Upregulation of Heat-Shock Protein (hsp)-27 in a Patient with Heterozygous SPG11 c.1951C>T and SYNJ1 c.2614G>T Mutations Causing Clinical Spastic Paraplegia
Abstract
1. Introduction
2. Methods
2.1. Ethics Approval and Informed Consent
2.2. Patient Sequencing
2.3. Hsp27 Analysis in the CSF
2.4. Measurement of Dopamine Levels in CSF
2.5. Statistical Analysis
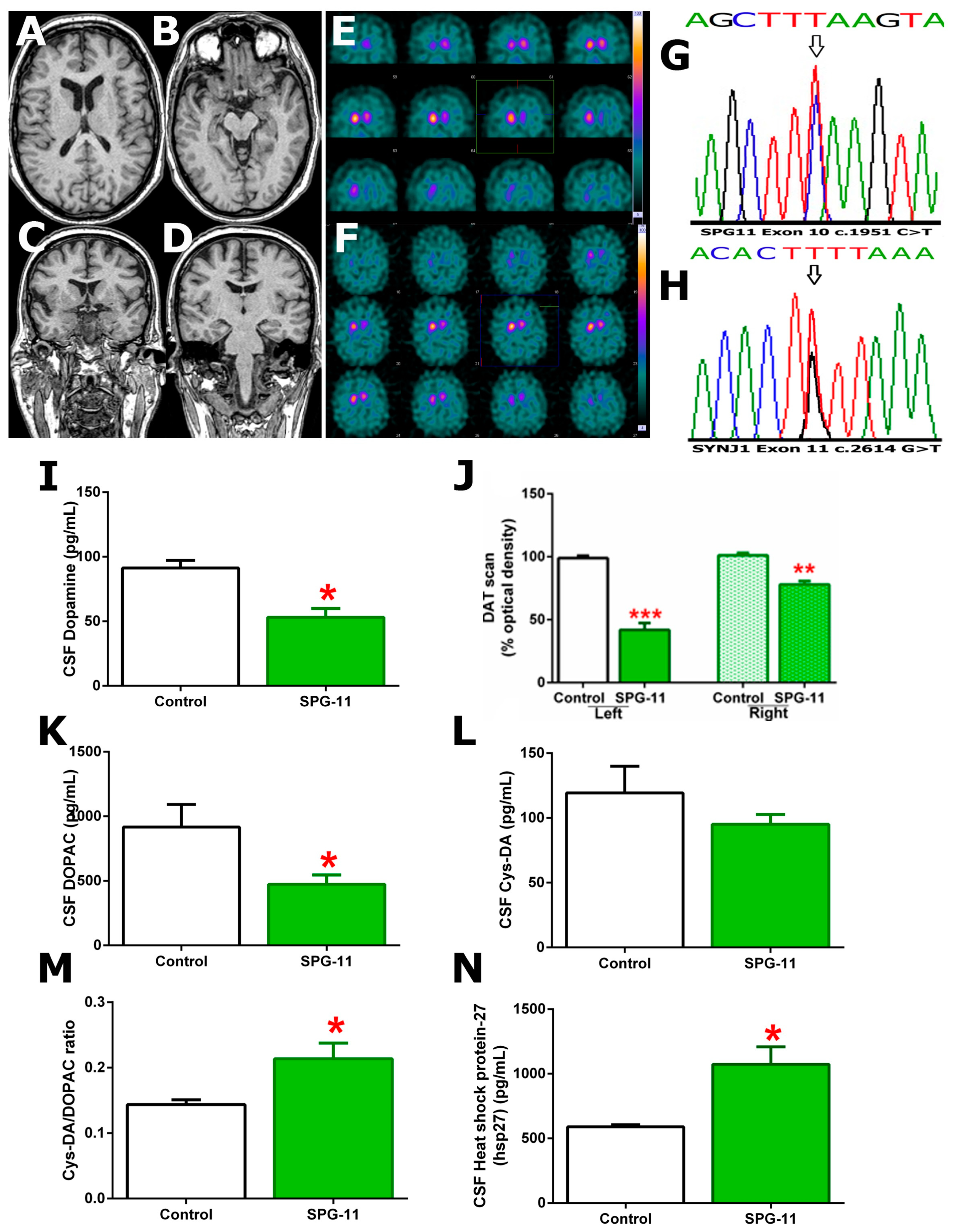
3. Results
DA, DA Oxidation, and Heat-Shock Protein 27
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyyazhagan, A.; Orlacchio, A. Hereditary Spastic Paraplegia: An Update. Int. J. Mol. Sci. 2022, 23, 1697. [Google Scholar] [CrossRef] [PubMed]
- Shribman, S.; Reid, E.; Crosby, A.H.; Houlden, H.; Warner, T.T. Hereditary spastic paraplegia: From diagnosis to emerging therapeutic approaches. Lancet Neurol. 2019, 18, 1136–1146. [Google Scholar] [CrossRef]
- Faber, I.; Pereira, E.R.; Martínez, A.R.; França, M., Jr.; Teive, H.A.G. Hereditary spastic paraplegia from 1880 to 2017: An historical review. Arq. Neuropsiquiatr. 2017, 75, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Parodi, L.; Fenu, S.; Stevanin, G.; Durr, A. Hereditary spastic paraplegia: More tan upper motor neuron disease. Rev. Neurol. 2017, 173, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Boutry, M.; Morais, S.; Stevanin, G. Update on the Genetics of Spastic Paraplegias. Curr. Neurol. Neurosci. Rep. 2019, 19, 18. [Google Scholar] [CrossRef]
- Finsterer, J.; Loscher, W.; Quasthoff, S.; Wanschitz, J.; Auer-Grumbach, M.; Stevanin, G. Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance. J. Neurol. Sci. 2012, 318, 1–18. [Google Scholar] [CrossRef]
- Schüle, R.; Wiethoff, S.; Martus, P.; Karle, K.N.; Otto, S.; Klebe, S.; Klimpe, S.; Gallenmüller, C.; Kurzwelly, D.; Henkel, D.; et al. Hereditary spastic paraplegia: Clinicogenetic lessons from 608 patients. Ann. Neurol. 2016, 79, 646–658. [Google Scholar] [CrossRef]
- Méreaux, J.L.; Banneau, G.; Papin, M.; Coarelli, G.; Valter, R.; Raymond, L.; Kol, B.; Ariste, O.; Parodi, L.; Tissier, L.; et al. Clinical and genetic spectra of 1550 index patients with hereditary spastic paraplegia. Brain 2022, 145, 1029–1037. [Google Scholar] [CrossRef]
- Kara, E.; Tucci, A.; Manzoni, C.; Lynch, D.S.; Elpidorou, M.; Bettencourt, C.; Chelban, V.; Manole, A.; Hamed, S.A.; Haridy, N.A.; et al. Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain 2016, 139 Pt 7, 1904–1918. [Google Scholar] [CrossRef]
- D’Amore, A.; Tessa, A.; Casali, C.; Dotti, M.T.; Filla, A.; Silvestri, G.; Antenora, A.; Astrea, G.; Barghigiani, M.; Battini, R.; et al. Next Generation Molecular Diagnosis of Hereditary Spastic Paraplegias: An Italian Cross-Sectional Study. Front. Neurol. 2018, 9, 981. [Google Scholar] [CrossRef]
