Hypoxia-Inducible Pathway Polymorphisms and Their Role in the Complications of Prematurity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Genotyping
2.3. Data Analysis
3. Results
3.1. Known Risk Factors and Comorbidities
3.2. Frequency of HIF1A and VEGFA SNPs
3.3. HIF1A and VEGFA SNPs and Comorbidities
3.4. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. 2022. Available online: https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth (accessed on 12 December 2022).
- Walani, S.R. Global burden of preterm birth. Int. J. Gynaecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef] [PubMed]
- WHO. Recommendations for Care of the Preterm or Low-Birth-Weight Infant; World Health Organization: Geneva, Switzerland, 2022.
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P. Pathogenesis and prevention of intraventricular hemorrhage. Clin. Perinatol. 2014, 41, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Walsh, M.C.; Vohr, B.R.; Jobe, A.H.; Wright, L.L.; Fanaroff, A.A.; Wrage, L.A.; Poole, K. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005, 116, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 2015, 122, 200–210. [Google Scholar] [CrossRef]
- Tanner, S.M.; Berryhill, T.F.; Ellenburg, J.L.; Jilling, T.; Cleveland, D.S.; Lorenz, R.G.; Martin, C.A. Pathogenesis of necrotizing enterocolitis: Modeling the innate immune response. Am. J. Pathol. 2015, 185, 4–16. [Google Scholar] [CrossRef]
- Ozsurekci, Y.; Aykac, K. Oxidative Stress Related Diseases in Newborns. Oxidative Med. Cell. Longev. 2016, 2016, 2768365. [Google Scholar] [CrossRef]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Buonocore, G.; Perrone, S.; Longini, M.; Vezzosi, P.; Marzocchi, B.; Paffetti, P.; Bracci, R. Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr. Res. 2002, 52, 46–49. [Google Scholar] [CrossRef]
- Buonocore, G.; Perrone, S.; Longini, M.; Terzuoli, L.; Bracci, R. Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies. Pediatr. Res. 2000, 47, 221–224. [Google Scholar] [CrossRef]
- Frajewicki, A.; Laštůvka, Z.; Borbélyová, V.; Khan, S.; Jandová, K.; Janišová, K.; Otáhal, J.; Mysliveček, J.; Riljak, V. Perinatal hypoxic-ischemic damage: Review of the current treatment possibilities. Physiol. Res. 2020, 69 (Suppl. S3), S379–S401. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Oxygen homeostasis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. HIFα targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef]
