Polymorphism, Genetic Effect, and Association with Egg-Laying Performance of Chahua Chickens Matrix Metalloproteinases 13 Promoter
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimentation Ethical Statement
2.2. Experimental Animal, Sample Collection, and Preparation
2.3. PCR Amplification and Sequencing
2.4. Construction of Fluorescent Dual Reporter Vector
2.5. Double Fluorescence Report Detection
2.6. RT-qPCR
2.7. Western Blot
2.8. Data Statistics and Analysis
3. Results
3.1. Correlation Analysis of Egg-Laying Performance in Chahua Chickens
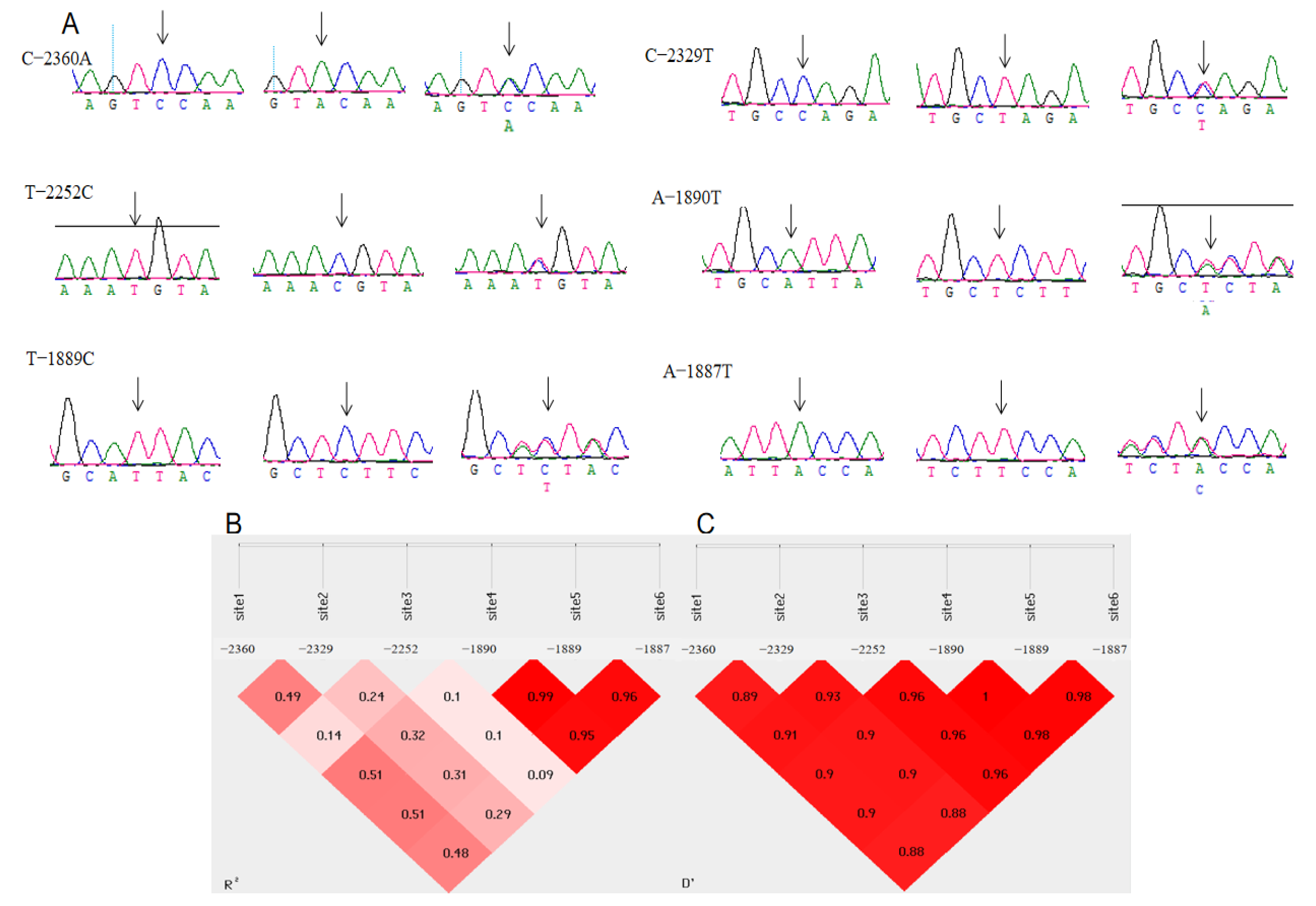
3.2. Polymorphisms of the 5′ Regulatory Region in Chahua Chickens’ MMP13 Gene
3.3. Analysis of the Association of Different Genotypes of the MMP13 Gene with the Egg-Laying Performance of Chahua Chickens
3.4. Analysis of the Association of Different Haplotypes of the MMP13 Gene with Egg-Laying Performance of Chahua Chicken
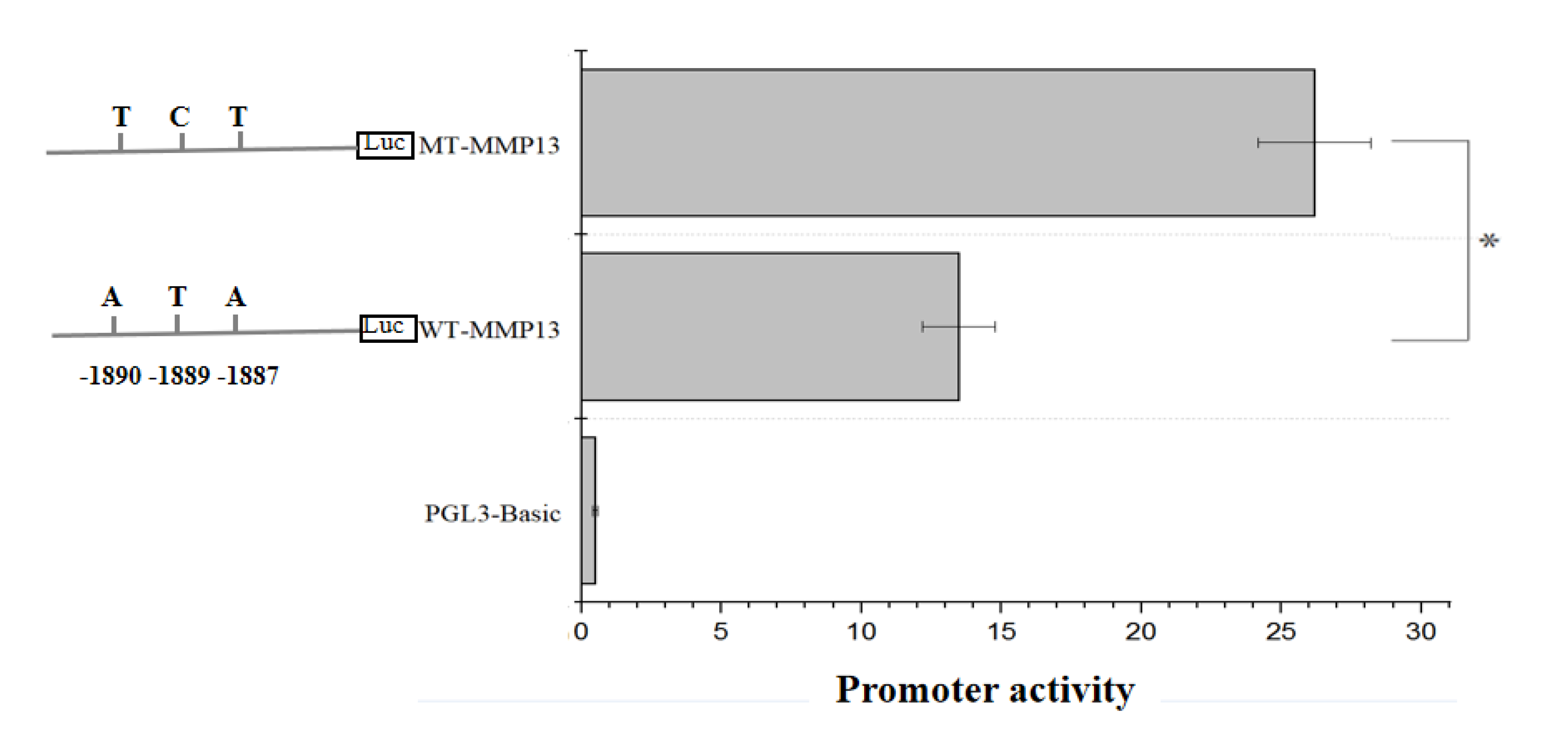
3.5. The Effect of SNPs on the Promoter Activity of the MMP13 Gene
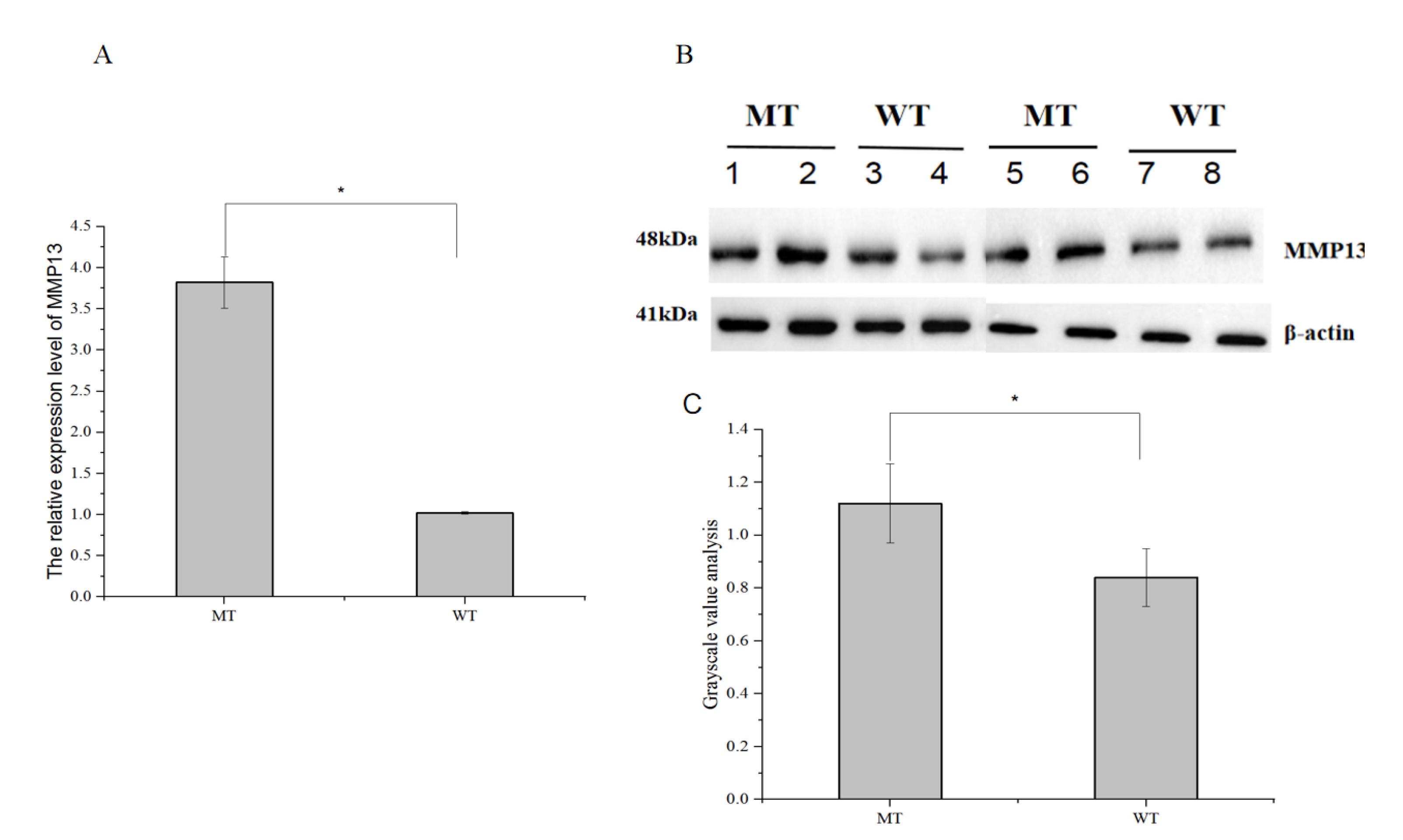
3.6. Analysis of the MMP13 Gene Expression Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zhao, Z.; Xiang, D.; Zhang, B.; Ning, T.; Duan, T.; Rao, J.; Yang, L.; Zhang, X.; Xiong, F. Expression of APOB, ADFP and FATP1 and their correlation with fat deposition in Yunnan’s top six famous chicken breeds. Br. Poult. Sci. 2018, 59, 494–505. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; Liu, Y.; Wang, K.; Shi, H.; He, Y.; Long, Y.; Sun, D.; Wu, H.; Zi, X.; et al. Ovary transcriptome profiling in high- and low-yielding Chinese Chahua laying chickens. Czech J. Anim. Sci. 2022, 67, 327–338. [Google Scholar] [CrossRef]
