Revisiting the Role of Autophagy in Cardiac Differentiation: A Comprehensive Review of Interplay with Other Signaling Pathways
Abstract
1. Introduction
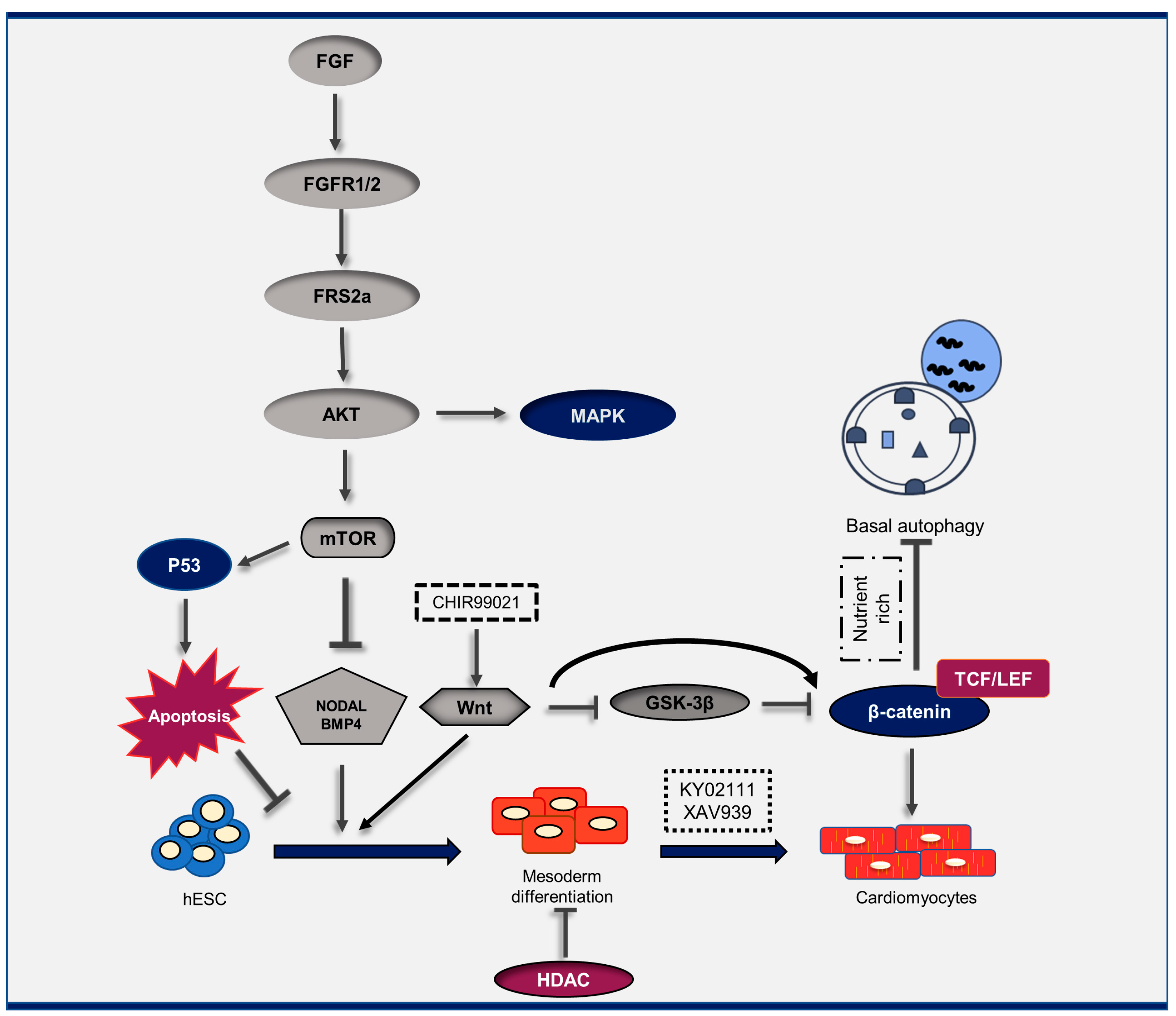
2. Crosstalk between Autophagy, Cardiac Differentiation, and Other Signaling Pathways
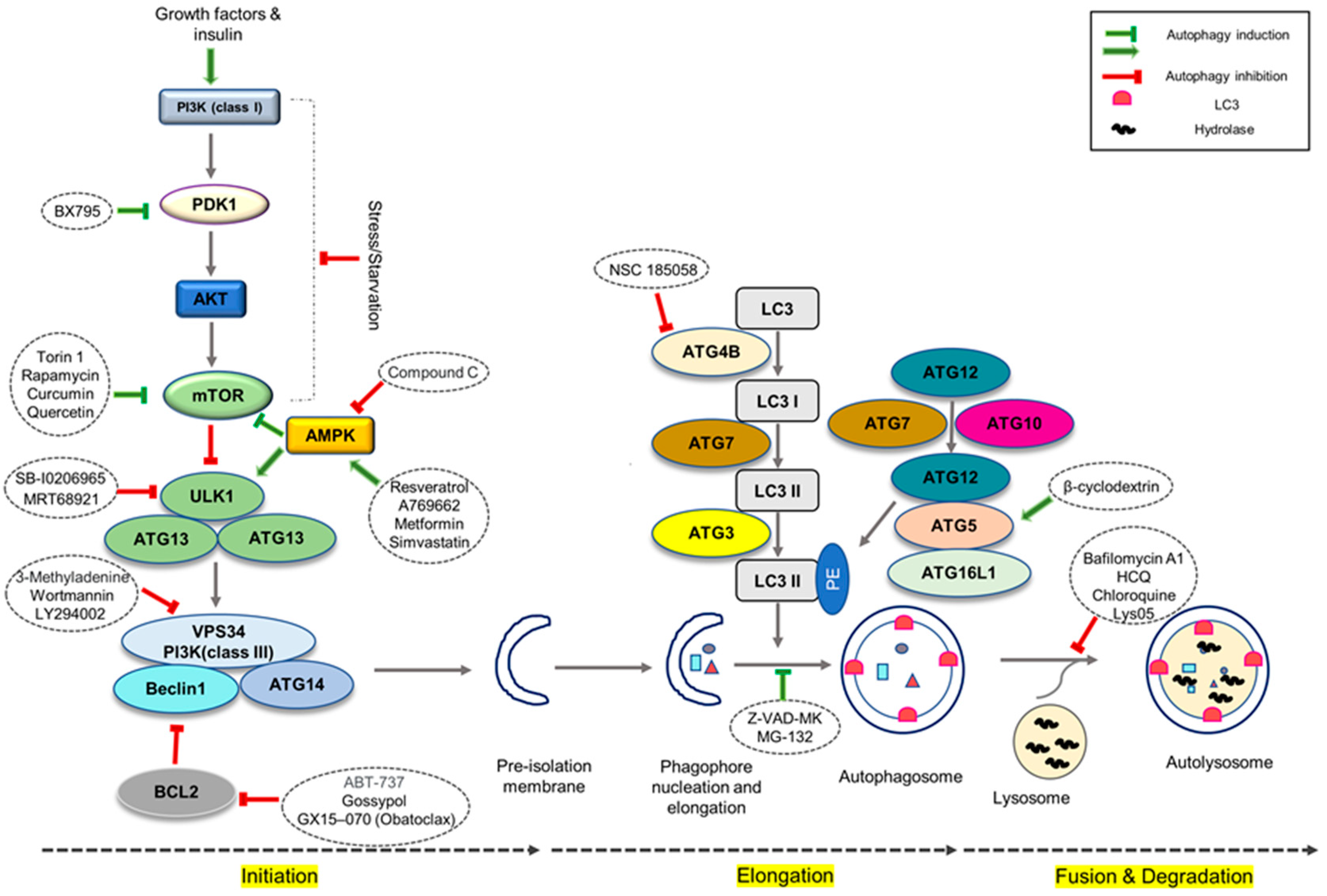
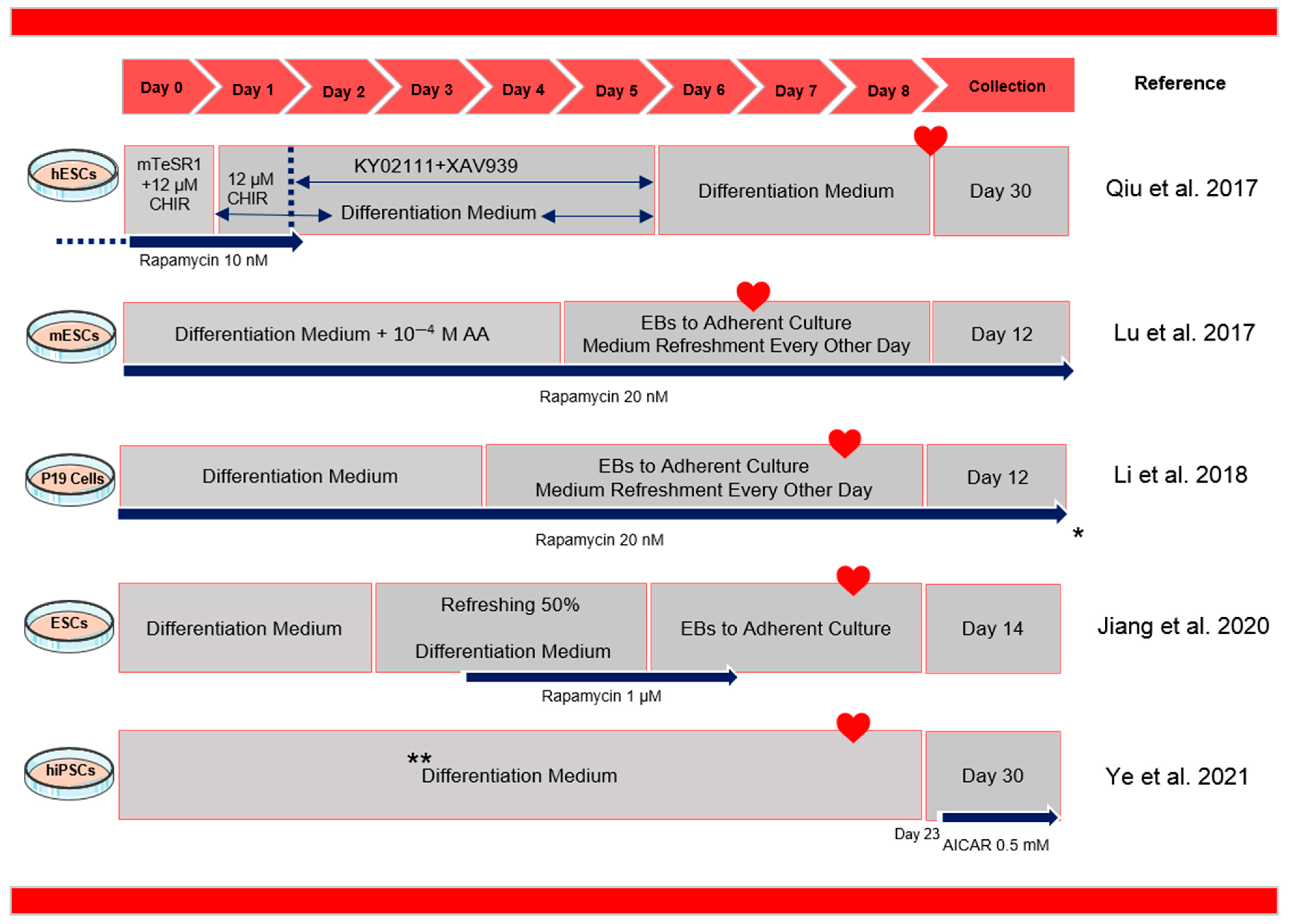
3. How Does Manipulation of Autophagy Improve Cardiac Differentiation?
4. Other Forms of Autophagy and Cardiac Differentiation
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lu, Q.; Liu, Y.; Wang, Y.; Yang, Z.; Li, T.; Tian, Y.; Chen, P.; Ma, K.; Jia, Z.; Zhou, C. Rapamycin efficiently promotes cardiac differentiation of mouse embryonic stem cells. Biosci. Rep. 2017, 37, BSR20160552. [Google Scholar] [CrossRef] [PubMed]
- Takano-Ohmuro, H.; Mukaida, M.; Kominami, E.; Morioka, K. Autophagy in embryonic erythroid cells: Its role in maturation. Eur. J. Cell Biol. 2000, 79, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.C.; Zhao, Z.; Stephenson, L.M.; Cadwell, K.; Pua, H.H.; Lee, H.K. The autophagy gene ATG5 plays an essential role in B lymphocyte development. Autophagy 2008, 4, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Goldman, S.; Baerga, R.; Zhao, Y.; Komatsu, M.; Jin, S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 19860–19865. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Zhang, W.; Xu, N.; Zhu, J.-Y.; Jia, J.; Sun, Z.-J.; Wang, Y.-N.; Zhao, Y.-F. Autophagy regulates hypoxia-induced osteoclastogenesis through the HIF-1α/BNIP3 signaling pathway. J. Cell. Physiol. 2012, 227, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Aymard, E.; Barruche, V.; Naves, T.; Bordes, S.; Closs, B.; Verdier, M.; Ratinaud, M.H. Autophagy in human keratinocytes: An early step of the differentiation? Exp. Dermatol. 2011, 20, 263–268. [Google Scholar] [CrossRef]
- Chang, D.H.; Deng, H.; Matthews, P.; Krasovsky, J.; Ragupathi, G.; Spisek, R.; Mazumder, A.; Vesole, D.H.; Jagannath, S.; Dhodapkar, M.V. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Am. J. Hematol. 2008, 112, 1308–1316. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, L.; McKeehan, W.L.; Wang, F. The fibroblast growth factor signaling axis controls cardiac stem cell differentiation through regulating autophagy. Autophagy 2012, 8, 690–691. [Google Scholar] [CrossRef]
- Simon, H.U. Autophagy in myocardial differentiation and cardiac development. Am. Heart Assoc. 2012, 110, 524–525. [Google Scholar] [CrossRef]
