Diagnostic Yield of Exome Sequencing in Fetuses with Sonographic Features of Skeletal Dysplasias but Normal Karyotype or Chromosomal Microarray Analysis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Strategy Searches
2.3. Study Selection
2.4. Data Extraction
2.5. Data Synthesis
2.6. Subgroup Analysis
- Abnormal curvature of long bones;
- Suspected fracture of bones, including those with angulated long bones;
- Reduced or abnormal ossification of bones;
- Absent long bones (including radius, tibia, etc.), absent or abnormal phalanges;
- Abnormal joint posture, including talipes, contractures;
- Abnormal skull, including abnormal skull shape, macrocephaly;
- Abnormal facial profile, including flattened face, absent nasal bone, retro/micrognathia;
- Small chest, including bell-shaped thorax, small chest circumference
- Abnormal spine, including scoliosis;
- Hydropic features, including cystic hygroma, subcutaneous edema, pleural effusion.
3. Results
3.1. Characteristics of This Study Cohort
3.2. Pooled Diagnostic Yield with ES
3.3. Subgorup Analysis—Isolated and Nonisolated Short Bone
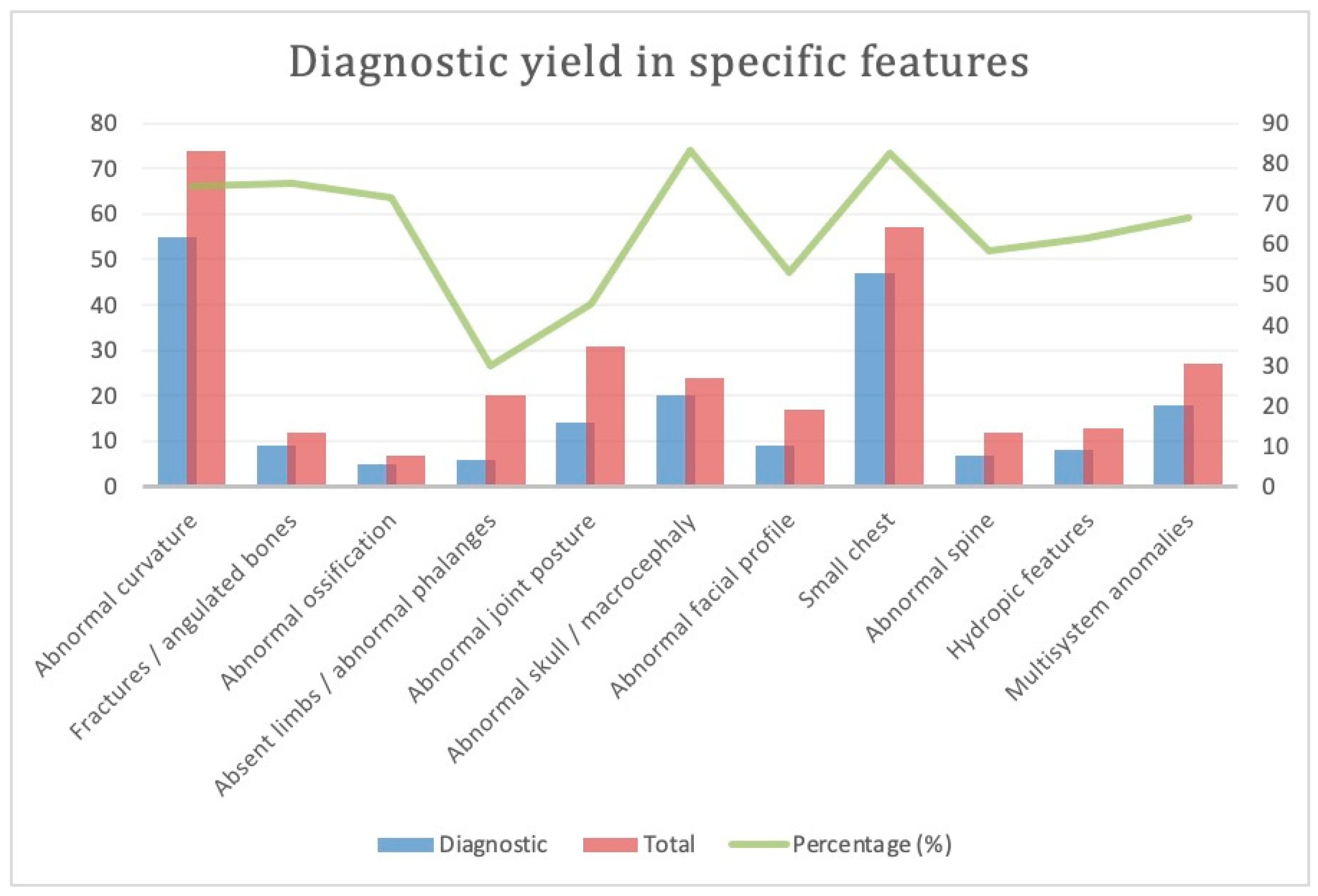
3.4. Subgroup Analysis: Various Phenotypes for Skeletal Dysplasia (Figure 3)
3.4.1. Abnormal Curvature
3.4.2. Suspected Fracture or Angulated Long Bones
3.4.3. Reduced or Abnormal Ossification
3.4.4. Absent Long Bone, Absent or Abnormal Phalanges
3.4.5. Abnormal Joint Posture, Contractures
3.4.6. Abnormal Skull
3.4.7. Abnormal Face
3.4.8. Small Chest
3.4.9. Abnormal Spine
3.4.10. Hydropic Features
3.4.11. Multisystem Anomalies
4. Discussion
4.1. Diagnostic Yield
4.2. Optimization of Prenatal ES in the Era of CMA
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Fetal Medicine Foundation. Skeletal Dysplasia. Available online: https://fetalmedicine.org/education/fetal-abnormalities/skeleton/skeletal-dysplasia (accessed on 15 April 2023).
- Rasmusen, S.A.; Bieber, F.R.; Benecerraf, B.R.; Lachman, R.S.; Rimoin, D.L.; Holmes, L.B. Epidemiology of osteochondrodysplasias: Changing trends due to advances in prenatal diagnosis. Am. J. Med. Genet. 1996, 61, 49–58. [Google Scholar] [CrossRef]
- Camera, G.; Mastroiacovo, P. Birth prevalence of skeletal dysplasias in the Italian multicentric monitoring system for birth defects. Prog. Clin. Biol. Res. 1982, 104, 441–449. [Google Scholar]
- Mortier, G.R.; Cohn, D.H.; Cormier-Daire, V.; Hall, C.; Krakow, D.; Mundlos, S.; Nishimura, G.; Robertson, S.; Sangiorgi, L.; Savarirayan, R.; et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am. J. Med. Genet. Part. A 2019, 179, 2393–2419. [Google Scholar] [CrossRef]
