Effects of Salinity Stress on Growth and Physiological Parameters and Related Gene Expression in Different Ecotypes of Sesuvium portulacastrum on Hainan Island
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Morphological Measures
2.3. Determination of Relative Water Content (RWC)
2.4. Determination of Fresh and Dry Weight
2.5. Determination of Proline Content
2.6. Determination of Malondialdehyde (MDA)
2.7. Determination of Chlorophyll Content
2.8. Total RNA Extraction and Reverse Transcription
2.9. Acquisition of Gene-Coding Sequences
2.10. Quantitative Real-Time PCR (qRT-PCR)
2.11. Data Analysis
2.12. A Comprehensive Evaluation of the Salt Resistance of Different Ecotypes
3. Results
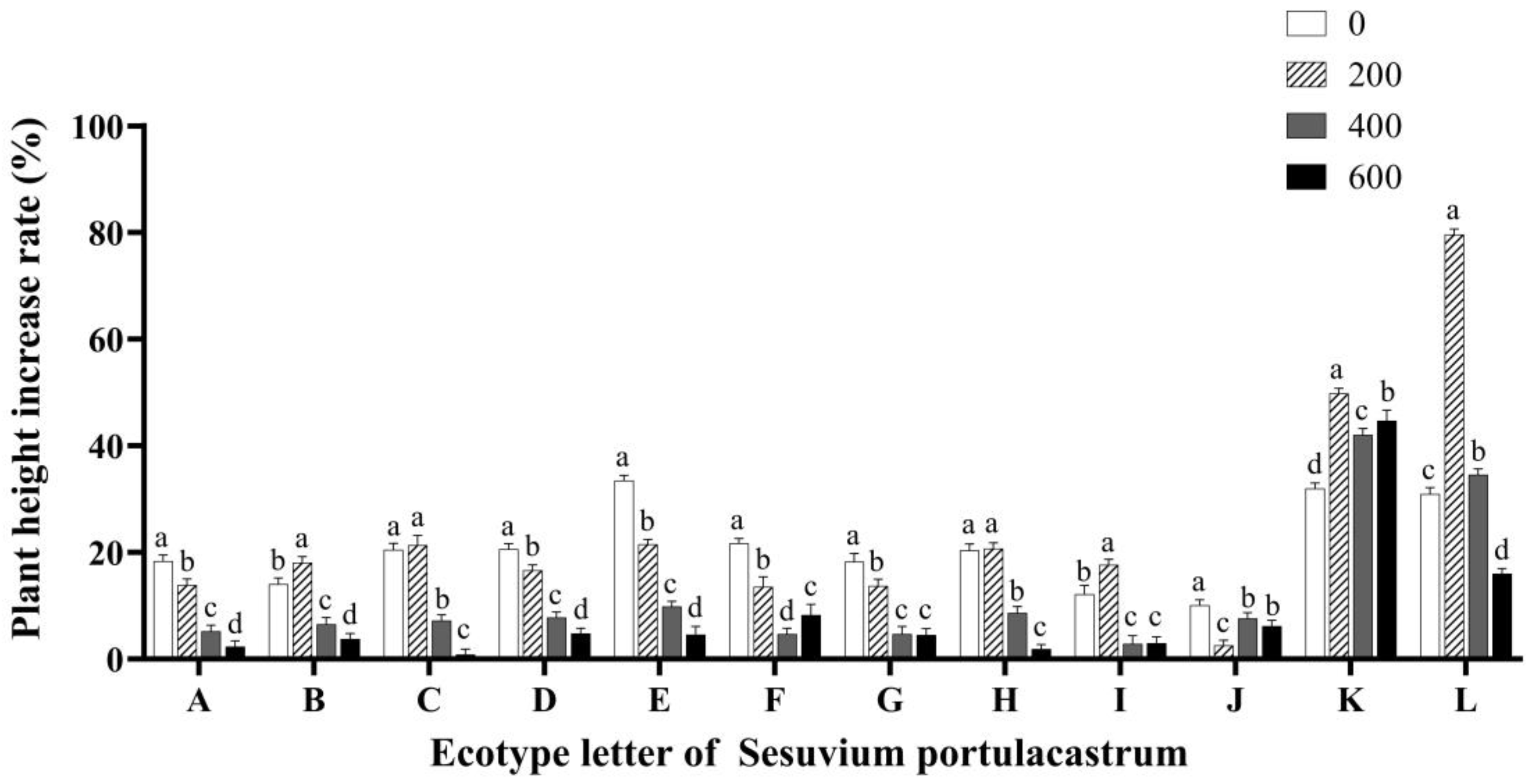
3.1. Effects of Salt Stress on Plant Height
3.2. Effects of Salt Stress on Internode Length
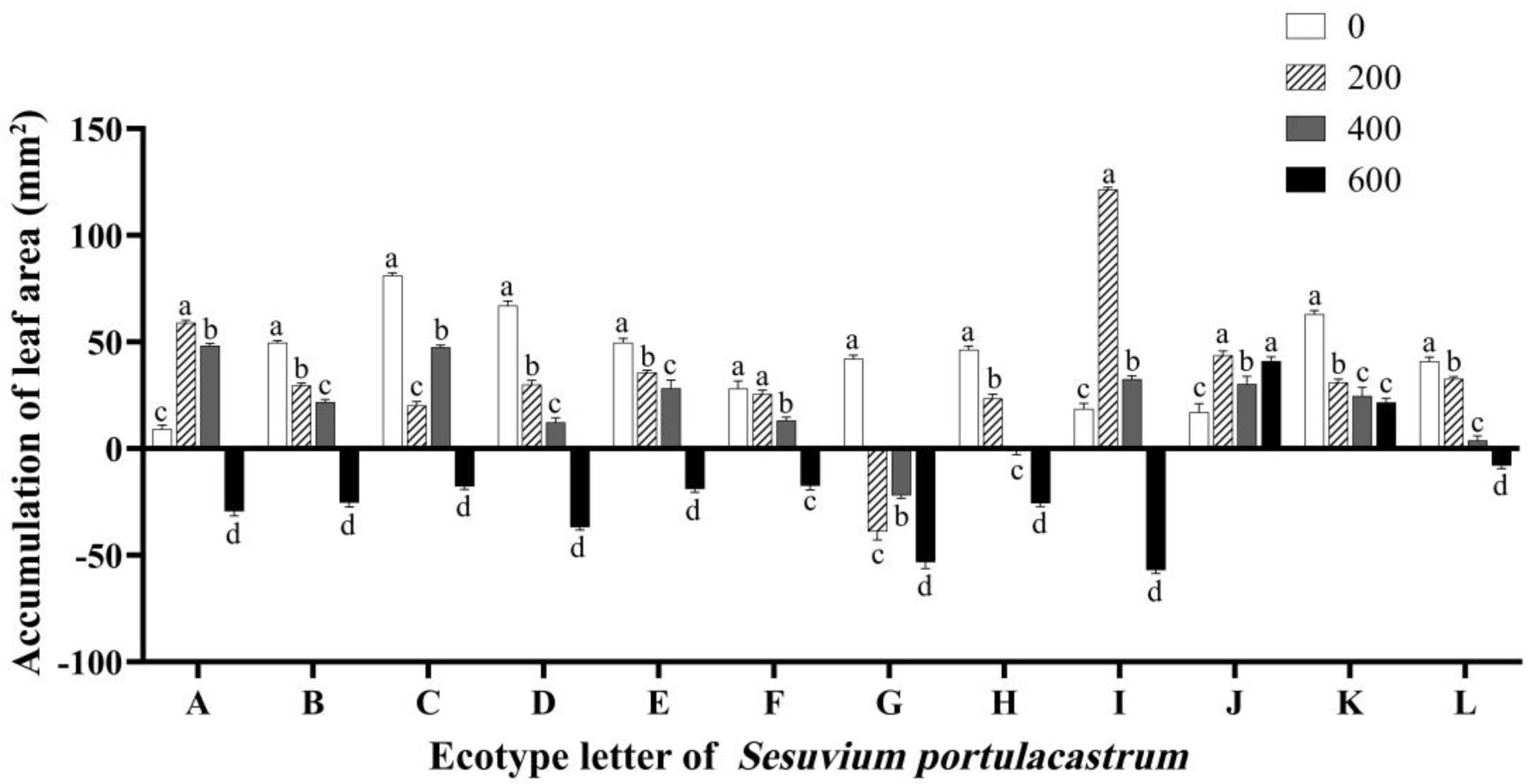
3.3. Effects of Salt Stress on Leaf Area
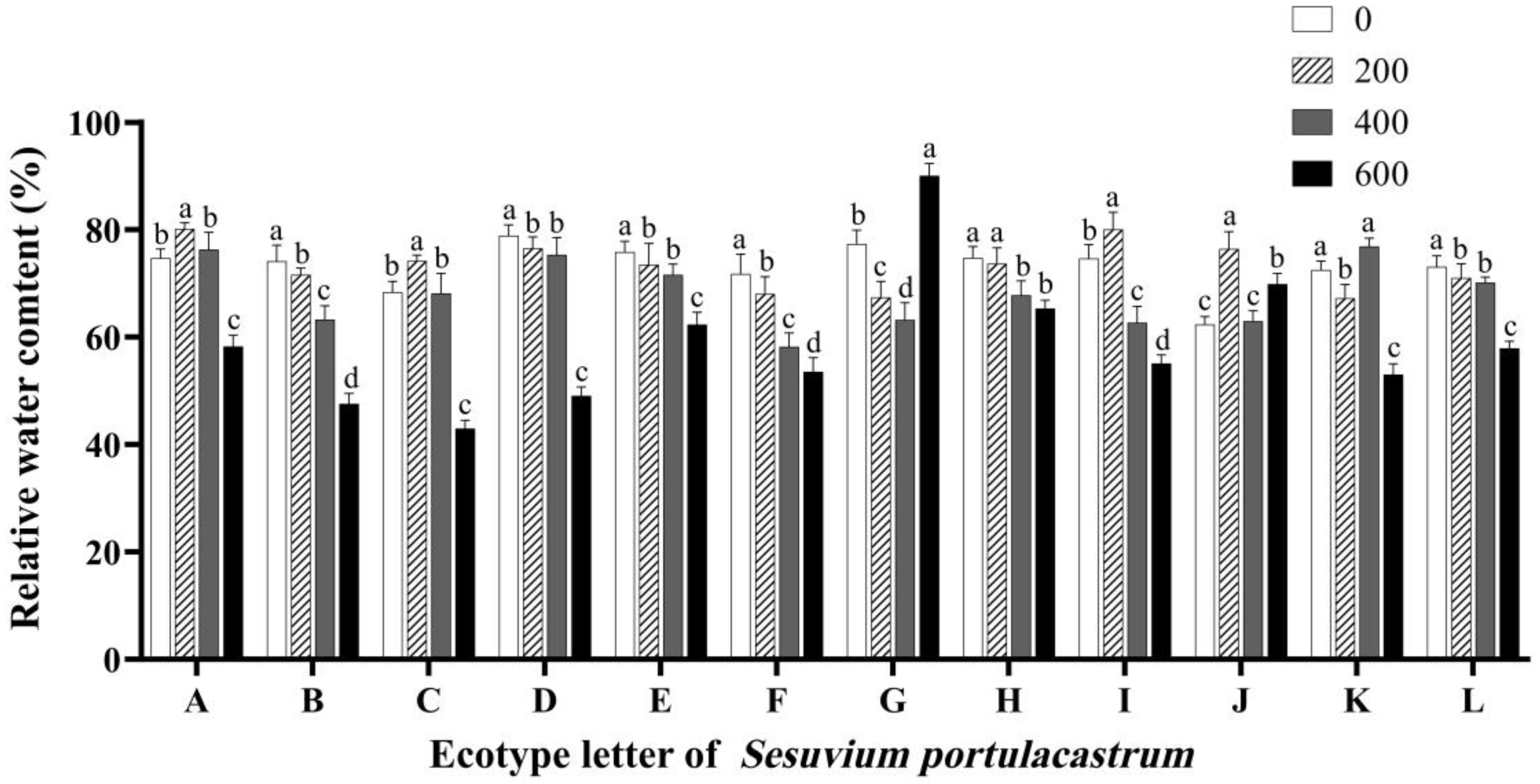
3.4. Effects of Salt Stress on the Relative Water Content (RWC)
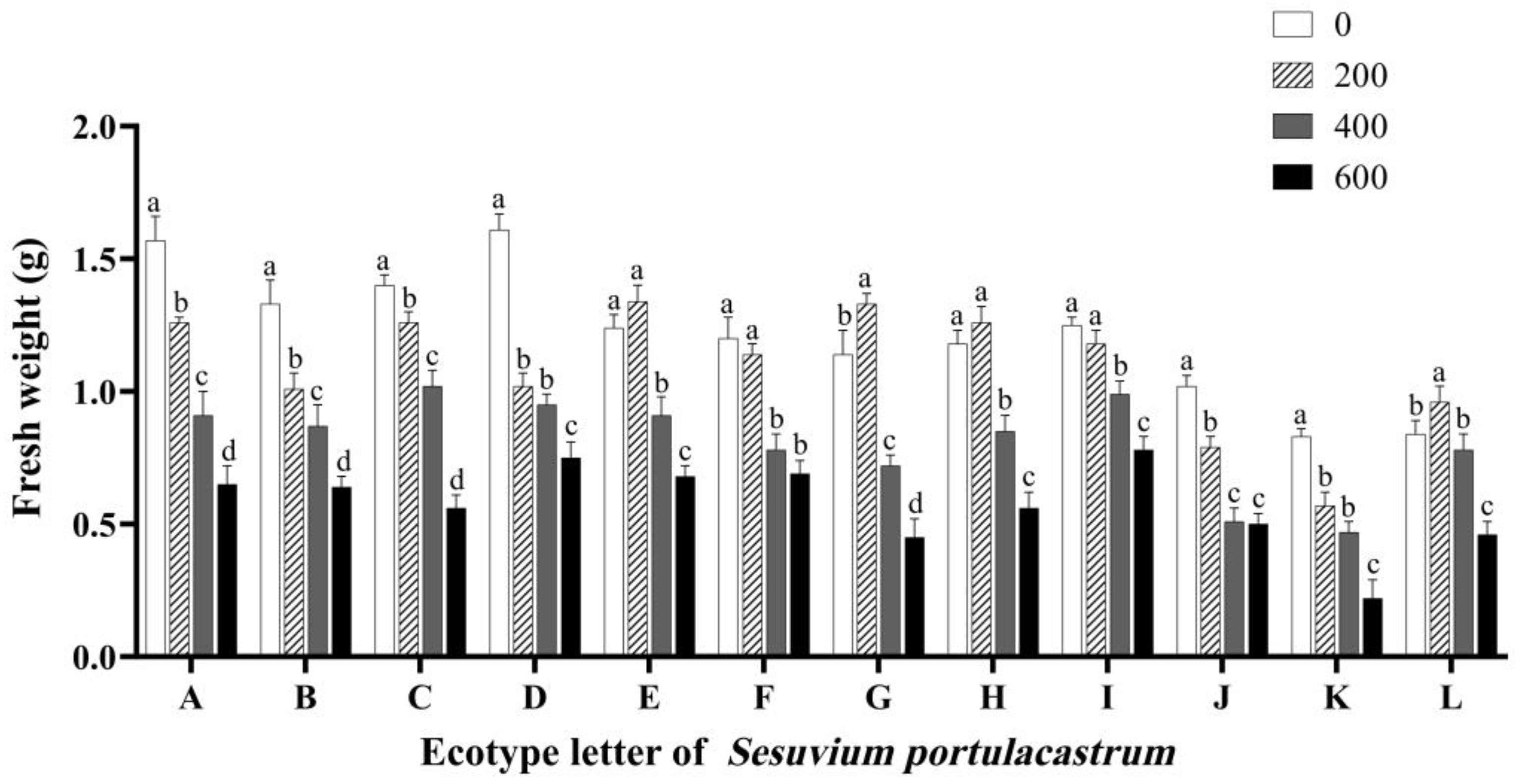
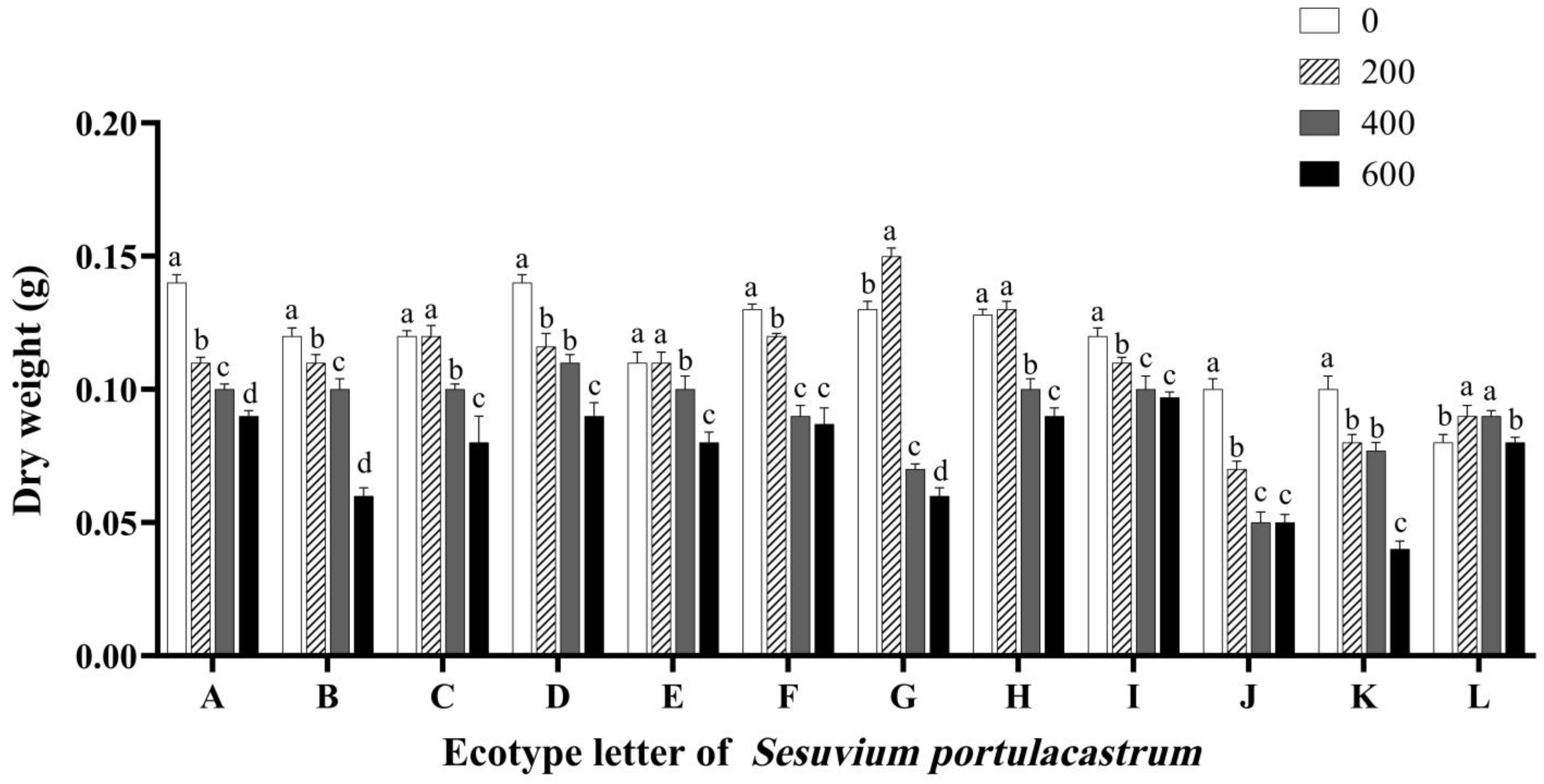
3.5. Effects of Salt Stress on the Fresh and Dry Weight
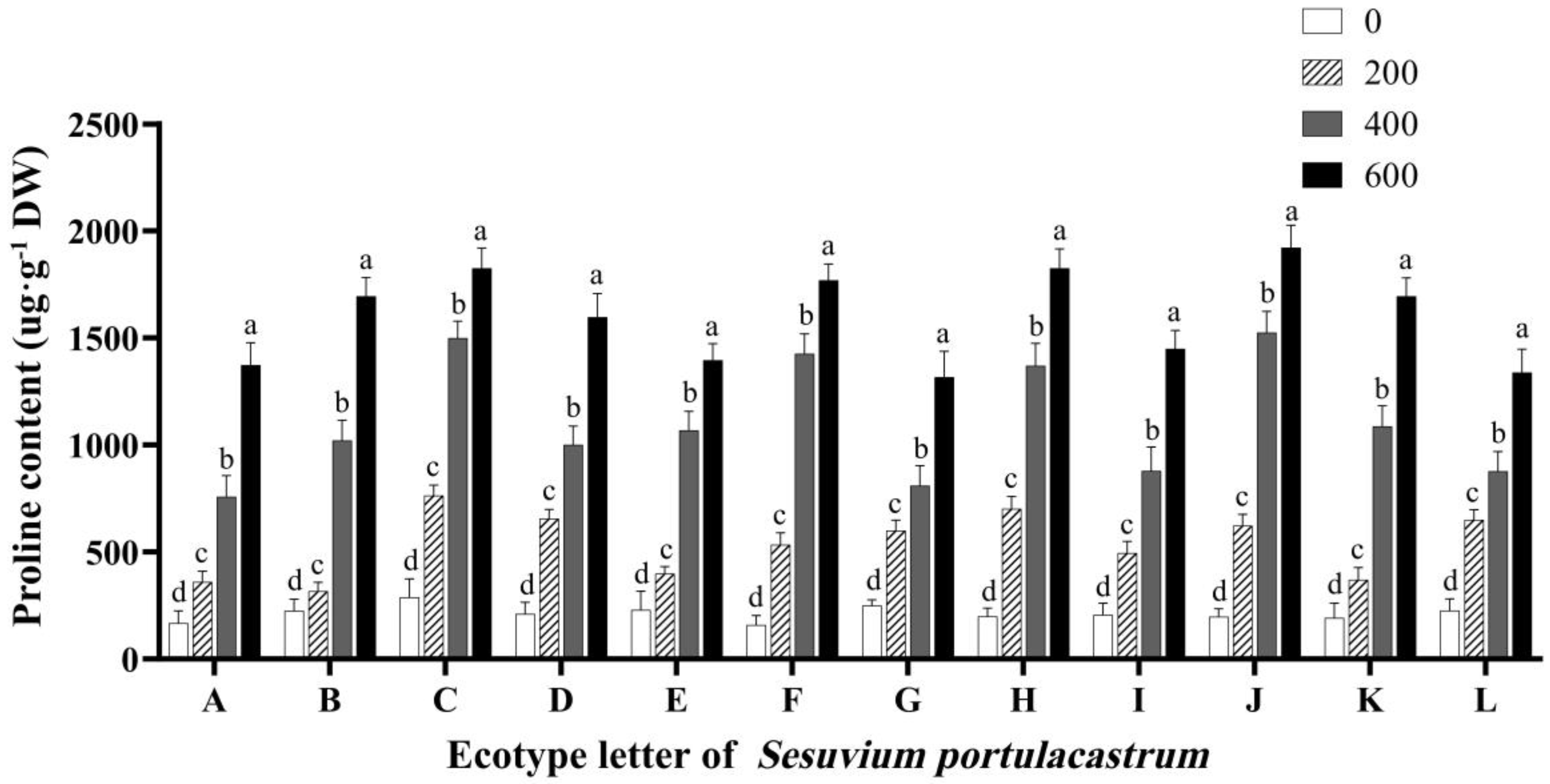
3.6. Effects of Salt Stress on Proline Content
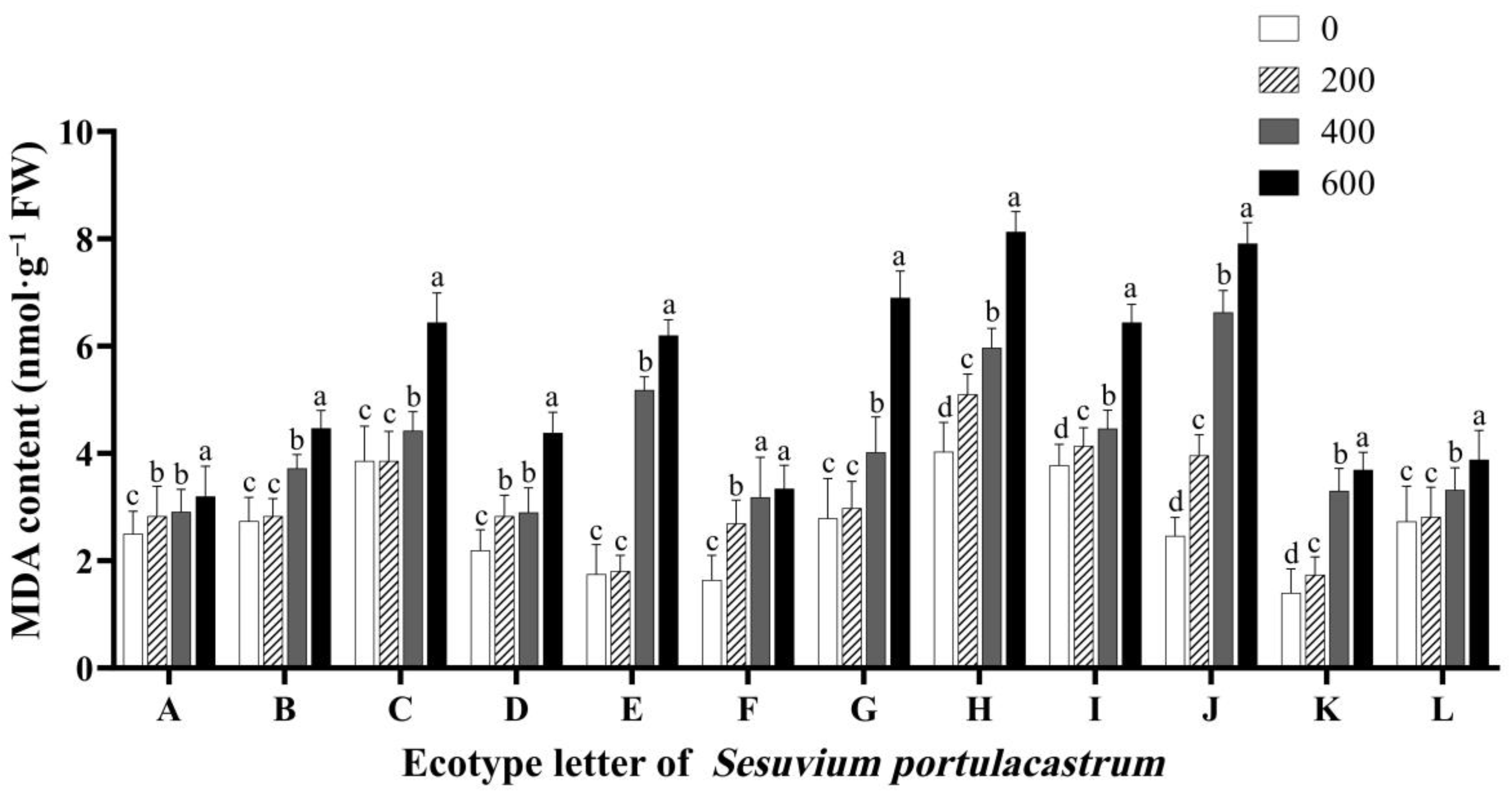
3.7. Effects of Salt Stress on Malondialdehyde (MDA) Content
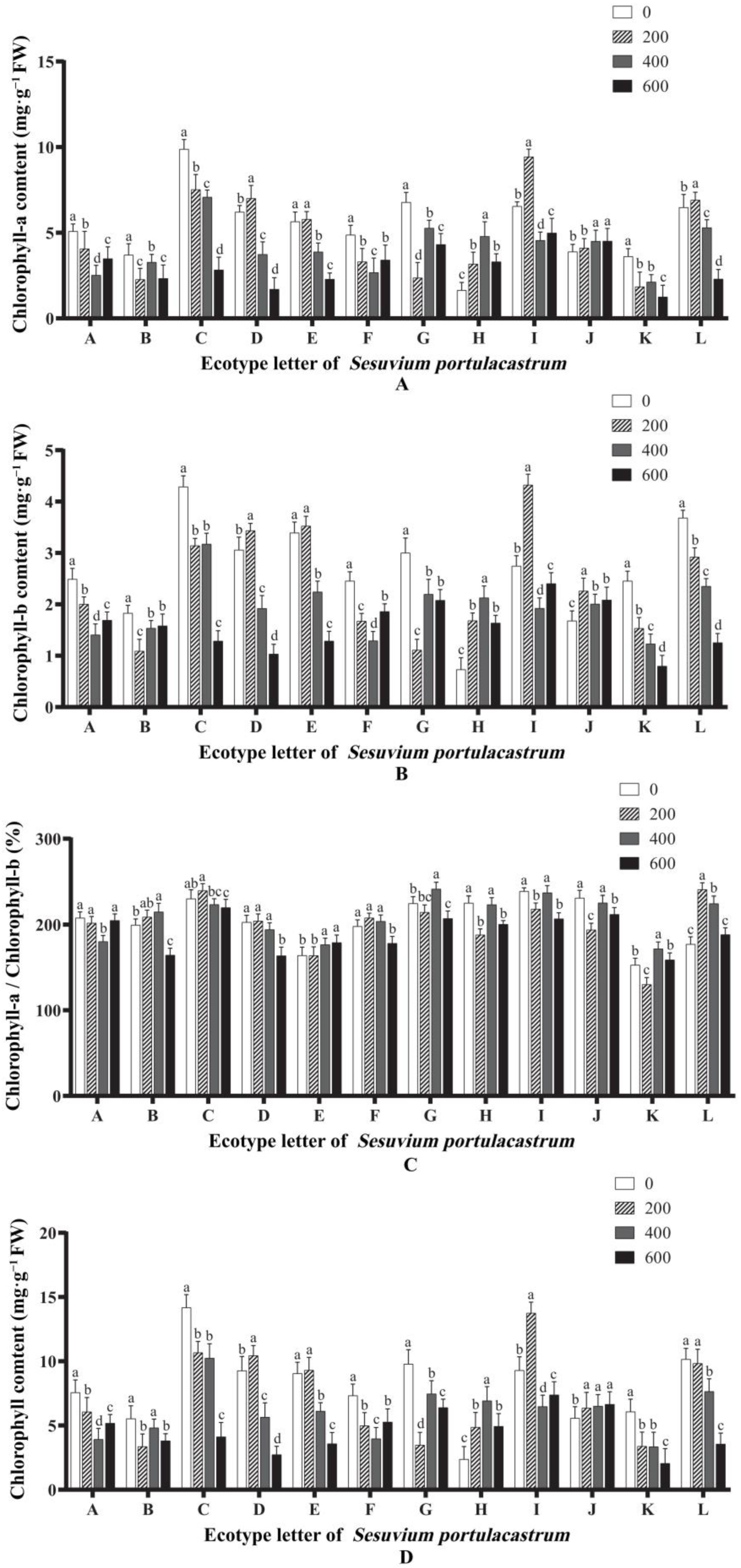
3.8. Effects of Salt Stress on Chlorophyll a Content
3.9. Effects of Salt Stress on Chlorophyll b Content
3.10. Effects of Salt Stress on the Ratio of Chlorophyll a to Chlorophyll b (Rab)
3.11. Effects of Salt Stress on Total Chlorophyll Content
3.12. Comprehensive Evaluation of Salt Resistance Based on Principal Component Analysis
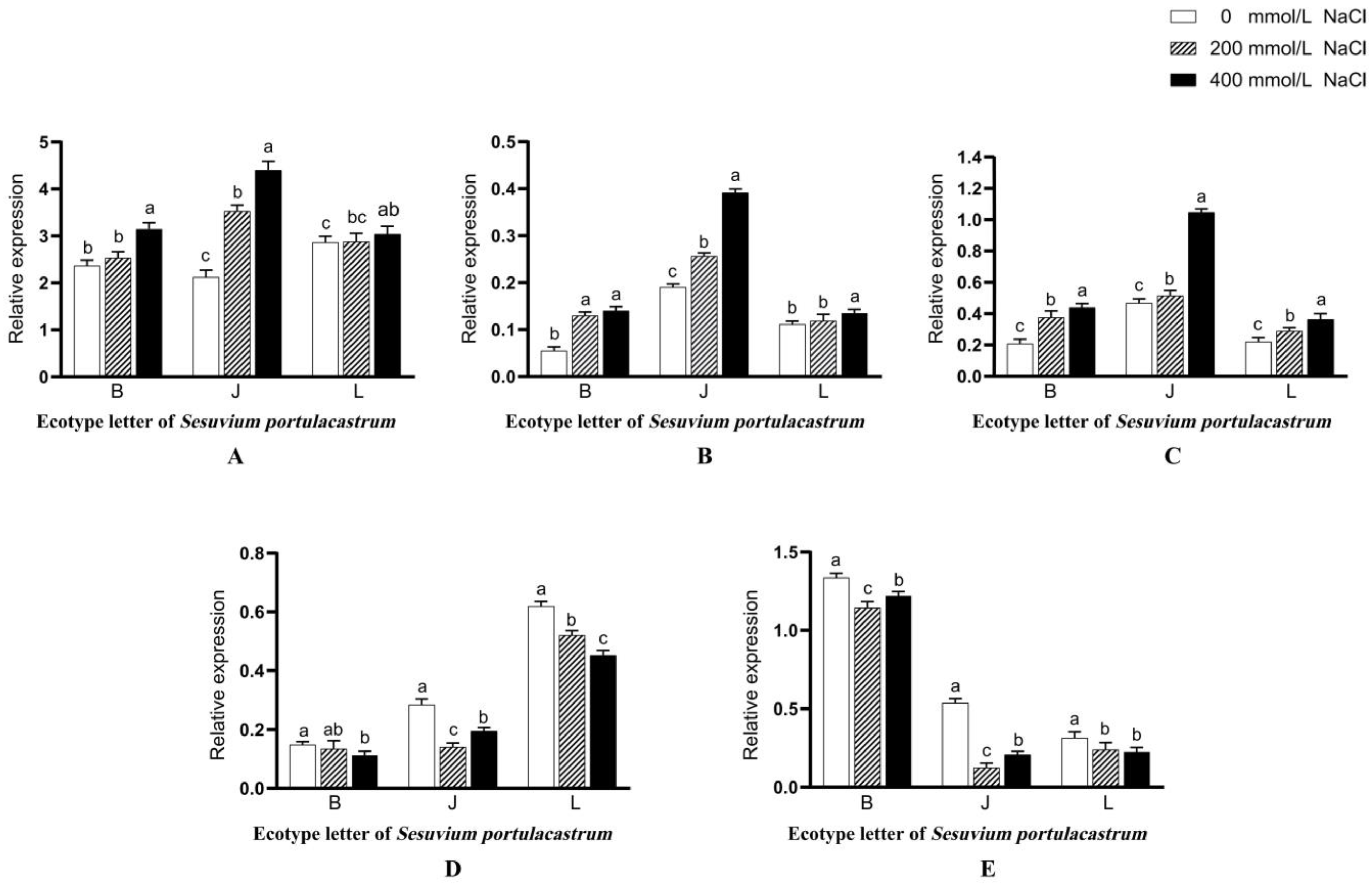
3.13. Analysis of Differential Expression of S. portulacastrum Metabolism-Related Genes under Different Salt Concentrations
3.14. Changes in the Relative mRNA Expression Levels of SpP5CS1, SpCHL1a, and SpCHL1b
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.Q.; Zhou, Y.; Cai, Y.; Feng, Y.; Zhong, C.; Fang, Z.S.; Zhang, Y. De Novo Transcriptome Analysis of High-Salinity Stress-Induced Antioxidant Activity and Plant Phytohormone Alterations in Sesuvium portulacastrum. Front. Plant Sci. 2022, 13, 995855. [Google Scholar] [CrossRef] [PubMed]
- Lonard, R.I.; Judd, F.W.; Stalter, R. The Biological Flora of Coastal Dunes and Wetlands: Distichlis spicata (C. Linnaeus) E. Greene. J. Coast. Res. 2013, 29, 105–117. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Nikam, T.D.; Suprasanna, P. Sesuvium portulacastrum (L.) L. a Promising Halophyte: Cultivation, Utilization and Distribution in India. Genet. Resour. Crop Evol. 2009, 56, 741–747. [Google Scholar] [CrossRef]