- Guan, J.L.; Simon, A.K.; Prescott, M.; Menendez, J.A.; Liu, F.; Wang, F.; Wang, C.; Wolvetang, E.; Vasquez-Martin, A.; Zhang, J. Autophagy in stem cells. Autophagy 2013, 9, 830–849. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Lu, F. Autophagy in stem cell biology: A perspective on stem cell self-renewal and differentiation. Stem Cells Int. 2018, 9131397. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouzmohammadi, M.; Totonchi, M.; Pahlavan, S. The Role of iPSC Modeling Toward Projection of Autophagy Pathway in Disease Pathogenesis: Leader or Follower. Stem Cell Rev. Rep. 2021, 17, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Finn, P.F.; Dice, J.F. Proteolytic and lipolytic responses to starvation. Nutrition 2006, 22, 830–844. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar] [CrossRef]
- Karthik, L.; Kumar, G.; Keswani, T.; Bhattacharyya, A.; Chandar, S.S.; Bhaskara Rao, K.V. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PLoS ONE 2014, 9, e90972. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Jiang, K.; Liu, J.; Liu, Z. Dural effects of oxidative stress on cardiomyogenesis via Gata4 transcription and protein ubiquitination. Cell Death Dis. 2018, 9, 246. [Google Scholar] [CrossRef]
- Kolahdouzmohammadi, M.; Pahalavan, S.; Sotoodehnejadnematalahi, F.; Tahamtani, Y.; Totonchi, M. Activation of AMPK promotes cardiac differentiation by stimulating the autophagy pathway. J. Cell Commun. Signal. 2023. [Google Scholar] [CrossRef] [PubMed]
- Garbern, J.C.; Lee, R.T. Mitochondria and metabolic transitions in cardiomyocytes: Lessons from development for stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2021, 12, 177. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, X.; Zhou, Q.; Tan, B.; Xu, H.; Yi, Q.; Yan, L.; Xie, M.; Zhang, Y.; Tian, J.; et al. Activation of AMPK promotes maturation of cardiomyocytes derived from human induced pluripotent stem cells. Front. Cell Dev. Biol. 2021, 9, 644667. [Google Scholar] [CrossRef]
- Sarikhani, M.; Garbern, J.C.; Ma, S.; Sereda, R.; Conde, J.; Krähenbühl, G.; Escalante, G.O.; Ahmed, A.; Buenrostro, J.D.; Lee, R.T. Sustained activation of AMPK enhances differentiation of human iPSC-derived cardiomyocytes via sirtuin activation. Stem Cell Rep. 2020, 15, 498–514. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, T.; Zhang, J.; Gao, S.; Tao, B.; Cao, R.; Qiu, Y.; Liu, J.; Li, Y.; Wang, Y.; et al. Rapamycin promotes cardiomyocyte differentiation of human induced pluripotent stem cells in a stage-dependent manner. Stem Cells Dev. 2020, 29, 1229–1239. [Google Scholar] [CrossRef]
- Qiu, X.X.; Liu, Y.; Zhang, Y.F.; Guan, Y.N.; Jia, Q.Q.; Wang, C.; Liang, H.; Li, Y.-Q.; Yang, H.-T.; Qin, Y.-W.; et al. Rapamycin and CHIR 99021 Coordinate Robust Cardiomyocyte Differentiation from Human Pluripotent Stem Cells Via Reducing p53-Dependent Apoptosis. J. Am. Heart Assoc. 2017, 6, e005295. [Google Scholar] [CrossRef] [PubMed]