- Panza, E.; Meyyazhagan, A.; Orlacchio, A. Hereditary spastic paraplegia: Genetic heterogeneity and common pathways. Exp. Neurol. 2022, 357, 114203. [Google Scholar] [CrossRef] [PubMed]
- Darios, F.; Mochel, F.; Stevanin, G. Lipids in the Physiopathology of Hereditary Spastic Paraplegias. Front. Neurosci. 2020, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, Z.; O’Kane, C.J.; Pérez-Moreno, J.J. Axonal Endoplasmic Reticulum Dynamics and Its Roles in Neurodegeneration. Front. Neurosci. 2020, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.Y.; Zhu, Y.; Shen, Y.; Yue, Z. Crosstalk between presynaptic trafficking and autophagy in Parkinson’s disease. Neurobiol. Dis. 2019, 122, 64–71. [Google Scholar] [CrossRef]
- Chang, J.; Lee, S.; Blackstone, C. Spastic paraplegia proteins spastizin and spatacsin mediate autophagic lysosome reformation. J. Clin. Investig. 2014, 124, 5249–5262. [Google Scholar] [CrossRef] [PubMed]
- Vantaggiato, C.; Panzeri, E.; Castelli, M.; Citterio, A.; Arnoldi, A.; Santorelli, F.M.; Liguoru, R.; Scarlato, M.; Musumeci, O.; Toscano, A.; et al. ZFYVE26/SPASTIZIN and SPG11/SPATACSIN mutations in hereditary spastic paraplegia types AR-SPG15 and AR-SPG11 have different effects on autophagy and endocytosis. Autophagy 2019, 15, 34–57. [Google Scholar] [CrossRef]
- Treweek, T.M.; Meehan, S.; Ecroyd, H.; Carver, J.A. Small heat-shock proteins: Important players in regulating cellular proteostasis. Cell. Mol. Life Sci. 2014, 72, 429–451. [Google Scholar] [CrossRef]
- Cox, D.; Carver, J.A.; Ecroyd, H. Preventing synuclein aggregation: The role of the small heat-shock molecular chaperone proteins. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1830–1843. [Google Scholar] [CrossRef]
- Xilouri, M.; Brekk, O.R.; Polissidis, A.; Chrysanthou-Piterou, M.; Kloukina, I.; Stefanis, L. Impairment of chaperone-mediated autophagy induces dopaminergic neurodegeneration in rats. Autophagy 2016, 12, 2230–2247. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, B.; Liu, W.; Xu, Q.; Hou, L.; Song, J.; Guo, Q.; Ning, L. Dysfunction of chaperone-mediated autophagy in human diseases. Mol. Cell. Biochem. 2021, 476, 1439–1454. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Cuervo, A.M. Entering the lysosome through a transient gate by chaperone-mediated autophagy. Autophagy 2008, 4, 1101–1103. [Google Scholar] [CrossRef]