- Ghnaya, T.; Nouairi, I.; Slama, I.; Messedi, D.; Grignon, C.; Abdelly, C.; Ghorbel, M.H. Cadmium Effects on Growth and Mineral Nutrition of Two Halophytes: Sesuvium portulacastrum and Mesembryanthemum crystallinum. J. Plant Physiol. 2005, 162, 1133–1140. [Google Scholar] [CrossRef]
- Slama, I.; Ghnaya, T.; Hessini, K.; Messedi, D.; Savouré, A.; Abdelly, C. Comparative Study of the Effects of Mannitol and PEG Osmotic Stress on Growth and Solute Accumulation in Sesuvium portulacastrum. Environ. Exp. Bot. 2007, 61, 10–17. [Google Scholar] [CrossRef]
- Wali, M.; Gunsè, B.; Llugany, M.; Corrales, I.; Abdelly, C.; Poschenrieder, C.; Ghnaya, T. High Salinity Helps the Halophyte Sesuvium portulacastrum in Defense against Cd Toxicity by Maintaining Redox Balance and Photosynthesis. Planta 2016, 244, 333–346. [Google Scholar] [CrossRef]
- Zhang, H.; Shin, P.K.S.; Cheung, S.G. Physiological Responses and Scope for Growth upon Medium-Term Exposure to the Combined Effects of Ocean Acidification and Temperature in a Subtidal Scavenger Nassarius conoidalis. Mar. Environ. Res. 2015, 106, 51–60. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Nikam, T.D.; Penna, S. Biochemical, Physiological and Growth Changes in Response to Salinity in Callus Cultures of Sesuvium portulacastrum L. Plant Cell Tissue Organ Cult. 2010, 102, 17–25. [Google Scholar] [CrossRef]
- Peng, C.; Chang, L.; Yang, Q.; Tong, Z.; Wang, D.; Tan, Y.; Sun, Y.; Yi, X.; Ding, G.; Xiao, J.; et al. Comparative Physiological and Proteomic Analyses of the Chloroplasts in Halophyte Sesuvium portulacastrum under Differential Salt Conditions. J. Plant Physiol. 2019, 232, 141–150. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zhao, K.F. Effects of Salt Stress on the Growth and Na+ and K+ Contents in Aeluropus Littoralis Var. Sinensis Debeaux. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 2005, 31, 311–316. [Google Scholar]
- Messedi, D.; Sleimi, N.; Abdelly, C. Some Physiological and Biochemical Aspects of Salt Tolerance of Sesuvium portulacastrum. In Cash Crop Halophytes: Recent Studies; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Fourati, E.; Vogel-Mikuš, K.; Bettaieb, T.; Kavčič, A.; Kelemen, M.; Vavpetič, P.; Pelicon, P.; Abdelly, C.; Ghnaya, T. Physiological Response and Mineral Elements Accumulation Pattern in Sesuvium portulacastrum L. Subjected in Vitro to Nickel. Chemosphere 2019, 219, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhuo, J.; Guo, W.; Spencer, R.G.M.; Zhang, Z.; Xu, J. Tracing Organic Matter Removal in Polluted Coastal Waters via Floating Bed Phytoremediation. Mar. Pollut. Bull. 2013, 71, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, Z.; Guo, Q.; Mao, S.; Li, S.; Sun, F.; Wang, H.; Yang, C. Transcriptomic Profiling and Physiological Responses of Halophyte Kochia sieversiana Provide Insights into Salt Tolerance. Front. Plant Sci. 2017, 8, 1085. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Pirjan, K.; Qasim, M.; Mahmood, A.; Javed, T.; Muhammad, H.; Yang, S.; Dong, R.; Ali, B.; Rahimi, M. Salinity Stress Improves Antioxidant Potential by Modulating Physio-Biochemical Responses in Moringa Oleifera Lam. Sci. Rep. 2023, 13, 2895. [Google Scholar] [CrossRef] [PubMed]
- Faryal, S.; Ullah, R.; Khan, M.N.; Ali, B.; Hafeez, A.; Jaremko, M.; Qureshi, K.A. Thiourea-Capped Nanoapatites Amplify Osmotic Stress Tolerance in Zea mays L. by Conserving Photosynthetic Pigments, Osmolytes Biosynthesis and Antioxidant Biosystems. Molecules 2022, 27, 5744. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Gillani, M.; Shah, A.A.; Shah, A.N.; Kauser, N.; Jamil, M.; Ahmad, R.T.; Ullah, S. Triticum aestivum: Antioxidant Gene Profiling and Morpho-Physiological Studies under Salt Stress. Mol. Biol. Rep. 2023, 50, 2569–2580. [Google Scholar] [CrossRef]
- Saleem, K.; Asghar, M.A.; Saleem, M.H.; Raza, A.; Kocsy, G.; Iqbal, N.; Ali, B.; Albeshr, M.F.; Bhat, E.A. Chrysotile-Asbestos-Induced Damage in Panicum virgatum and Phleum pretense Species and Its Alleviation by Organic-Soil Amendment. Sustainability 2022, 14, 10824. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, H.; Chu, Y.; Sun, C.; Wei, N.; Yang, M.; Zheng, C. Effects of Salt Stress on Chlorophyll Fluorescence and the Antioxidant System in Ginkgo biloba L. Seedlings. HortScience 2019, 54, 2125–2133. [Google Scholar] [CrossRef]
- Bidalia, A.; Hanief, M.; Rao, K.S. Tolerance of Mitragyna parvifolia (Roxb.) Korth. Seedlings to NaCl Salinity. Photosynthetica 2017, 55, 231–239. [Google Scholar] [CrossRef]
- He, W.; Wang, D.; Yang, N.; Cao, D.; Chen, X.; Chen, J.; Wei, X. In Vitro Shoot Culture of Sesuvium portulacastrum: An Important Plant for Phytoremediation. Agriculture 2022, 12, 47. [Google Scholar] [CrossRef]
- Ran, X.; Wang, X.; Huang, X.; Ma, C.; Liang, H.; Liu, B. Study on the Relationship of Ions (Na, K, Ca) Absorption and Distribution to Photosynthetic Response of Salix matsudana Koidz Under Salt Stress. Front. Plant Sci. 2022, 13, 860111. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yin, X.; Xie, Q.; Xia, Y.; Wang, Z.; Song, J.; Zhou, Y.; Jiang, X. Co-Expression of SpSOS1 and SpAHA1 in Transgenic Arabidopsis Plants Improves Salinity Tolerance. BMC Plant Biol. 2019, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ni, R.; Yang, S.; Pu, Y.; Qian, M.; Yang, Y.; Yang, Y. Functional Characterization of the Stipa Purpurea P5cs Gene under Drought Stress Conditions. Int. J. Mol. Sci. 2021, 22, 9599. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, N.; Zhang, C.; He, W.; Ye, G.; Chen, J.; Wei, X. Transcriptome Analysis Reveals Molecular Mechanisms Underlying Salt Tolerance in Halophyte Sesuvium portulacastrum. Front. Plant Sci. 2022, 13, 973419. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, Z.Z.; Zhou, X.F.; Yin, H.B.; Li, X.; Xin, X.F.; Hong, X.H.; Zhu, J.K.; Gong, Z. Overexpression of SOS (Salt Overly Sensitive) Genes Increases Salt Tolerance in Transgenic Arabidopsis. Mol. Plant 2009, 2, 22–31. [Google Scholar] [CrossRef]
- Kariola, T.; Brader, G.; Li, J.; Palva, E.T. Chlorophyllase 1, a Damage Control Enzyme, Affects the Balance between Defense Pathways in Plants. Plant Cell 2005, 17, 282–294. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll Degradation during Senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, S.M.; Kumar, N. Role of Chlorophyllase in Chlorophyll Homeostasis and Post-Harvest Breakdown in Piper betle L. Leaf. Indian J. Biochem. Biophys. 2011, 48, 353–360. [Google Scholar]
- Jacob-Wilk, D.; Holland, D.; Goldschmidt, E.E.; Riov, J.; Eyal, Y. Chlorophyll Breakdown by Chlorophyllase: Isolation and Functional Expression of the Chlase1 Gene from Ethylene-Treated Citrus Fruit and Its Regulation during Development. Plant J. 1999, 20, 653–661. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Suzuki, T.; Yamada, T.; Shimada, H.; Masuda, T.; Ohta, H.; Takamiya, K.I. Chlorophyllase as a Serine Hydrolase: Identification of a Putative Catalytic Triad. Plant Cell Physiol. 2003, 44, 96–101. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Ohta, H.; Okawa, K.; Iwamatsu, A.; Shimada, H.; Masuda, T.; Takamiya, K.I. Cloning of Chlorophyllase, the Key Enzyme in Chlorophyll Degradation: Finding of a Lipase Motif and the Induction by Methyl Jasmonate. Proc. Natl. Acad. Sci. USA 1999, 96, 15362–15367. [Google Scholar] [CrossRef]
- Takamiya, K.I.; Tsuchiya, T.; Ohta, H. Degradation Pathway(s) of Chlorophyll: What Has Gene Cloning Revealed? Trends Plant Sci. 2000, 5, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil the college of agriculture. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in the water relations of the cotton plant: I. the field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of Malondialdehyde (MDA) by Thiobarbituric Acid (TBA) Assay. In Plant-Microbe Interactions; Humana: New York, NY, USA, 2021; pp. 103–105. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Shen, L.; Yang, C.; Guo, J.; Duan, R. Cloning and Expression Analysis of Δ1-Pyrroline-5-Carboxylate Synthetase (SpP5CS) from Sesuvium portulacastrum. Plant Physiol. J. 2015, 51, 1933–1941. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cheeseman, J.M. Mechanisms of Salinity Tolerance in Plants. Plant Physiol. 1988, 87, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Singroha, G.; Kumar, S.; Gupta, O.P.; Singh, G.P.; Sharma, P. Uncovering the Epigenetic Marks Involved in Mediating Salt Stress Tolerance in Plants. Front. Genet. 2022, 13, 811732. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Ruan, C.J.; da Silva, J.A.T.; Mopper, S.; Pei, Q.; Lutts, S. Halophyte Improvement for a Salinized World. CRC. Crit. Rev. Plant Sci. 2010, 29, 329–359. [Google Scholar] [CrossRef]
- Acharya, B.R.; Sandhu, D.; Dueñas, C.; Dueñas, M.; Pudussery, M.; Kaundal, A.; Ferreira, J.F.S.; Suarez, D.L.; Skaggs, T.H. Morphological, Physiological, Biochemical, and Transcriptome Studies Reveal the Importance of Transporters and Stress Signaling Pathways during Salinity Stress in Prunus. Sci. Rep. 2022, 12, 1274. [Google Scholar] [CrossRef]
- Ji, X.; Tang, J.; Zhang, J. Effects of Salt Stress on the Morphology, Growth and Physiological Parameters of Juglans microcarpa L. Seedlings. Plants 2022, 11, 2381. [Google Scholar] [CrossRef]
- Banakar, M.H.; Amiri, H.; Sarafraz Ardakani, M.R.; Ranjbar, G.H. Susceptibility and Tolerance of Fenugreek (Trigonella foenum-graceum L.) to Salt Stress: Physiological and Biochemical Inspections. Environ. Exp. Bot. 2022, 194, 104748. [Google Scholar] [CrossRef]
- Mahmood, U.; Hussain, S.; Hussain, S.; Ali, B.; Ashraf, U.; Zamir, S.; Al-Robai, S.A.; Alzahrani, F.O.; Hano, C.; El-Esawi, M.A. Morpho-Physio-Biochemical and Molecular Responses of Maize Hybrids to Salinity and Waterlogging during Stress and Recovery Phase. Plants 2021, 10, 1345. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Sümer, A.; Zörb, C.; Yan, F.; Schubert, S. Evidence of Sodium Toxicity for the Vegetative Growth of Maize (Zea mays L.) during the First Phase of Salt Stress. J. Appl. Bot. Food Qual. 2004, 78, 135–139. [Google Scholar]
- Karimi, R.; Ebrahimi, M.; Amerian, M. Abscisic Acid Mitigates NaCl Toxicity in Grapevine by Influencing Phytochemical Compounds and Mineral Nutrients in Leaves. Sci. Hortic. 2021, 288, 110336. [Google Scholar] [CrossRef]
- Sajid, Z.A. Effect of Salinity on Growth and Physiology of Thellungiella halophila L. Ecotypes. Pak. J. Bot. 2023, 55, 1–8. [Google Scholar] [CrossRef]
- Munns, R. Genes and Salt Tolerance: Bringing Them Together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.Q.; Sun, D.W.; Lu, Q.; Yang, L.; Wang, H.W.; Fu, X.X. Responses of Physiology, Photosynthesis, and Related Genes to Saline Stress in Cornus hongkongensis subsp. Tonkinensis (W. P. Fang) Q. Y. Xiang. Plants 2022, 11, 940. [Google Scholar] [CrossRef]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badeck, F.W.; Citerne, S.; Tangama, M.; David, A.; Ghashghaie, J. Stomatal and Non-Stomatal Limitations Are Responsible in down-Regulation of Photosynthesis in Melon Plants Grown under the Saline Condition: Application of Carbon Isotope Discrimination as a Reliable Proxy. Plant Physiol. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef]