- Foley, A.; Mercola, M. Heart induction: Embryology to cardiomyocyte regeneration. Trends Cardiovasc. Med. 2004, 14, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jia, Z.; Wang, T.; Wang, W.; Zhang, C.; Chen, P.; Ma, K.; Zhou, C. Interaction of Wnt/β-catenin and notch signaling in the early stage of cardiac differentiation of P19CL6 cells. J. Cell. Biochem. 2012, 113, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Weidinger, G.; Osugi, T.; Kohn, A.D.; Golob, J.L.; Pabon, L.; Reinecke, H.; Moon, R.T.; Murry, C.E. Biphasic role for Wnt/β-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9685–9690. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Liu, Z.; Chen, Z.; Wang, J.; Chen, T.; Zhao, X.; Ma, Y.; Qin, L.; Kang, J.; Wei, B.; et al. Ascorbic acid enhances the cardiac differentiation of induced pluripotent stem cells through promoting the proliferation of cardiac progenitor cells. Cell Res. 2012, 22, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Li, T.Y.; Liu, Q.; Zhang, C.; Li, X.; Chen, Y.; Zhang, S.-M.; Lian, G.; Liu, Q.; Ruan, K.; et al. GSK3-TIP60-ULK1 signaling pathway links growth factor deprivation to autophagy. Science 2012, 336, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, H.; Liu, P.; Yin, D.; Zhang, S.; Zhao, J. Sphingosylphosphorylcholine promotes the differentiation of resident Sca-1 positive cardiac stem cells to cardiomyocytes through lipid raft/JNK/STAT3 and β-catenin signaling pathways. BBA Mol. Cell Res. 2016, 1863, 1579–1588. [Google Scholar] [CrossRef]
- Rojanasakul, Y. Linking JNK-STAT3-Akt signaling axis to EZH2 phosphorylation: A novel pathway of carcinogenesis. Cell Cycle 2013, 12, 202. [Google Scholar] [CrossRef]
- Moslehi, M.; Yazdanparast, R. SK-N-MC cell death occurs by distinct molecular mechanisms in response to hydrogen peroxide and superoxide anions: Involvements of JAK2-STAT3, JNK, and p38 MAP kinases pathways. Cell Biochem. Biophys. 2013, 66, 817–829. [Google Scholar] [CrossRef]
- Nakamura, T.; Sano, M.; Songyang, Z.; Schneider, M.D. A Wnt-and β-catenin-dependent pathway for mammalian cardiac myogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 5834–5839. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.; Qian, L.; Cheng, P.; Nigam, V.; Arnold, J.; Srivastava, D. A regulatory pathway involving Notch1/β-catenin/Isl1 determines cardiac progenitor cell fate. Nat. Cell Biol. 2009, 11, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, A.; Cipolat, S.; Chen, Y.; Dorn, G.W.; Scorrano, L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science 2013, 342, 734–737. [Google Scholar] [CrossRef]
- Saravanakumar, M.; Devaraj, H. Notch signalling in cardiovasculogenesis: Insight into their role in early cardiovascular development. Mol. Biol. Rep. 2013, 40, 3537–3547. [Google Scholar] [CrossRef] [PubMed]