- Kallio, P.J.; Wilson, W.J.; O’Brien, S.; Makino, Y.; Poellinger, L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J. Biol. Chem. 1999, 274, 6519–6525. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Hon, W.C.; Wilson, M.I.; Harlos, K.; Claridge, T.D.; Schofield, C.J.; Pugh, C.W.; Maxwell, P.H.; Ratcliffe, P.J.; Stuart, D.I.; Jones, E.Y. Structural basis for the recognition of hydroxyproline in HIF-1 α by pVHL. Nature 2002, 417, 975–978. [Google Scholar] [CrossRef]
- Gladek, I.; Ferdin, J.; Horvat, S.; Calin, G.A.; Kunej, T. HIF1A gene polymorphisms and human diseases: Graphical review of 97 association studies. Genes Chromosomes Cancer 2017, 56, 439–452. [Google Scholar] [CrossRef]
- Tanimoto, K.; Yoshiga, K.; Eguchi, H.; Kaneyasu, M.; Ukon, K.; Kumazaki, T.; Oue, N.; Yasui, W.; Imai, K.; Nakachi, K.; et al. Hypoxia-inducible factor-1alpha polymorphisms associated with enhanced transactivation capacity, implying clinical significance. Carcinogenesis 2003, 24, 1779–1783. [Google Scholar] [CrossRef]
- Fu, X.S.; Choi, E.; Bubley, G.J.; Balk, S.P. Identification of hypoxia-inducible factor-1α (HIF-1α) polymorphism as a mutation in prostate cancer that prevents normoxia-induced degradation. Prostate 2005, 63, 215–221. [Google Scholar] [CrossRef]
- Kunej, T. Integrative Map of HIF1A Regulatory Elements and Variations. Genes 2021, 12, 1526. [Google Scholar] [CrossRef]
- Wei, M.H.; Popescu, N.C.; Lerman, M.I.; Merrill, M.J.; Zimonjic, D.B. Localization of the human vascular endothelial growth factor gene, VEGF, at chromosome 6p12. Hum. Genet. 1996, 97, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.; Soden, J.; Brenchley, P.E.; Ralph, S.; Ray, D.W. Haplotype analysis of the polymorphic human vascular endothelial growth factor gene promoter. Cancer Res. 2003, 63, 812–816. [Google Scholar] [PubMed]
- Koukourakis, M.I.; Papazoglou, D.; Giatromanolaki, A.; Bougioukas, G.; Maltezos, E.; Sivridis, E. VEGF gene sequence variation defines VEGF gene expression status and angiogenic activity in non-small cell lung cancer. Lung Cancer 2004, 46, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Luo, J.Q.; Gao, Y.C.; Chen, M.Y.; Chen, X.P.; Zhou, H.H.; Jiang, Y.; Zhang, W. Genetic association of hypoxia inducible factor 1-α (HIF1A) Pro582Ser polymorphism with risk of diabetes and diabetic complications. Aging 2020, 12, 12783–12798. [Google Scholar] [CrossRef]