- Tao, R.; Ding, J.; Li, C.; Zhu, X.; Guo, W.; Zhu, M. Evaluating and Screening of Agro-Physiological Indices for Salinity Stress Tolerance in Wheat at the Seedling Stage. Front. Plant Sci. 2021, 12, 646175. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, Y.; Mi, P.; Wang, B.; Yuan, F. Salt-Tolerance Screening in Limonium sinuatum Varieties with Different Flower Colors. Sci. Rep. 2021, 11, 14562. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.N.; Lew, R.R. Turgor Regulation in Osmotically Stressed Arabidopsis Epidermal Root Cells. Direct Support for the Role of Inorganic Ion Uptake as Revealed by Concurrent Flux and Cell Turgor Measurements. Plant Physiol. 2002, 129, 290–299. [Google Scholar] [CrossRef]
- Ghaffari, A. Role of Inorganic and Organic Ions in Response to Salt and Drought Stresses. J. Plant Stress Physiol. 2022, 8, 17–25. [Google Scholar] [CrossRef]
- Bai, X.; Dai, L.; Sun, H.; Chen, M.; Sun, Y. Effects of Moderate Soil Salinity on Osmotic Adjustment and Energy Strategy in Soybean under Drought Stress. Plant Physiol. Biochem. 2019, 139, 307–313. [Google Scholar] [CrossRef]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline Accumulation Influenced by Osmotic Stress in Arbuscular Mycorrhizal Symbiotic Plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef]
- Igarashi, Y.; Yoshiba, Y.; Sanada, Y.; Yamaguchi-Shinozaki, K.; Wada, K.; Shinozaki, K. Characterization of the Gene for Δ1-Pyrroline-5-Carboxylate Synthetase and Correlation between the Expression of the Gene and Salt Tolerance in Oryza sativa L. Plant Mol. Biol. 1997, 33, 857–865. [Google Scholar] [CrossRef]
- Brini, F.; Saibi, W.; Brini, F. Proline, A Peculiar Amino Acid with Astucious Functions in Development and Salt Tolerance Process in Plants. J. Food Nutr. Metab. 2020, 8, e0140. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Javid, M.G.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, S.M.; Trifonova, E.A.; Filipenko, E.A.; Shymny, V.K. Evaluation of Salt Tolerance of Transgenic Tobacco Plants Bearing with P5CS1 Gene of Arabidopsis Thaliana. Genetika 2015, 51, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Aycan, M.; Baslam, M.; Mitsui, T.; Yildiz, M. The TaGSK1, TaSRG, TaPTF1, and TaP5CS Gene Transcripts Confirm Salinity Tolerance by Increasing Proline Production in Wheat (Triticum aestivum L.). Plants 2022, 11, 3401. [Google Scholar] [CrossRef]