- Nàger, M.; Sallán, M.C.; Visa, A.; Pushparaj, C.; Santacana, M.; Macià, A.; Yeramian, A.; Canti, C.; Herreros, J. Inhibition of WNT-CTNNB1 signaling upregulates SQSTM1 and sensitizes glioblastoma cells to autophagy blockers. Autophagy 2018, 14, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Petherick, K.J.; Williams, A.C.; Lane, J.D.; Ordóñez-Morán, P.; Huelsken, J.; Collard, T.J.; Smartt, H.J.M.; Batson, J.; Malik, K.; Paraskeva, C.; et al. Autolysosomal β-catenin degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J. 2013, 32, 1903–1916. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, J.; Wang, W.; Tian, Y.; XiangWei, W.; Chen, P.; Ma, K.; Zhou, C. Autophagy eliminates cytoplasmic β-catenin and NICD to promote the cardiac differentiation of P19CL6 cells. Cell Signal. 2014, 26, 2299–2305. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Liu, K.; He, Q.; Han, S.; Sun, X.; Li, T.; Shen, L. Timely inhibition of Notch signaling by DAPT promotes cardiac differentiation of murine pluripotent stem cells. PLoS ONE 2014, 9, e109588. [Google Scholar] [CrossRef]
- Karamboulas, C.; Swedani, A.; Ward, C.; Al-Madhoun, A.S.; Wilton, S.; Boisvenue, S.; Ridgeway, A.G.; Skerjanc, I.S. HDAC activity regulates entry of mesoderm cells into the cardiac muscle lineage. J. Cell Sci. 2006, 119, 4305–4314. [Google Scholar] [CrossRef]
- Dossou, A.S.; Basu, A. The emerging roles of mTORC1 in macromanaging autophagy. Cancers 2019, 11, 1422. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, J.; Tang, L.; Shi, J.; Zhu, D. mTORC1 and mTORC2 play different roles in regulating cardiomyocyte differentiation from embryonic stem cells. Int. J. Dev. Biol. 2017, 61, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Proud, C.G. Crosstalk between mTOR complexes. Nat. Cell Biol. 2013, 15, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev. Physiol. Biochem. Pharmacol. 2014, 166, 43–95. [Google Scholar] [PubMed]
- Burnett, P.E.; Barrow, R.K.; Cohen, N.A.; Snyder, S.H.; Sabatini, D.M. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl. Acad. Sci. USA 1998, 95, 1432–1437. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.-H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Song, M.; Csordas, G.; Kelly, D.P.; Matkovich, S.J.; Dorn, G.W. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science 2015, 350, aad2459. [Google Scholar] [CrossRef]
- Lampert, M.A.; Orogo, A.M.; Najor, R.H.; Hammerling, B.C.; Leon, L.J.; Wang, B.J.; Kim, T.; Sussman, M.A.; Gustafsson, Å. B BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy 2019, 15, 1182–1198. [Google Scholar] [CrossRef]
- Rodger, C.E.; McWilliams, T.G.; Ganley, I.G. Mammalian mitophagy–from in vitro molecules to in vivo models. FEBS J. 2018, 285, 1185–1202. [Google Scholar] [CrossRef]