- Sajjadi, M.S.; Ghandil, P.; Shahbazian, N.; Saberi, A. Association of vascular endothelial growth factor A polymorphisms and aberrant expression of connexin 43 and VEGFA with idiopathic recurrent spontaneous miscarriage. J. Obstet. Gynaecol. Res. 2020, 46, 369–375. [Google Scholar] [CrossRef]
- Wang, X.; Sun, T.; Chen, G.; Gao, H. Association between Vascular Endothelial Growth Factor Gene Polymorphisms and Pre-Eclampsia Susceptibility: An Updated Meta-Analysis. Immunol. Investig. 2020, 49, 120–133. [Google Scholar] [CrossRef]
- Langmia, I.M.; Apalasamy, Y.D.; Omar, S.Z.; Mohamed, Z. Association of VEGFA gene polymorphisms and VEGFA plasma levels with spontaneous preterm birth. Pharm. Genom. 2015, 25, 199–204. [Google Scholar] [CrossRef]
- Mahlman, M.; Huusko, J.M.; Karjalainen, M.K.; Kaukola, T.; Marttila, R.; Ojaniemi, M.; Haataja, R.; Lavoie, P.M.; Rämet, M.; Hallman, M. Genes Encoding Vascular Endothelial Growth Factor A (VEGF-A) and VEGF Receptor 2 (VEGFR-2) and Risk for Bronchopulmonary Dysplasia. Neonatology 2015, 108, 53–59. [Google Scholar] [CrossRef]
- Kosik, K.; Szpecht, D.; Al-Saad, S.R.; Karbowski, L.M.; Kurzawińska, G.; Szymankiewicz, M.; Drews, K.; Wolski, H.; Seremak-Mrozikiewicz, A. Single nucleotide vitamin D receptor polymorphisms (FokI, BsmI, ApaI, and TaqI) in the pathogenesis of prematurity complications. Sci. Rep. 2020, 10, 21098. [Google Scholar] [CrossRef]
- Holme, N.; Chetcuti, P. The pathophysiology of respiratory distress syndrome in neonates. Paediatr. Child Health 2012, 22, 507–512. [Google Scholar] [CrossRef]
- Thekkeveedu, K.; Guaman, M.C.; Shivanna, B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir. Med. 2017, 132, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Corsini, I.; Bertini, G.; Pratesi, S.; Barp, J.; Rubaltelli, F.F. Effect of multiple INSURE procedures in extremely preterm infants. J. Matern. Fetal Neonatal Med. 2011, 24, 1427–1431. [Google Scholar] [CrossRef]
