.; Francescatto, L.; Bartesaghi, L.; Ortega-Cubero, S.; Kousi, M.; Lorenzo-Betancor, O.; Jiménez-Jiménez, F.J.; Gironell, A.; Clarimón, J.; Drechsel, O.; et al. Missense mutations in TENM4, a regulator of axon guidance and central myelination, cause essential tremor. Hum. Mol. Genet. 2015, 24, 5677–5686. [Google Scholar] [CrossRef]
- Sánchez, E.; Bergareche, A.; Krebs, C.E.; Gorostidi, A.; Makarov, V.; Ruiz-Martinez, J.; Chorny, A.; Lopez de Munain, A.; Marti Masso, J.F.; Paisán-Ruiz, C. SORT1 Mutation resulting in sortilin deficiency and p75(NTR) upregulation in a family with essential tremor. ASN Neuro 2015, 7, 1759091415598290. [Google Scholar] [CrossRef]
- Leng, X.R.; Qi, X.H.; Zhou, Y.T.; Wang, Y.P. Gain-of-function mutation p.Arg225Cys in SCN11A causes familial episodic pain and contributes to essential tremor. J. Hum. Genet. 2017, 62, 641–646. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Xu, Q.; Tian, Y.; Hu, Z.M.; Qin, L.X.; Yang, J.X.; Huang, W.; Xue, J.; Li, J.C.; Zeng, S.; et al. Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain 2020, 143, 222–233. [Google Scholar] [CrossRef]
- Odgerel, Z.; Sonti, S.; Hernandez, N.; Park, J.; Ottman, R.; Louis, E.D.; Clark, L.N. Whole genome sequencing and rare variant analysis in essential tremor families. PLoS ONE 2019, 14, e0220512. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hernandez, N.; Kisselev, S.; Floratos, A.; Sawle, A.; Ionita-Laza, I.; Ottman, R.; Louis, E.D.; Clark, L.N. Identification of candidate genes for familial early-onset essential tremor. Eur. J. Hum. Genet. 2016, 24, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Diez-Fairen, M.; Houle, G.; Ortega-Cubero, S.; Bandres-Ciga, S.; Alvarez, I.; Carcel, M.; Ibañez, L.; Fernandez, M.V.; Budde, J.P.; Trotta, J.R.; et al. Exome-wide rare variant analysis in familial essential tremor. Park. Relat. Disord. 2021, 82, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Álvarez, I.; Pastor, P.; Agúndez, J.A.G. Genomic Markers for Essential Tremor. Pharmaceuticals 2021, 14, 516. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, D.; Raethjen, J.; Maria Stamelou, M.; Testa, C.M.; Guenther, D. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef]
- Ariano, A.; D’Apolito, M.; Bova, M.; Bellanti, F.; Loffredo, S.; D’Andrea, G.; Intrieri, M.; Petraroli, A.; Maffione, A.B.; Spadaro, G.; et al. A myoferlin gain-of-function variant associates with a new type of hereditary angioedema. Allergy 2020, 75, 2989–2992. [Google Scholar] [CrossRef]
- Sue, R.; Nazneen, A.; Sherri, B.; David, B.; Soma, D.; Gastier-Foster, J.; Grody, W.W.; Madhuri, H.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar]
- D’Andrea, G.; Colaizzo, D.; Vecchione, G.; Grandone, E.; Di Minno, G.; Margaglione, M.; GLAnzmann’s Thrombasthenia Italian Team (GLATIT). Glanzmann’s thrombasthenia: Identification of 19 new mutations in 30 patients. Thromb. Haemost. 2002, 87, 1034–1042. [Google Scholar] [CrossRef]
- Park, Y.-G.; Jang, W.; Youn, J.; Ki, C.-S.; Kim, B.J.; Kim, H.T.; Louis, E.D.; Cho, J.W. Prevalence of fragile X-associated tremor/ataxia syndrome: A survey of essential tremor patients with cerebellar signs or extrapyramidal signs. Brain Behav. 2019, 9, e01337. [Google Scholar] [CrossRef]
- Desai, R.; Peretz, A.; Idelson, H.; Lazarovici, P.; Attali, B. Ca2+-activated K+ channels in human leukemic Jurkat T cells. Molecular cloning, biochemical and functional characterization. J. Biol. Chem. 2000, 275, 39954–39963. [Google Scholar] [CrossRef]
- Ishii, T.M.; Maylie, J.; Adelman, J.P. Determinants of apamin and d-tubocurarine block in SK potassium channels. J. Biol. Chem. 1997, 272, 23195–23200. [Google Scholar] [CrossRef] [PubMed]