- Montava-Garriga, L.; Ganley, I.G. Outstanding questions in mitophagy: What we do and do not know. J. Mol. Biol. 2020, 432, 206–230. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Padman, B.S.; Lazarou, M. Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends Cell Biol. 2016, 26, 733–744. [Google Scholar] [CrossRef]
- Ishihara, T.; Ban-Ishihara, R.; Maeda, M.; Maeda, Y.; Ichimura, A.; Kyogoku, S.; Aoki, H.; Katada, S.; Nakada, K.; Nomura, M.; et al. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol. Cell Biol. 2015, 35, 211–223. [Google Scholar] [CrossRef]
- Spaan, J.A.; Piek, J.J.; Hoffman, J.I.E.; Siebes, M. Physiological basis of clinically used coronary hemodynamic indices. Circulation 2006, 113, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Manzella, N.; Santin, Y.; Maggiorani, D.; Martini, H.; Douin-Echinard, V.; Passos, J.F.; Lezoualc’H, F.; Binda, C.; Parini, A.; Mialet-Perez, J. Monoamine oxidase-A is a novel driver of stress-induced premature senescence through inhibition of parkin-mediated mitophagy. Aging Cell 2018, 17, e12811. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Semenza, G.L. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: Implications for models of hypoxia signal transduction. Blood 1993, 82, 3610–3615. [Google Scholar] [CrossRef]
- Zhao, J.F.; Rodger, C.E.; Allen, G.F.; Weidlich, S.; Ganley, I.G. HIF1α-dependent mitophagy facilitates cardiomyoblast differentiation. Cell Stress 2020, 4, 99. [Google Scholar] [CrossRef]
- Tapaswi, D. Liposomes as a potential drug delivery system: A Review. Int. Res. J. Pharm. 2013, 4, 1–7. [Google Scholar]
- Shi, X.; Li, W.; Liu, H.; Yin, D.; Zhao, J. β-Cyclodextrin induces the differentiation of resident cardiac stem cells to cardiomyocytes through autophagy. BBA-Mol. Cell Res. 2017, 1864, 1425–1434. [Google Scholar] [CrossRef]
- Hatani, T.; Miki, K.; Yoshida, Y. Induction of human induced pluripotent stem cells to cardiomyocytes using embryoid bodies. In Experimental Models of Cardiovascular Diseases; Ishikawa, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1816, pp. 79–92. [Google Scholar]
- Tran, T.H.; Wang, X.; Browne, C.; Zhang, Y.; Schinke, M.; Izumo, S.; Burcin, M. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells 2009, 27, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Ichikawa, H.; Johkura, K.; Mogi, A.; No, H.; Yoshie, S.; Tomotsune, D.; Sasaki, K. Bone morphogenetic protein-4 promotes induction of cardiomyocytes from human embryonic stem cells in serum-based embryoid body development. Am. J. Physiol. Circ. Physiol. 2009, 296, H1793–H1803. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Huang, Y.; Chang, J.Y.; Liu, L.; McKeehan, W.L.; Martin, J.F.; Wang, F. FRS2α-mediated FGF signals suppress premature differentiation of cardiac stem cells through regulating autophagy activity. Circ. Res. 2012, 110, e29–e39. [Google Scholar] [CrossRef]