- Hirsch, K.; Taglauer, E.; Seedorf, G.; Callahan, C.; Mandell, E.; White, C.W.; Kourembanas, S.; Abman, S.H. Perinatal Hypoxia-Inducible Factor Stabilization Preserves Lung Alveolar and Vascular Growth in Experimental Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2020, 202, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Gong, D.; Nguyen, D.N.; Zhang, X.; Hu, Q.; Lu, H.; Fredholm, M.; Sangild, P.T.; Gao, F. Early microbial colonization affects DNA methylation of genes related to intestinal immunity and metabolism in preterm pigs. DNA Res. 2018, 25, 287–296. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Xu, J.; Kim, B.; Deng, W.; Guo, F. HIFα Regulates Developmental Myelination Independent of Autocrine Wnt Signaling. J. Neurosci. 2021, 41, 251–268. [Google Scholar] [CrossRef]
- Zaghloul, N.; Patel, H.; Ahmed, M.N. A model of Periventricular Leukomalacia (PVL) in neonate mice with histopathological and neurodevelopmental outcomes mimicking human PVL in neonates. PLoS ONE 2017, 12, e0175438. [Google Scholar] [CrossRef]
- Bel, F.V.; Groenendaal, F. Birth asphyxia-induced brain damage: The long road to optimal reduction and prevention! Pediatr. Med. 2020, 3, 3. [Google Scholar]
- Kumar, N.; Akangire, G.; Sullivan, B.; Fairchild, K.; Sampath, V. Continuous vital sign analysis for predicting and preventing neonatal diseases in the twenty-first century: Big data to the forefront. Pediatr. Res. 2020, 87, 210–220. [Google Scholar] [CrossRef]
- Song, J.E.; Park, S.J.; Lee, K.Y.; Lee, W.J. Amniotic fluid HIF1α and exosomal HIF1α in cervical insufficiency patients with physical examination-indicated cerclage. J. Matern. Fetal Neonatal Med. 2019, 32, 2287–2294. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; McAdams, R.M. Influence of infection during pregnancy on fetal development. Reproduction 2013, 146, R151–R162. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.X.; Sun, X.C.; Wang, S.W.; Hu, B. Association of polymorphisms of 1772 (C-->T) and 1790 (G-->A) in HIF1A gene with hypoxia adaptation in high altitude in Sherpas. Chin. J. Med. Genet. 2007, 24, 230–232. [Google Scholar]