- Wapner, R.J.; Martin, C.L.; Levy, B.; Ballif, B.C.; Eng, C.M.; Zachary, J.M.; Savage, M.; Platt, L.D.; Saltzman, D.; Grobman, W.A.; et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N. Engl. J. Med. 2012, 367, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Srebniak, M.I.; Joosten, M.; Knapen, M.F.C.M.; Arends, L.R.; Polak, M.; van Veen, S.; Go, A.T.J.I.; Van Opstal, D. Frequency of submicroscopic chromosomal aberrations in pregnancies without increased risk for structural chromosomal aberrations: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, L.G.; Dabell, M.P.; Fisher, A.J.; Coppinger, J.; Bandholz, A.M.; Ellison, J.W.; Ravnan, J.B.; Torchia, B.S.; Ballif, B.C.; Rosenfeld, J.A. Experience with microarray-based comparative genomic hybridization for prenatal diagnosis in over 5000 pregnancies. Prenat. Diagn. 2012, 10, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, G.A.; Ross, J.P.; Dion, P.A. Exome sequencing in genetic disease: Recent advances and considerations. F1000Research 2020, 9, 1–9. [Google Scholar]
- Lord, J.; McMullan, D.J.; Eberhardt, R.Y.; Rinck, G.; Hamilton, S.J.; Quinlan-Jones, E.; Prigmore, E.; Keelagher, R.; Best, S.K.; Carey, G.K.; et al. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): A cohort study. Lancet 2019, 393, 747–757. [Google Scholar] [CrossRef]
- Petrovski, S.; Aggarwal, V.; Giordano, J.L.; Stosic, M.; Wou, K.; Bier, L.; Spiegel, E.; Brennan, K.; Stong, N.; Jobanputra, V.; et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: A prospective cohort study. Lancet 2019, 393, 758–767. [Google Scholar] [CrossRef]
- Van den Veyver, I.B.; Chandler, N.; Wilkins-Haug, L.E.; Wapner, R.J.; Chitty, L.S. International Society for Prenatal Diagnosis Updated Position Statement on the use of genome-wide sequencing for prenatal diagnosis. Prenat. Diagn. 2022, 42, 796–803. [Google Scholar] [CrossRef]
- de Wit, M.C.; Srebniak, M.I.; Govaerts, L.C.; Van Opstal, D.; Galjaard, R.J.; Go, A.T. Additional value of prenatal genomic array testing in fetuses with isolated structural ultrasound abnormalities and a normal karyotype: A systematic review of the literature. Ultrasound Obstet. Gynecol. 2014, 43, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chandler, N.; Best, S.; Hayward, J.; Faravelli, F.; Mansour, S.; Kivuva, E.; Tapon, D.; Male, A.; DeVile, C.; Chitty, L.S. Rapid prenatal diagnosis using targeted exome sequencing: A cohort study to assess feasibility and potential impact on prenatal counseling and pregnancy management. Anesth. Analg. 2018, 20, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Shen, M.; Yan, Y.; Tan, Y.; Zhang, J.; Wu, J.; Yang, G.; Li, S.; Wang, J.; Ren, Z.; et al. Genetic Analysis in Fetal Skeletal Dysplasias by Trio Whole-Exome Sequencing. BioMed. Res. Int. 2019, 2019, 2492590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Yang, Y.