- Abu-Issa, R.; Smyth, G.; Smoak, I.; Yamamura, K.I.; Meyers, E.N. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 2002, 129, 4613–4625. [Google Scholar] [CrossRef]
- Vervliet, T.; Duelen, R.; Rovere, R.L.; Roderick, H.L.; Sampaolesi, M. Cardiomyocyte differentiation from iPS cells is delayed following knockout of Bcl-2. bioRxiv 2022. [Google Scholar] [CrossRef]
- Krantz, S.; Kim, Y.-M.; Srivastava, S.; Leasure, J.W.; Toth, P.T.; Marsboom, G.; Rehman, J. Mitophagy mediates metabolic reprogramming of induced pluripotent stem cells undergoing endothelial differentiation. J. Biol. Chem. 2021, 297, 101410. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, B. Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 2011, 27, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Hardie, D.G. AMPK: Sensing glucose as well as cellular energy status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Rodriguez, M.L.; Leonard, A.; Sun, L.; Fischer, K.A.; Wang, Y.; Ritterhoff, J.; Zhao, L.; Kolwicz, S.C., Jr.; Pabon, L.; et al. Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. 2019, 13, 657–668. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Hill, B.G.; Dranka, B.P.; Zou, L.; Chatham, J.C.; Darley-Usmar, V.M. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem. J. 2009, 424, 99–107. [Google Scholar] [CrossRef]
- Tatti, M.; Motta, M.; Scarpa, S.; Di Bartolomeo, S.; Cianfanelli, V.; Tartaglia, M.; Salvioli, R. BCM-95 and (2-hydroxypropyl)-β-cyclodextrin reverse autophagy dysfunction and deplete stored lipids in Sap C-deficient fibroblasts. Hum. Mol. Genet. 2015, 24, 4198–4211. [Google Scholar] [CrossRef]
- Onodera, R.; Motoyama, K.; Tanaka, N.; Ohyama, A.; Okamatsu, A.; Higashi, T.; Kariya, R.; Okada, S.; Arima, H. Involvement of autophagy in antitumor activity of folate-appended methyl-β-cyclodextrin. Sci. Rep. 2014, 4, 4417. [Google Scholar] [CrossRef]
- Soares, C.P.; Portilho, D.M.; da Silva Sampaio, L.; Einicker-Lamas, M.; Morales, M.M.; Costa, M.L.; dos Santos Mermelstein, C. Membrane cholesterol depletion by methyl-β-cyclodextrin enhances the expression of cardiac differentiation markers. Cells Tissues Organs 2010, 192, 187–199. [Google Scholar] [CrossRef]
- Ng, M.Y.W.; Wai, T.; Simonsen, A. Quality control of the mitochondrion. Dev. Cell 2021, 56, 881–905. [Google Scholar] [CrossRef]
- Mathieu, J.; Ruohola-Baker, H. Metabolic remodeling during the loss and acquisition of pluripotency. Development 2017, 144, 541–551. [Google Scholar] [CrossRef]