- Kim, M.H.; Seo, D.S.; Ko, Y. Relationship Between Egg Productivity and Insulin-Like Growth Factor-I Genotypes in Korean Native Ogol Chickens. Poult. Sci. 2004, 83, 1203–1208. [Google Scholar] [CrossRef]
- Evans, A.C. Characteristics of Ovarian Follicle Development in Domestic Animals. Reprod. Domest. Anim. 2003, 38, 240–246. [Google Scholar] [CrossRef]
- Regan, S.L.; Knight, P.G.; Yovich, J.L.; Leung, Y.; Arfuso, F.; Dharmarajan, A. Involvement of Bone Morphogenetic Proteins (BMP) in the Regulation of Ovarian Function. Vitam. Horm. 2018, 107, 227–261. [Google Scholar] [CrossRef]
- Gilbert, A.B.; Perry, M.M.; Waddington, D.; Hardie, M.A. Role of atresia in establishing the follicular hierarchy in the ovary of the domestic hen (Gallus domesticus). Reproduction 1983, 69, 221–227. [Google Scholar] [CrossRef]
- Hocking, P.M. Biology of breeding poultry. In Poultry Science Symposium Series Bodmin; CAB International: Wallingford, UK, 2009; pp. 75–76. [Google Scholar]
- Onagbesan, O.; Bruggeman, V.; Decuypere, E. Intra-ovarian growth factors regulating ovarian function in avian species: A review. Anim. Reprod. Sci. 2009, 111, 121–140. [Google Scholar] [CrossRef]
- Ghanem, K.; Johnson, A. Follicle dynamics and granulosa cell differentiation in the turkey hen ovary. Poult. Sci. 2018, 97, 3755–3761. [Google Scholar] [CrossRef]
- Hrabia, A.; Wolak, D.; Kwaśniewska, M.; Kieronska, A.; Socha, J.K.; Sechman, A. Expression of gelatinases (MMP-2 and MMP-9) and tissue inhibitors of metalloproteinases (TIMP-2 and TIMP-3) in the chicken ovary in relation to follicle development and atresia. Theriogenology 2018, 125, 268–276. [Google Scholar] [CrossRef]