-K.; Liang, Y.; Zhang, T.-J.; Liang, N.; Yang, L.-M.; Li, S.-J.; Shan, D.; Wu, Q.-Q. Prenatal diagnosis of fetal skeletal dysplasia using targeted next-generation sequencing: An analysis of 30 cases. Diagn. Pathol. 2019, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhou, C.; Shi, H.; Mo, Y.; Tan, W.; Sun, T.; Zhu, J.; Li, Q.; Li, H.; Li, Y.; et al. Prenatal diagnosis of skeletal dysplasias using whole exome sequencing in China. Clin. Chim. Acta 2020, 507, 187–193. [Google Scholar] [CrossRef]
- Han, J.; Yang, Y.D.; He, Y.; Liu, W.J.; Zhen, L.; Pan, M.; Yang, X.; Zhang, V.W.; Liao, C.; Li, D.Z. Rapid prenatal diagnosis of skeletal dysplasia using medical trio exome sequencing: Benefit for prenatal counseling and pregnancy management. Prenat. Diagn. 2020, 40, 577–584. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, S.; Huang, X.; Pang, J.; Liu, J.; Hu, J.; Shen, X.; Tang, C.; Wang, H. Whole Exome Sequencing Analysis in Fetal Skeletal Dysplasia Detected by Ultrasonography: An Analysis of 38 Cases. Front. Genet. 2021, 12, 728544. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, Q.; Xiang, J.; Yin, L.; Wang, J.; Wang, T. Whole Exome Sequencing Aids the Diagnosis of Fetal Skeletal Dysplasia. Front. Genet. 2021, 12, 599863. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, Y.; Song, R.; Wang, L.; Xu, H.; Xie, X.; Zhou, H.; Sun, P.; Zhang, M.; Zhao, Q.; et al. Combined exome sequencing and deep phenotyping in highly selected fetuses with skeletal dysplasia during the first and second trimesters improves diagnostic yield. Prenat. Diagn. 2021, 41, 1401–1413. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Teng, Y.; Liang, D.; Li, Z.; Wu, L. Molecular diagnosis for 55 fetuses with skeletal dysplasias by whole-exome sequencing: A retrospective cohort study. Clin. Genet. 2021, 100, 219–226. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M.; Wang, H. Prenatal trio-based whole exome sequencing in fetuses with abnormalities of the skeletal system. Mol. Genet. Genom. 2022, 297, 1017–1026. [Google Scholar] [CrossRef]
- Hui, A.S.; Chau, M.H.K.; Chan, Y.M.; Cao, Y.; Kwan, A.H.; Zhu, X.; Kwok, Y.K.; Chen, Z.; Lao, T.T.; Choy, K.W.; et al. The role of chromosomal microarray analysis among fetuses with normal karyotype and single system anomaly or nonspecific sonographic findings. Acta Obstet. Gynecol. Scand. 2021, 100, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Scocchia, A.; Kangas-Kontio, T.; Irving, M.; Hero, M.; Saarinen, I.; Pelttari, L.; Gall, K.; Valo, S.; Huusko, J.M.; Tallila, J.; et al. Diagnostic utility of next-generation sequencing-based panel testing in 543 patients with suspected skeletal dysplasia. Orphanet J. Rare Dis. 2021, 16, 412. [Google Scholar] [CrossRef] [PubMed]