- Feng, X.J.; Li, J.R.; Qi, S.L.; Lin, Q.F.; Jin, J.B.; Hua, X.J. Light Affects Salt Stress-Induced Transcriptional Memory of P5CS1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, E8335–E8343. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.; Hayat, S. Specific Roles of Lipoxygenases in Development and Responses to Stress in Plants. Plants 2022, 11, 979. [Google Scholar] [CrossRef]
- Keller, N.P.; Hohn, T.M. Metabolic Pathway Gene Clusters in Filamentous Fungi. Fungal Genet. Biol. 1997, 21, 17–29. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Q.; Ervin, E.H.; Yang, Z.; Zhang, X. Physiological Mechanism of Enhancing Salt Stress Tolerance of Perennial Ryegrass by 24-Epibrassinolide. Front. Plant Sci. 2017, 10, 1017. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, A.; Feng, Z. Amelioration of Chilling Stress by Triadimefon in Cucumber Seedlings. Plant Growth Regul. 2003, 39, 277–283. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The Response of Salinity Stress-Induced A. Tricolor to Growth, Anatomy, Physiology, Non-Enzymatic and Enzymatic Antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]
- Turan, S.; Tripathy, B.C. Salt-Stress Induced Modulation of Chlorophyll Biosynthesis during de-Etiolation of Rice Seedlings. Physiol. Plant. 2015, 153, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Han, L.; Yin, S.; Jiang, Y. Chlorophyll Metabolism and Gene Expression in Response to Submergence Stress and Subsequent Recovery in Perennial Ryegrass Accessions Differing in Growth Habits. J. Plant Physiol. 2020, 251, 153195. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, K.; Taïbi, F.; Ait Abderrahim, L.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of Salt Stress on Growth, Chlorophyll Content, Lipid Peroxidation and Antioxidant Defence Systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
| Ecotype Number | Place | Latitude (N) | Longitude (E) | Altitude (m) | Habitat |
|---|---|---|---|---|---|
| A | Xialing Village, Qionghai City | 19°23′ | 110°40′ | 30 | Beach |
| B | Shuigoupo Village Lingshui County | 18°26′ | 110°03′ | 22 | Beach |
| C | Wanglougang Village, Ledong County | 18°26′ | 108°51′ | 2 | Wetland side |
| D | Dongjiao Port Wengtian Town Wenchang City | 19°53′ | 110°59′ | 2 | Bare sand |
| E | Hongsha Waste Water Treatment Works Sanya City | 18°14′ | 109°33′ | 3 | Mudflat |
| F | East Water Port Chengmai County | 19°58 | 110°04′ | 0 | Sand beside the river |
| G | Xingying Port Yangpu Danzhou City | 19°44′ | 109°12′ | 1 | Rock crevice of salt fields |
| H | Gangbei Port Wanning City | 18°53′ | 110°30′ | 3 | Beach |
| I | Jiangnancheng District Haikou City | 20°04′ | 110°20′ | 0.4 | Beach sand |