- Chung, S.; Arrell, D.K.; Faustino, R.S.; Terzic, A.; Dzeja, P.P. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J. Mol. Cell. Cardiol. 2010, 48, 725–734. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1α signaling pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Jedrzejas, M.J. Structure, function, and evolution of phosphoglycerate mutases: Comparison with fructose-2, 6-bisphosphatase, acid phosphatase, and alkaline phosphatase. Prog. Biophys. Mol. 2000, 73, 263–287. [Google Scholar] [CrossRef]
- Bernkopf, D.B.; Jalal, K.; Brückner, M.; Knaup, K.X.; Gentzel, M.; Schambony, A.; Behrens, J. Pgam5 released from damaged mitochondria induces mitochondrial biogenesis via Wnt signaling. J. Cell Biol. 2018, 217, 1383–1394. [Google Scholar] [CrossRef]
- Lorzadeh, S.; Kohan, L.; Ghavami, S.; Azarpira, N. Autophagy and the Wnt signaling pathway: A focus on Wnt/β-catenin signaling. BBA-Mol. Cell Res. 2021, 1868, 118926. [Google Scholar] [CrossRef]
- Ding, Q.; Qi, Y.; Tsang, S.Y. Mitochondrial biogenesis, mitochondrial dynamics, and mitophagy in the maturation of cardiomyocytes. Cells 2021, 10, 2463. [Google Scholar] [CrossRef]
- Yang, M.; Fu, J.-D.; Zou, J.; Sridharan, D.; Zhao, M.-T.; Singh, H. Assessment of mitophagy in human iPSC-derived cardiomyocytes. Autophagy 2022, 18, 2481–2494. [Google Scholar] [CrossRef]
| No. | Organism/Cell | Autophagy Inducer/Inhibitor | Genetic Model/Modification | Key Result | In Vivo/In Vitro | Ref. |
|---|---|---|---|---|---|---|
| 1 | Mouse | - | yes | FGF signaling promotes cardiac lineage determination at early stages and suppresses premature differentiation at late stages. In addition, autophagy plays a crucial role in mediating growth factor signaling pathways in regulating heart progenitor differentiation. | In vivo/In vitro | [62] |
| 2 | P19CL6 cell line | Rapamycin 3-MA MG-132 | yes | The main effectors of Wnt and Notch signaling pathways, β-catenin and NICD, could form a complex with LC3 and P62 and could then be degraded by autophagy | In vitro | [37] |
| 3 | H9c2 cells SH-SY5Y cells | bafilomycin A1 | no | During differentiation, HIF1α was upregulated and was required for cardiomyoblast differentiation as well as mitophagy. | In vitro | [55] |
| 4 | mES cells (D3) | shRNA-Raptor or shRNA-Rictor | Knockdown of both Raptor- and Rictor-suppressed mES cell differentiation into cardiomyocytes, which was similar to the effect of shRNA-Rictor in mES cells. | In vitro | [41] | |
| 5 | hiPSCs | Rapamycin/NH4Cl | - | Rapamycin promoted EB-based cardiomyocyte differentiation of hiPSCs in a stage-dependent manner. | In vitro | [21] |
| 6 | mESCs | Rapamycin/Ku0063794/AA | - | Potential role of mTOR signaling in cardiac differentiation | In vitro | [1] |