- Droma, Y.; Ota, M.; Hanaoka, M.; Katsuyama, Y.; Basnyat, B.; Neupane, P.; Arjyal, A.; Pandit, A.; Sharma, D.; Ito, M.; et al. Two hypoxia sensor genes and their association with symptoms of acute mountain sickness in Sherpas. Aviat. Space Environ. Med. 2008, 79, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- López-Reyes, A.; Rodríguez-Pérez, J.M.; Fernández-Torres, J.; Martínez-Rodríguez, N.; Pérez-Hernández, N.; Fuentes-Gómez, A.J.; Aguilar-González, C.A.; Alvarez-León, E.; Posadas-Romero, C.; Villarreal-Molina, T.; et al. The HIF1A rs2057482 polymorphism is associated with risk of developing premature coronary artery disease and with some metabolic and cardiovascular risk factors. The Genetics of Atherosclerotic Disease (GEA) Mexican Study. Exp. Mol. Pathol. 2014, 96, 405–410. [Google Scholar] [CrossRef]
- Andraweera, P.H.; Dekker, G.A.; Thompson, S.D.; Dissanayake, V.H.; Jayasekara, R.W.; Roberts, C.T. Hypoxia-inducible factor-1α gene polymorphisms in early and late onset preeclampsia in Sinhalese women. Placenta 2014, 35, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Sanders, T.A.; Maltepe, E. Hypoxia-inducible factor (HIF) and HIF-stabilizing agents in neonatal care. Semin. Fetal Neonatal Med. 2010, 15, 196–202. [Google Scholar] [CrossRef]
- Hoppe, G.; Yoon, S.; Gopalan, B.; Savage, A.R.; Brown, R.; Case, K.; Vasanji, A.; Chan, E.R.; Silver, R.B.; Sears, J.E. Comparative systems pharmacology of HIF stabilization in the prevention of retinopathy of prematurity. Proc. Natl. Acad. Sci. USA 2016, 113, E2516–E2525. [Google Scholar] [CrossRef]
- Miwa, Y.; Hoshino, Y.; Shoda, C.; Jiang, X.; Tsubota, K.; Kurihara, T. Pharmacological HIF inhibition prevents retinal neovascularization with improved visual function in a murine oxygen-induced retinopathy model. Neurochem. Int. 2019, 128, 21–31. [Google Scholar] [CrossRef]
- Al-Habboubi, H.H.; Sater, M.S.; Almawi, A.W.; Al-Khateeb, G.M.; Almawi, W.Y. Contribution of VEGF polymorphisms to variation in VEGF serum levels in a healthy population. Eur. Cytokine Netw. 2011, 22, 154–158. [Google Scholar] [CrossRef]
- Kosik, K.; Szpecht, D.; Karbowski, Ł.; Al-Saad, S.R.; Chmielarz-Czarnocińska, A.; Minta, M.; Sowińska, A.; Strauss, E. Hemangioma-related gene polymorphisms in the pathogenesis of intraventricular hemorrhage in preterm infants. Child’s Nerv. Syst. 2023. [Google Scholar] [CrossRef]
| Parameter | Premature Infants N = 334 | Non-Hypoxic N = 180 | Hypoxic N = 154 | p | |
|---|---|---|---|---|---|
| Gestational age [wk.] | M (SD) | 27.6 (2.0) | 28.3 (1.8) | 26.9 (2.0) | <0.0001 |