- Muranova, L.K.; Ryzhavskaya, A.S.; Sudnitsyna, M.V.; Shatov, V.M.; Gusev, N.B. Small heat shock proteins and human neurodegenerative diseases. Biochemistry 2019, 84, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 2017, 357, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Huenchuguala, S.; Paris, I.; Segura-Aguila, J. Dopamine oxidation and autophagy. Park. Dis. 2012, 2012, 920953. [Google Scholar] [CrossRef]
- Stefani, M.; Dobson, C.M. Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 2003, 81, 678–699. [Google Scholar] [CrossRef] [PubMed]
- Schule, R.; Holland-Letz, T.; Klimpe, S.; Kassubek, J.; Klopsock, T.; Mall, V.; Otto, S.; Winner, B.; Schols, L. The Spastic Paraplegia Rating Scale (SPRS): A reliable and valid measure of disease severity. Neurology 2006, 67, 430–434. [Google Scholar] [CrossRef] [PubMed]
- García-Carmona, J.A.; Yousefzadeh, M.J.; Alarcón-Soldevilla, F.; Fages-Caravaca, E.; Kieu, T.L.; Witt, M.A.; López-Ávila, Á.; Niedernhofer, L.J.; Pérez-Vicente, J.A. Case Report: Identification of a Heterozygous XPA c.553C>T Mutation Causing Neurological Impairment in a Case of Xeroderma Pigmentosum Complementation Group A. Front. Genet. 2021, 12, 717361. [Google Scholar] [CrossRef]
- García-Carmona, J.A.; Georgiou, P.; Zanos, P.; Bailey, A.; Laorden, M.L. Methamphetamine withdrawal induces activation of CRF neurons in the brain stress system in parallel with an increased activity of cardiac sympathetic pathways. Naunyn Schmiedeberg’s Arch. Pharmacol. 2018, 391, 423–434. [Google Scholar] [CrossRef]
- González-Sepulveda, M.; Laguna, A.; Carballo-Carbajal, I.; Galiano-Landeira, J.; Romero-Gimenez, J.; Cuadros, T.; Parent, A.; Peñuelas, N.; Compte, J.; Nicolau, A.; et al. Validation of a Reversed Phase UPLC-MS/MS Method to Determine Dopamine Metabolites and Oxidation Intermediates in Neuronal Differentiated SH-SY5Y Cells and Brain Tissue. ACS Chem. Neurosci. 2020, 11, 2679–2687. [Google Scholar] [CrossRef]
- Stevanin, G.; Azzedine, H.; Denora, P.; Boukhris, A.; Tazir, M.; Lossos, A.; Rosa, A.L.; Lerer, I.; Hamri, A.; Alegria, P.; et al. Mutations in SPG11 are frequent in autosomal recessive spastic paraplegia with thin corpus callosum, cognitive decline and lower motor neuron degeneration. Brain 2008, 131, 772–784. [Google Scholar] [CrossRef]
- Santos, M.; Damásio, J.; Carmona, S.; Nieto, J.L.; Dehghani, N.; Correia Guedes, L.; Barbot, C.; Barros, J.; Brás, J.; Sequeiros, J.; et al. Molecular characterization of Portuguese patients with hereditary cerebellar ataxia. Cells 2022, 11, 981. [Google Scholar] [CrossRef]
- Pérez-Brangulí, F.; Mishra, H.K.; Prots, I.; Havlicek, S.; Kohl, Z.; Saul, D.; Rummel, C.; Dorca-Arevalo, J.; Regensburger, M.; Graef, D.; et al. Dysfunction of spatacsin leads to axonal pathology in SPG11-linked hereditary spastic paraplegia. Hum. Mol. Genet. 2014, 23, 4859–4874. [Google Scholar] [CrossRef]