- Wolak, D.; Sechman, A.; Hrabia, A. Effect of eCG treatment on gene expression of selected matrix metalloproteinases (MMP-2, MMP-7, MMP-9, MMP-10, and MMP-13) and the tissue inhibitors of metalloproteinases (TIMP-2 and TIMP-3) in the chicken ovary. Anim. Reprod. Sci. 2021, 224, 106666. [Google Scholar] [CrossRef]
- Ingman, W.; Owens, P.; Armstrong, D. Differential regulation by FSH and IGF-I of extracellular matrix IGFBP-5 in bovine granulosa cells: Effect of association with the oocyte. Mol. Cell. Endocrinol. 2000, 164, 53–58. [Google Scholar] [CrossRef]
- Huet, C.; Pisselet, C.; Mandon-Pépin, B.; Monget, P.; Monniaux, D. Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: Relationships between cell shape and function. J. Endocrinol. 2001, 169, 347–360. [Google Scholar] [CrossRef]
- Curry, T.E., Jr.; Osteen, K.G. The matrix metalloproteinase system: Changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr. Rev. 2003, 24, 428–465. [Google Scholar] [CrossRef]
- Leśniak-Walentyn, A.; Hrabia, A. Expression and localization of matrix metalloproteinases (MMP-2, -7, -9) and their tissue inhibitors (TIMP-2, -3) in the chicken oviduct during pause in laying induced by tamoxifen. Theriogenology 2017, 88, 50–60. [Google Scholar] [CrossRef]
- Hrabia, A. Matrix Metalloproteinases (MMPs) and Inhibitors of MMPs in the Avian Reproductive System: An Overview. Int. J. Mol. Sci. 2021, 22, 8056. [Google Scholar] [CrossRef]
- Wolak, D.; Hrabia, A. Alternations in the expression of selected matrix metalloproteinases (MMP-2, -9, -10, and −13) and their tissue inhibitors (TIMP-2 and -3) and MMP-2 and -9 activity in the chicken ovary during pause in laying induced by fasting. Theriogenology 2020, 161, 176–186. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; He, Y.; Dou, T.; Jia, J.; Ge, C. Endocrine and genetic factors affecting egg laying performance in chickens: A review. Br. Poult. Sci. 2020, 61, 538–549. [Google Scholar] [CrossRef]