- Mellis, R.; Oprych, K.; Scotchman, E.; Hill, M.; Chitty, L.S. Diagnostic yield of exome sequencing for prenatal diagnosis of fetal structural anomalies: A systematic review and meta-analysis. Prenat. Diagn. 2022, 42, 662–685. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, C.; Ding, H.; Wang, Y.; Yu, L.; Guo, F.; Li, F.; Shi, X.; Zhang, Y.; Yin, A. Exome sequencing in fetuses with short long bones detected by ultrasonography: A retrospective cohort study. Front. Genet. 2023, 14, 1032346. [Google Scholar] [CrossRef]


| Authors | Year | Total Sample | Diagnostic Yield | Reference |
|---|---|---|---|---|
| Chandler et al. | 2018 | 16 | 13 [81.3%] | [13] |
| Yang K et al. | 2019 | 8 | 6 [75%] | [14] |
| Liu et al. | 2019 | 28 | 16 [57.1] | [15] |
| Tang et al. | 2020 | 8 | 6 [75%] | [16] |
| Han et al. | 2020 | 26 | 23 [88.5%] | [17] |
| Peng et al. | 2021 | 38 | 24 [63.2] | [18] |
| Tang H et al. | 2021 | 15 | 10 [66.7] | [19] |
| Zhang X et al. | 2021 | 27 | 19 [70.4] | [20] |
| Zhang L et al. | 2021 | 55 | 35 [63.6] | [21] |
| Yang et al. | 2022 | 5 | 4 [80%] | [22] |
| Total | 226 | 156 [69.0%] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tse, K.Y.; Surya, I.U.; Irwinda, R.; Leung, K.Y.; Ting, Y.H.; Cao, Y.; Choy, K.W. Diagnostic Yield of Exome Sequencing in Fetuses with Sonographic Features of Skeletal Dysplasias but Normal Karyotype or Chromosomal Microarray Analysis: A Systematic Review. Genes 2023, 14, 1203. https://doi.org/10.3390/genes14061203
Tse KY, Surya IU, Irwinda R, Leung KY, Ting YH, Cao Y, Choy KW. Diagnostic Yield of Exome Sequencing in Fetuses with Sonographic Features of Skeletal Dysplasias but Normal Karyotype or Chromosomal Microarray Analysis: A Systematic Review. Genes. 2023; 14(6):1203. https://doi.org/10.3390/genes14061203
Chicago/Turabian StyleTse, Kai Yeung, Ilham Utama Surya, Rima Irwinda, Kwok Yin Leung, Yuen Ha Ting, Ye Cao, and Kwong Wai Choy. 2023. "Diagnostic Yield of Exome Sequencing in Fetuses with Sonographic Features of Skeletal Dysplasias but Normal Karyotype or Chromosomal Microarray Analysis: A Systematic Review" Genes 14, no. 6: 1203. https://doi.org/10.3390/genes14061203
APA StyleTse, K. Y., Surya, I. U., Irwinda, R., Leung, K. Y., Ting, Y. H., Cao, Y., & Choy, K. W. (2023). Diagnostic Yield of Exome Sequencing in Fetuses with Sonographic Features of Skeletal Dysplasias but Normal Karyotype or Chromosomal Microarray Analysis: A Systematic Review. Genes, 14(6), 1203. https://doi.org/10.3390/genes14061203