- Pozner, T.; Regensburger, M.; Engelhorn, T.; Winkler, J.; Winner, B. Janus-faced spatacsin (SPG11): Involvement in neurodevelopment and multisystem neurodegeneration. Brain 2020, 143, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Mani, M.; Lee, S.Y.; Lucast, L.; Cremona, O.; Di Paolo, G.; De Camilli, P.; Ryan, T.A. The dual phosphatase activity of synaptojanin1 is required for both efficient synaptic vesicle endocytosis and reavailability at nerve terminals. Neuron 2007, 56, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Vanhauwaert, R.; Kuenen, S.; Masius, R.; Bademosi, A.; Manetsberger, J.; Schoovaerts, N.; Bounti, L.; Gontcharenko, S.; Swerts, J.; Vilain, S.; et al. The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals. EMBO J. 2017, 36, 1392–1411. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.Y.; Sheehan, P.; Wang, Q.; Zhu, X.; Zhang, Y.; Choi, I.; Li, X.; Saenz, J.; Zhu, J.; Wang, J.; et al. Synj1 haploinsufficiency causes dopamine neuron vulnerability and α-synuclein accumulation in mice. Hum. Mol. Genet. 2020, 29, 2300–2312. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Sullivan, P.; Holmes, C.; Mash, D.C.; Kopin, I.J.; Sharabi, Y. Determinants of denervation-independent depletion of putamen dopamine in Parkinson’s disease and multiple system atrophy. Park. Relat. Disord. 2017, 35, 88–91, Erratum in Park. Relat. Disord. 2017, 39, 95. [Google Scholar] [CrossRef]
- Riederer, P.; Youdim, M.B. Monoamine oxidase activity and monoamine metabolism in brains of parkinsonian patients treated with l-deprenyl. J. Neurochem. 1986, 46, 1359–1365. [Google Scholar] [CrossRef]
- Fornstedt, B.; Rosengren, E.; Carlsson, A. Occurrence and distribution of 5-S-cysteinyl derivatives of dopamine, dopa and dopac in the brains of eight mammalian species. Neuropharmacology 1986, 25, 451–454. [Google Scholar] [CrossRef]
- Carlsson, A.; Fornstedt, B. Catechol metabolites in the cerebrospinal fluid as possible markers in the early diagnosis of Parkinson’s disease. Neurology 1991, 41, 50–52. [Google Scholar]
- Webster, J.M.; Darling, A.L.; Uversky, V.N.; Blair, L.J. Small heat shock proteins, big impact on protein aggregation in neurodegenerative disease. Front. Pharm. 2019, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, J.; Ton, J.; Negi, S.; Stephens, D.; Pountney, D.L.; Preiss, T.; Carver, J.A. The Effect of Oxidized Dopamine on the Structure and Molecular Chaperone Function of the Small Heat-Shock Proteins, B-Crystallin and Hsp27. Int. J. Mol. Sci. 2021, 22, 3700. [Google Scholar] [CrossRef] [PubMed]
| Mutations | SPG11 c.1951C>T, p.R651* (Ch. 15, exon 10) |