- Taylor, S.C.; Posch, A. The Design of a Quantitative Western Blot Experiment. BioMed Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef]
- Liu, J.; Miao, X.M.; Li, F.G.; Zhu, Q.; Wang, Y.Y.; Yin, H.D.; Shu, G.; Ye, L.; Zhao, X.L. The curves fitting for laying rate and cumulative egg production, and the correlation analysis for laying performance of Jiuyuan Black Chickens. J. Yunnan Agric. Univ. 2020, 35, 40–47. [Google Scholar] [CrossRef]
- Zou, K.; Asiamah, C.A.; Lu, L.-L.; Liu, Y.; Pan, Y.; Chen, T.; Zhao, Z.; Su, Y. Ovarian transcriptomic analysis and follicular development of Leizhou black duck. Poult. Sci. 2020, 99, 6173–6187. [Google Scholar] [CrossRef]
- Zhou, F.; Shi, L.-B.; Zhang, S.-Y. Ovarian Fibrosis: A Phenomenon of Concern. Chin. Med. J. 2017, 130, 365–371. [Google Scholar] [CrossRef]
- Cooke, R.G.I.; Nothnick, W.B.; Komar, C.; Burns, P.; Curry, T.E. Collagenase and Gelatinase Messenger Ribonucleic Acid Expression and Activity During Follicular Development in the Rat Ovary1. Biol. Reprod. 1999, 61, 1309–1316. [Google Scholar] [CrossRef]
- Zhu, G.; Kang, L.; Wei, Q.; Cui, X.; Wang, S.; Chen, Y.; Jiang, Y. Expression and Regulation of MMP1, MMP3, and MMP9 in the Chicken Ovary in Response to Gonadotropins, Sex Hormones, and TGFB11. Biol. Reprod. 2014, 90, 57. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, Y.; Chen, Q.; Guo, M.; Kang, L.; Zhu, G.; Jiang, Y. Characterization of Chicken MMP13 Expression and Genetic Effect on Egg Production Traits of Its Promoter Polymorphisms. G3 Genes|Genomes|Genet. 2016, 6, 1305–1312. [Google Scholar] [CrossRef]