| 7 | hESC (H9, H7)/hiPSCs (hiPS-U-Q1) | LiCl, HN4Cl, rapamycin, LY294002, Wortmannin, PD98059, PD0325901, SB431542, SB203580, SP600125, retinoic acid, Asiatic acid, Y27632, thiazovivin, z-VAD-FMK, VPA, TSA, VO-OHpic, SF1670, KU-55933, resveratrol, STR1720, CX-4945, ABT-737, nutlin-3, pifithrin-a, pifithrin-l, GSK1904529A, and FG-4592. | - | mTOR possesses a wide variety of effects on cardiogenesis from hPSCs | In vitro | [22] |
| 8 | hiPSCs | AICAR | - | Activation of AMPK by AICAR is an effective means to promote morphological and metabolic maturation of hiPSC-CMs | [19] | |
| 9 | P19 | H2O2, Rapamycin, Tunicamycin | - | The activation of Gata4 in transcription is promoted by appropriate ROS | In vitro | [16] |
| 10 | hiPSC (A18945) | - | CRISPR/Cas9-mediated knock out of Bcl-2 | Loss of Bcl-2 delays cardiomyocyte development from hiPSC by regulating c-Myc expression | In vitro | [64] |
| 11 | hiPSC | Glucose starvation | Mfn2 shRNA PGAM5 shRNA | Mitophagy could help generate mature, differentiated cells | In vitro | [65] |
| 12 | Cardiac Sca-1+ cells from adult C57BL/6 mice | β-cyclodextrin (β-CD), 3-methyladenine (3MA), Bafilomycin A1 (Baf A1) | Atg5 knock down by siRNA | β-CD treatment induced autophagy differentiation through the JNK/STAT3 pathway | In vivo | [57] |
| 13 | hESCs | Metformin, HCQ | - | While the induction of autophagy leads to the production of mature cardiomyocytes, the inhibition of autophagy leads to a dramatic decrease in the number of cardiac cells. | In vitro | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolahdouzmohammadi, M.; Kolahdouz-Mohammadi, R.; Tabatabaei, S.A.; Franco, B.; Totonchi, M. Revisiting the Role of Autophagy in Cardiac Differentiation: A Comprehensive Review of Interplay with Other Signaling Pathways. Genes 2023, 14, 1328. https://doi.org/10.3390/genes14071328
Kolahdouzmohammadi M, Kolahdouz-Mohammadi R, Tabatabaei SA, Franco B, Totonchi M. Revisiting the Role of Autophagy in Cardiac Differentiation: A Comprehensive Review of Interplay with Other Signaling Pathways. Genes. 2023; 14(7):1328. https://doi.org/10.3390/genes14071328
Chicago/Turabian StyleKolahdouzmohammadi, Mina, Roya Kolahdouz-Mohammadi, Seyed Abdolhossein Tabatabaei, Brunella Franco, and Mehdi Totonchi. 2023. "Revisiting the Role of Autophagy in Cardiac Differentiation: A Comprehensive Review of Interplay with Other Signaling Pathways" Genes 14, no. 7: 1328. https://doi.org/10.3390/genes14071328
APA StyleKolahdouzmohammadi, M., Kolahdouz-Mohammadi, R., Tabatabaei, S. A., Franco, B., & Totonchi, M. (2023). Revisiting the Role of Autophagy in Cardiac Differentiation: A Comprehensive Review of Interplay with Other Signaling Pathways. Genes, 14(7), 1328. https://doi.org/10.3390/genes14071328