- Faber, L. Functions and modulation of neuronal SK channels. Cell Biochem. Biophys. 2009, 55, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.T.; Herson, P.S.; Strassmaier, T.; Hammond, R.; Stackman, R.; Maylie, J.; Adelman, J.P. Small Conductance Ca2+-Activated K Channel Knock-Out Mice Reveal the Identity of Calcium-Dependent after Hyperpolarization Currents. J. Neurosci. 2004, 24, 5301–5306. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; MacKinnon, R. Activation mechanism of a human SK-calmodulin channel complex elucidated by cryo-EM structures. Science 2018, 360, 508–513. [Google Scholar] [CrossRef]
- Balinta, B.; Guerreiroc, R.; Carmonac, S.; Dehghanic, N.; Latorrea, A.; Cordivari, C. KCNN2 mutation in autosomal-dominant tremulous myoclonus-dystonia. Eur. J. Neurol. 2020, 27, 1471–1477. [Google Scholar] [CrossRef]
- Mochel, F.; Rastetter, A.; Ceulemans, B.; Platzer, K.; Yang, S.; Shinde, D.N.; Helbig, K.L.; Lopergolo, D.; Mari, F.; Renieri, A.; et al. Variants in the SK2 channel gene (KCNN2) lead to dominant neurodevelopmental movement disorders. Brain 2020, 143, 3564–3573. [Google Scholar] [CrossRef]
- Takashi, K.; Mayuko, Y.; Naofumi, K.; Kana, O.; Takahito, M.; Yuki, H.; Kazuto, Y.; Tomoji, M.; Miyuu, T.; Mitusru, K.; et al. Tremor dominant Kyoto (Trdk) rats carry a missense mutation in the gene encoding the SK2 subunit of small-conductance Ca2+-activated K+ channel. Brain Res. 2017, 1676, 38–45. [Google Scholar]

| V-1 (Index Case) | IV-3 | IV-5 | III-3 | |
|---|---|---|---|---|
| Sex, age (years) | M, 15 | F, 42 | M, 40 | F, 70 |
| Age at onset (years), First symptom | 14 upper extremity tremors—anxiety | 20 upper extremity tremors—anxiety | 15 upper extremity tremors—anxiety | 20 upper extremity tremors—anxiety |
| X Examination | -Upper extremity tremors -Worse impairment in the visual–spatial domain -Anxiety | -Upper extremity tremors -Worse impairment in the visual–spatial domain -Anxiety -Depression | -Upper extremity tremors -Anxiety | -Upper extremity tremors -Lewy body dementia (LBD) |
| Gene | Nucleotide | Amino Acid Change | SIFT | PoliPhen | CADD | REVEL | MetaLR | Mutation Assessor |
|---|---|---|---|---|---|---|---|---|
| KCNN2 | GGT/GAT | G382D | 0 | 0.969 | 32 | 0.709 | 0.58 | 0.948 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Apolito, M.; Ceccarini, C.; Savino, R.; Adipietro, I.; di Bari, I.; Santacroce, R.; Curcetti, M.; D’Andrea, G.; Croce, A.-I.; Cesarano, C.; et al. A Novel KCNN2 Variant in a Family with Essential Tremor Plus: Clinical Characteristics and In Silico Analysis. Genes 2023, 14, 1380. https://doi.org/10.3390/genes14071380
d’Apolito M, Ceccarini C, Savino R, Adipietro I, di Bari I, Santacroce R, Curcetti M, D’Andrea G, Croce A-I, Cesarano C, et al. A Novel KCNN2 Variant in a Family with Essential Tremor Plus: Clinical Characteristics and In Silico Analysis. Genes. 2023; 14(7):1380. https://doi.org/10.3390/genes14071380
Chicago/Turabian Styled’Apolito, Maria, Caterina Ceccarini, Rosa Savino, Iolanda Adipietro, Ighli di Bari, Rosa Santacroce, Maria Curcetti, Giovanna D’Andrea, Anna-Irma Croce, Carla Cesarano, and et al. 2023. "A Novel KCNN2 Variant in a Family with Essential Tremor Plus: Clinical Characteristics and In Silico Analysis" Genes 14, no. 7: 1380. https://doi.org/10.3390/genes14071380
APA Styled’Apolito, M., Ceccarini, C., Savino, R., Adipietro, I., di Bari, I., Santacroce, R., Curcetti, M., D’Andrea, G., Croce, A.-I., Cesarano, C., Polito, A. N., & Margaglione, M. (2023). A Novel KCNN2 Variant in a Family with Essential Tremor Plus: Clinical Characteristics and In Silico Analysis. Genes, 14(7), 1380. https://doi.org/10.3390/genes14071380