- Luk, I.Y.; Reehorst, C.M.; Mariadason, J.M. ELF3, ELF5, EHF and SPDEF Transcription Factors in Tissue Homeostasis and Cancer. Molecules 2018, 23, 2191. [Google Scholar] [CrossRef]
- Kameyama, N.; Kobayashi, K.; Shimizu, S.; Yamasaki, Y.; Endo, M.; Hashimoto, M.; Furihata, T.; Chiba, K. Involvement of ESE-3, epithelial-specific ETS factor family member 3, in transactivation of the ABCB1 gene via pregnane X receptor in intestine-derived LS180 cells but not in liver-derived HepG2 cells. Drug Metab. Pharmacokinet. 2016, 31, 340–348. [Google Scholar] [CrossRef]
- Hollenhorst, P.C.; Ferris, M.W.; Hull, M.A.; Chae, H.; Kim, S.; Graves, B.J. Oncogenic ETS proteins mimic activated RAS/MAPK signaling in prostate cells. Genes Dev. 2011, 25, 2147–2157. [Google Scholar] [CrossRef]
- Madison, B.J.; Clark, K.A.; Bhachech, N.; Hollenhorst, P.C.; Graves, B.J.; Currie, S.L. Electrostatic repulsion causes anticooperative DNA binding between tumor suppressor ETS transcription factors and JUN–FOS at composite DNA sites. J. Biol. Chem. 2018, 293, 18624–18635. [Google Scholar] [CrossRef]
| BWF | AFE | EW 30 | EN 30 | EN30–EN43 | EW 43 | EN 43 | |
|---|---|---|---|---|---|---|---|
| BWF | 1 | ||||||
| AFE | −0.324 ** | 1 | |||||
| EW 30 | 0.302 ** | 0.082 | 1 | ||||
| EN 30 | 0.194 ** | −0.577 ** | −0.09 | 1 | |||
| EN30–EN43 | 0.313 ** | −0.092 | −0.025 | 0.742 ** | 1 | ||
| EW 43 | 0.333 ** | −0.043 | 0.598 ** | −0.106 | −0.021 | 1 | |
| EN 43 | 0.119 * | −0.399 ** | −0.019 | 0.902 ** | 0.845 ** | −0.081 | 1 |
| SNPs | Quantity | Genotype | BWF (g) | AFE (Days) | EW 30 (g) | EN 30 (Count) | EW 43 (g) | EN 43 (Count) |
|---|---|---|---|---|---|---|---|---|