| J | Haidongfang Park Dongfang City | 19°08 | 108°39′ | 8 | Pond side |
| K | Yangjin Village, Lingshui County | 18°26′ | 109°59′ | 1 | Pond side |
| L | Xinglong Village, Danzhou City | 19°50′ | 109°30′ | 13 | Beach |
| Ecotype | 0 mmol/L | 200 mmol/L | 400 mmol/L | 600 mmol/L |
|---|---|---|---|---|
| A | 0.29a | 0.20b | 0.04d | 0.14c |
| B | 0.38a | 0.18b | 0.10c | 0.00d |
| C | 0.36b | 0.40a | 0.04c | 0.01d |
| D | 0.56a | 0.48b | 0.05c | 0.04c |
| E | 0.29b | 0.20c | 0.23c | 0.50a |
| F | 0.74a | 0.24b | 0.09d | 0.14c |
| G | 0.51a | 0.02d | 0.06c | 0.11b |
| H | 0.33b | 0.50a | 0.13c | 0.08d |
| I | 0.61a | 0.18c | 0.02d | 0.34b |
| J | 0.13b | 0.02c | 0.28a | 0.05c |
| K | 0.38c | 0.87b | 0.25d | 1.35a |
| L | 0.09d | 0.70a | 0.16c | 0.33b |
| Ecotype | Place | Overall Ratings | Rank |
|---|---|---|---|
| A | Xialing Village, Qionghai City | −0.218 | 8 |
| B | Shuigoupo Village Lingshui County | 0.136 | 6 |
| C | Wanglougang Village, Ledong County | −0.274 | 9 |
| D | Dongjiao Port Wengtian Town Wenchang City | −0.120 | 7 |
| E | Hongsha Waste Water Treatment Works Sanya City | 0.259 | 3 |
| F | East Water Port Chengmai County | 0.220 | 4 |
| G | Xingying Port Yangpu Danzhou City | −0.925 | 11 |
| H | Gangbei Port Wanning City | 0.176 | 5 |
| I | Jiangnancheng District Haikou City | 1.561 | 2 |
| J | Haidongfang Park Dongfang City | −0.975 | 12 |
| K | Yangjin Village, Lingshui County | −0.858 | 10 |
| L | Xinglong Village, Danzhou City | 1.573 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ma, W.; Fu, H.; Li, L.; Ruan, X.; Zhang, X. Effects of Salinity Stress on Growth and Physiological Parameters and Related Gene Expression in Different Ecotypes of Sesuvium portulacastrum on Hainan Island. Genes 2023, 14, 1336. https://doi.org/10.3390/genes14071336
Wang Y, Ma W, Fu H, Li L, Ruan X, Zhang X. Effects of Salinity Stress on Growth and Physiological Parameters and Related Gene Expression in Different Ecotypes of Sesuvium portulacastrum on Hainan Island. Genes. 2023; 14(7):1336. https://doi.org/10.3390/genes14071336
Chicago/Turabian StyleWang, Yong, Wei Ma, Haijiang Fu, Liting Li, Xueyu Ruan, and Xueyan Zhang. 2023. "Effects of Salinity Stress on Growth and Physiological Parameters and Related Gene Expression in Different Ecotypes of Sesuvium portulacastrum on Hainan Island" Genes 14, no. 7: 1336. https://doi.org/10.3390/genes14071336
APA StyleWang, Y., Ma, W., Fu, H., Li, L., Ruan, X., & Zhang, X. (2023). Effects of Salinity Stress on Growth and Physiological Parameters and Related Gene Expression in Different Ecotypes of Sesuvium portulacastrum on Hainan Island. Genes, 14(7), 1336. https://doi.org/10.3390/genes14071336