| SYNJ1 c.2614G>T, p.D872Y (Ch. 21, exon 11) | |
| Age | 48 |
| Ethnicity | Caucasian, Mediterranean European |
| Symptom prompting first neurological examination | Cognitive decline at 47 years of age |
| Sequence of neurological symptoms | 1 epilepsy crisis, cognitive decline, spasticity, gait impairment |
| Height (cm); weight (Kg) | 175 cm; 83 Kg; BMI = 27 |
| Developmental milestones | Normal |
| Education | Primary school |
| Activities of daily living | Independent until gait impairment |
| Resting tremor | No |
| Slurred speech | No |
| Tendon reflexes; plantar response | Hyperreflexia with clonus; extensor |
| Levodopa responsive | No |
| Urinary incontinence | No |
| Brain MR imaging | Normal |
| DAT-scan | DAT loss in substantia nigra |
| EMG/ENG | Sensorimotor polyneuropathy in lower limbs |
| SPRS on admission | 36/52 |
| SPRS 1 year before | 9/52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Carmona, J.A.; Amores-Iniesta, J.; Soler-Usero, J.; Cerdán-Sánchez, M.; Navarro-Zaragoza, J.; López-López, M.; Soria-Torrecillas, J.J.; Ballesteros-Arenas, A.; Pérez-Vicente, J.A.; Almela, P. Upregulation of Heat-Shock Protein (hsp)-27 in a Patient with Heterozygous SPG11 c.1951C>T and SYNJ1 c.2614G>T Mutations Causing Clinical Spastic Paraplegia. Genes 2023, 14, 1320. https://doi.org/10.3390/genes14071320
García-Carmona JA, Amores-Iniesta J, Soler-Usero J, Cerdán-Sánchez M, Navarro-Zaragoza J, López-López M, Soria-Torrecillas JJ, Ballesteros-Arenas A, Pérez-Vicente JA, Almela P. Upregulation of Heat-Shock Protein (hsp)-27 in a Patient with Heterozygous SPG11 c.1951C>T and SYNJ1 c.2614G>T Mutations Causing Clinical Spastic Paraplegia. Genes. 2023; 14(7):1320. https://doi.org/10.3390/genes14071320
Chicago/Turabian StyleGarcía-Carmona, Juan Antonio, Joaquín Amores-Iniesta, José Soler-Usero, María Cerdán-Sánchez, Javier Navarro-Zaragoza, María López-López, Juan José Soria-Torrecillas, Ainhoa Ballesteros-Arenas, José Antonio Pérez-Vicente, and Pilar Almela. 2023. "Upregulation of Heat-Shock Protein (hsp)-27 in a Patient with Heterozygous SPG11 c.1951C>T and SYNJ1 c.2614G>T Mutations Causing Clinical Spastic Paraplegia" Genes 14, no. 7: 1320. https://doi.org/10.3390/genes14071320
APA StyleGarcía-Carmona, J. A., Amores-Iniesta, J., Soler-Usero, J., Cerdán-Sánchez, M., Navarro-Zaragoza, J., López-López, M., Soria-Torrecillas, J. J., Ballesteros-Arenas, A., Pérez-Vicente, J. A., & Almela, P. (2023). Upregulation of Heat-Shock Protein (hsp)-27 in a Patient with Heterozygous SPG11 c.1951C>T and SYNJ1 c.2614G>T Mutations Causing Clinical Spastic Paraplegia. Genes, 14(7), 1320. https://doi.org/10.3390/genes14071320