| 2360C > A | 381 | AA | 1057.56 ± 12.69 a | 146.45 ± 1.37 | 39.74 ± 0.42 | 56.91 ± 1.54 a | 42.93 ± 0.42 | 84.43 ± 3.64 |
| AC | 1027.75 ± 7.91 ab | 148.95 ± 0.82 | 37.12 ± 0.41 | 52.88 ± 1.07 ab | 41.48 ± 0.37 | 89.13 ± 2.38 | ||
| CC | 1024.51 ± 10.82 b | 150.29 ± 1.45 | 37.47 ± 0.47 | 52.08 ± 1.83 b | 42.31 ± 0.71 | 87.2 ± 3.76 | ||
| 2329T > C | 381 | CC | 1062.45 ± 10.32 | 146.11 ± 1.05 | 38.94 ± 0.33 | 55.49 ± 1.26 | 42.4 ± 0.356 | 88.32 ± 2.804 |
| CT | 1020.86 ± 7.74 | 150.06 ± 0.88 | 37.19 ± 0.46 | 52.17 ± 1.27 | 41.71 ± 0.421 | 87.21 ± 2.607 | ||
| TT | 996.46 ± 12.24 | 150.55 ± 1.85 | 36.93 ± 0.61 | 52.89 ± 2.48 | 42.01 ± 0.975 | 85.75 ± 4.628 | ||
| 2252T > C | 381 | CC | 1040.23 ± 6.91 | 147.41 ± 0.72 | 38.14 ± 0.27 | 54.95 ± 0.88 | 41.99 ± 0.29 | 89.02 ± 1.952 |
| CT | 1018.47 ± 10.64 | 151.44 ± 1.38 | 36.91 ± 0.82 | 50.48 ± 1.85 | 42.31 ± 0.65 | 83.98 ± 4.10 | ||
| TT | 1004.78 ± 28.98 | 156.64 ± 3.77 | 37.22 ± 0.53 | 45.17 ± 5.61 | 41.56 ± 1.79 | 78.27 ± 10.50 | ||
| 1890A > T | 381 | AA | 1015.80 ± 9.55 | 151.02 ± 1.09 a | 36.60 ± 0.59 | 52.18 ± 1.35 | 41.64 ± 0.46 b | 83.99 ± 3.09 |
| AT | 1037.91 ± 8.53 | 147.77 ± 0.91 ab | 37.96 ± 0.27 | 54.28 ± 1.12 | 41.91 ± 0.39 ab | 89.57 ± 2.48 | ||
| TT | 1065.36 ± 13.09 | 146.31 ± 1.52 b | 40.09 ± 0.46 | 54.82 ± 2.1 | 43.24 ± 0.52 a | 89.03 ± 4.07 | ||
| 1889T > C | 381 | TT | 1017.48 ± 9.47 | 151.03 ± 1.08 a | 36.57 ± 0.58 | 51.92 ± 1.34 | 41.6 ± 0.45 b | 84.05 ± 3.05 |
| TC | 1036.95 ± 8.61 | 147.74 ± 0.92 ab | 38.02 ± 0.27 | 54.49 ± 1.13 | 41.95 ± 0.40 ab | 89.58 ± 2.50 | ||
| CC | 1065.36 ± 13.09 | 146.21 ± 1.52 b | 40.09 ± 0.46 | 54.82 ± 2.11 | 43.24 ± 0.52 a | 89.03 ± 4.01 | ||
| 1887A > T | 381 | AA | 1016.26 ± 9.37 | 151.07 ± 1.06 a | 36.61 ± 0.57 | 52.13 ± 1.33 | 41.6 ± 0.45 b | 83.73 ± 3.03 |
| AT | 1037.69 ± 8.76 | 147.78 ± 0.94 ab | 38.01 ± 0.27 | 54.49 ± 1.14 | 41.95 ± 0.40 ab | 90.09 ± 2.53 | ||
| TT | 1065.52941 ± 12.70 | 145.74 ± 1.48 b | 40.09 ± 0.46 | 54.49 ± 2.05 | 43.24 ± 0.52 a | 88.34 ± 4.032 |
| Diplotype | BWF | AFE | EW 30 | EN 30 | EW 43 | EN 43 |
|---|---|---|---|---|---|---|
| A | 1062.46 ± 12.96 a | 145.62 ± 1.49 b | 40.11 ± 0.46 | 55.17 ± 2.11 | 43.24 ± 0.44 | 90.67 ± 3.02 |
| B | 1015.30 ± 9.67 b | 150.94 ± 0.89 a | 38.19 ± 0.47 | 52.41 ± 1.36 | 41.11 ± 0.32 | 87.59 ± 3.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Cao, C.; Liu, Y.; Zi, X.; He, Y.; Shi, H.; Zhao, J.; Ge, C.; Wang, K. Polymorphism, Genetic Effect, and Association with Egg-Laying Performance of Chahua Chickens Matrix Metalloproteinases 13 Promoter. Genes 2023, 14, 1352. https://doi.org/10.3390/genes14071352
Du Y, Cao C, Liu Y, Zi X, He Y, Shi H, Zhao J, Ge C, Wang K. Polymorphism, Genetic Effect, and Association with Egg-Laying Performance of Chahua Chickens Matrix Metalloproteinases 13 Promoter. Genes. 2023; 14(7):1352. https://doi.org/10.3390/genes14071352
Chicago/Turabian StyleDu, Yanli, Changwei Cao, Yong Liu, Xiannian Zi, Yang He, Hongmei Shi, Jinbo Zhao, Changrong Ge, and Kun Wang. 2023. "Polymorphism, Genetic Effect, and Association with Egg-Laying Performance of Chahua Chickens Matrix Metalloproteinases 13 Promoter" Genes 14, no. 7: 1352. https://doi.org/10.3390/genes14071352
APA StyleDu, Y., Cao, C., Liu, Y., Zi, X., He, Y., Shi, H., Zhao, J., Ge, C., & Wang, K. (2023). Polymorphism, Genetic Effect, and Association with Egg-Laying Performance of Chahua Chickens Matrix Metalloproteinases 13 Promoter. Genes, 14(7), 1352. https://doi.org/10.3390/genes14071352