| Range | 22.0–31.9 | 23.0–31.9 | 22.0–31.9 | ||
| Body weight [g] | M (SD) | 1114.2 (335.0) | 1236.4 (323.2) | 971.4 (289.7) | <0.0001 |
| Range | 432–2340 | 570–2340 | 432–1900 | ||
| Intrauterine hypotrophy, n (%) | 22 (6.6) | 10 (5.6) | 12 (7.8) | 0.411 | |
| Male sex, n (%) | 186 (55.7) | 101 (56.1) | 85 (55.2) | 0.866 | |
| Risk factors at birth | |||||
| Ruptured fetal bladder, n (%) | 95 (28.4) | 58 (32.6) | 37 (24.0) | 0.085 | |
| Ruptured fetal bladder, [d], M (SD) | 3.2 (9.3) | 3.6 (9.4) | 2.7 (9.2) | 0.394 | |
| Delivery by caesarean section, n (%) | 179 (53.6) | 93 (51.7) | 86 (55.8) | 0.445 | |
| Apgar 1, Me (Q1, Q3) | 5 (2, 7) | 6 (5, 8) | 2 (1, 5) | <0.0001 | |
| Apgar 5, Me (Q1, Q3) | 7 (6, 8) | 8 (7, 9) | 6 (5, 7) | <0.0001 | |
| Apgar 5 ≤ 6, n (%) | 120 (35.9) | 8 (4.4) | 112 (72.7) | <0.0001 | |
| Acidemia, n (%) | 94 (28.1) | 11 (6.1) | 83 (53.9) | <0.0001 | |
| FiO2 ≥ 0.4, n (%) | 227 (68.0) | 80 (46.1) | 147 (95.5) | <0.0001 | |
| Hypoxia at birth, n (%) | 154 (46.1) | 0 (0.00) | 154 (100.0) | NA | |
| Parameters related to respiratory failure | |||||
| Surfactant treatment, n (%) | 156 (46.7) | 59 (32.8) | 97 (63.0) | <0.0001 | |
| Resuscitation, n (%) | 277 (82.9) | 136 (75,5) | 141 (91,5) | <0.0001 | |
| Mechanical ventilation, n (%) | 229 (68.6) | 115 (53.0) | 114 (97.44) | <0.0001 | |
| Mechanical ventilation period [d], M (SD) | 22.3 (24.5) | 16.8 (22.9) | 28.8 (24.8) | <0.0001 | |
| Oxygen supply period [d], M (SD) | 33.2 (34.7) | 24.7 (30.3) | 43.6 (34.8) | <0.0001 | |
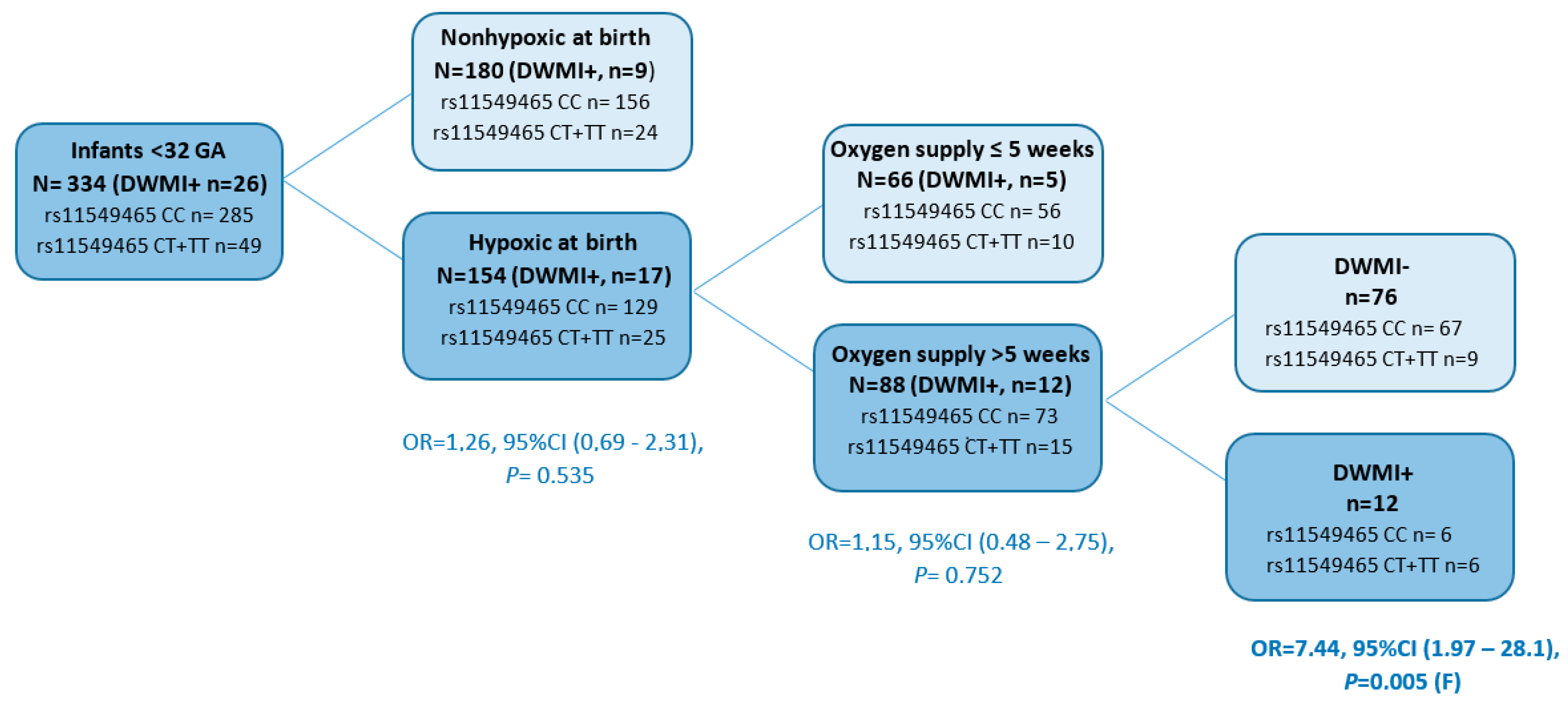
| Oxygen supply >5 weeks, n (%) | 141 (42.2) | 53 (29.4) | 88 (57.1) | <0.0001 | |
| Blood transfusions, n (%) | 4.1 (3.4) | 3.2 (3.6) | 4.8 (3.1) | 0.0005 | |
| Complications of prematurity, n (%) | |||||
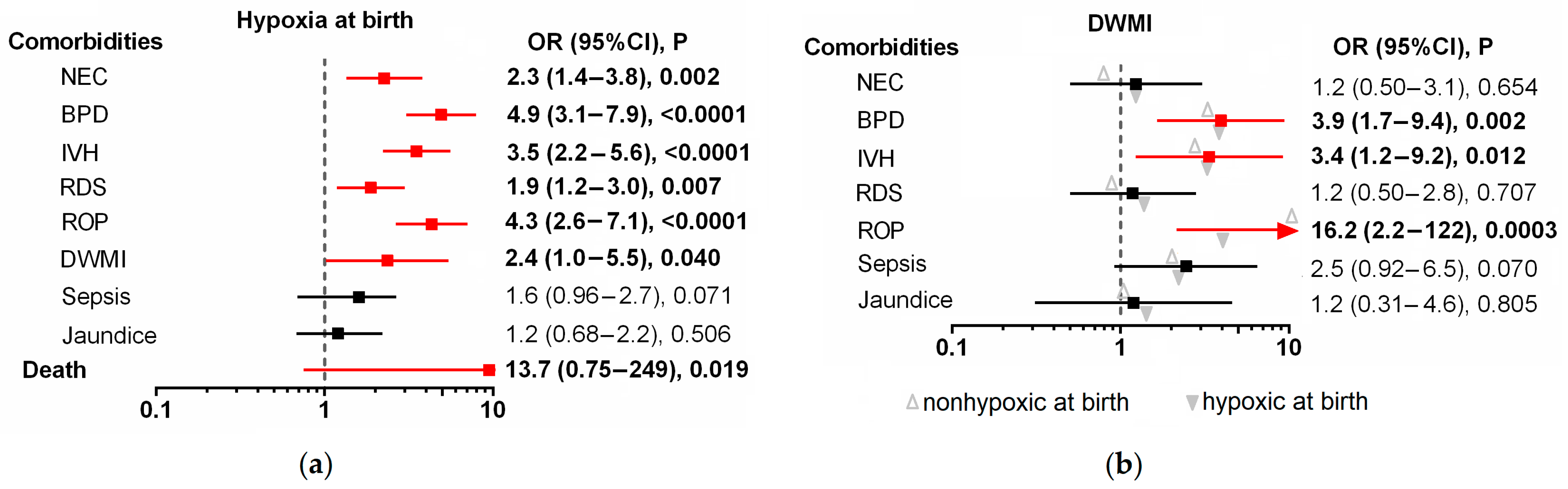
| NEC | 78 (23.4) | 30 (16.7) | 48 (31.2) | 0.002 | |
| BPD | 130 (38.9) | 40 (22.2) | 90 (58.4) | <0.0001 | |
| IVH | 192 (57.5) | 79 (43.9) | 113 (73.4) | <0.0001 | |
| RDS | 220 (66.5) | 107 (59.4) | 113 (73.4) | 0.007 | |
| ROP | 212 (63.5) | 88 (47.9) | 124 (80.5) | <0.0001 | |
| DWMI | 26 (7.9) | 9 (5.0) | 17 (11.0) | 0.040 | |
| Sepsis | 78 (23.4) | 35 (19.6) | 43 (27.9) | 0.068 | |
| Neonatal jaundice | 278 (83.2) | 147 (81.7) | 130 (84.4) | 0.506 | |
| Death | 5 (4.5) | 0 (0.0) | 5 (3.2) | 0.019 | |
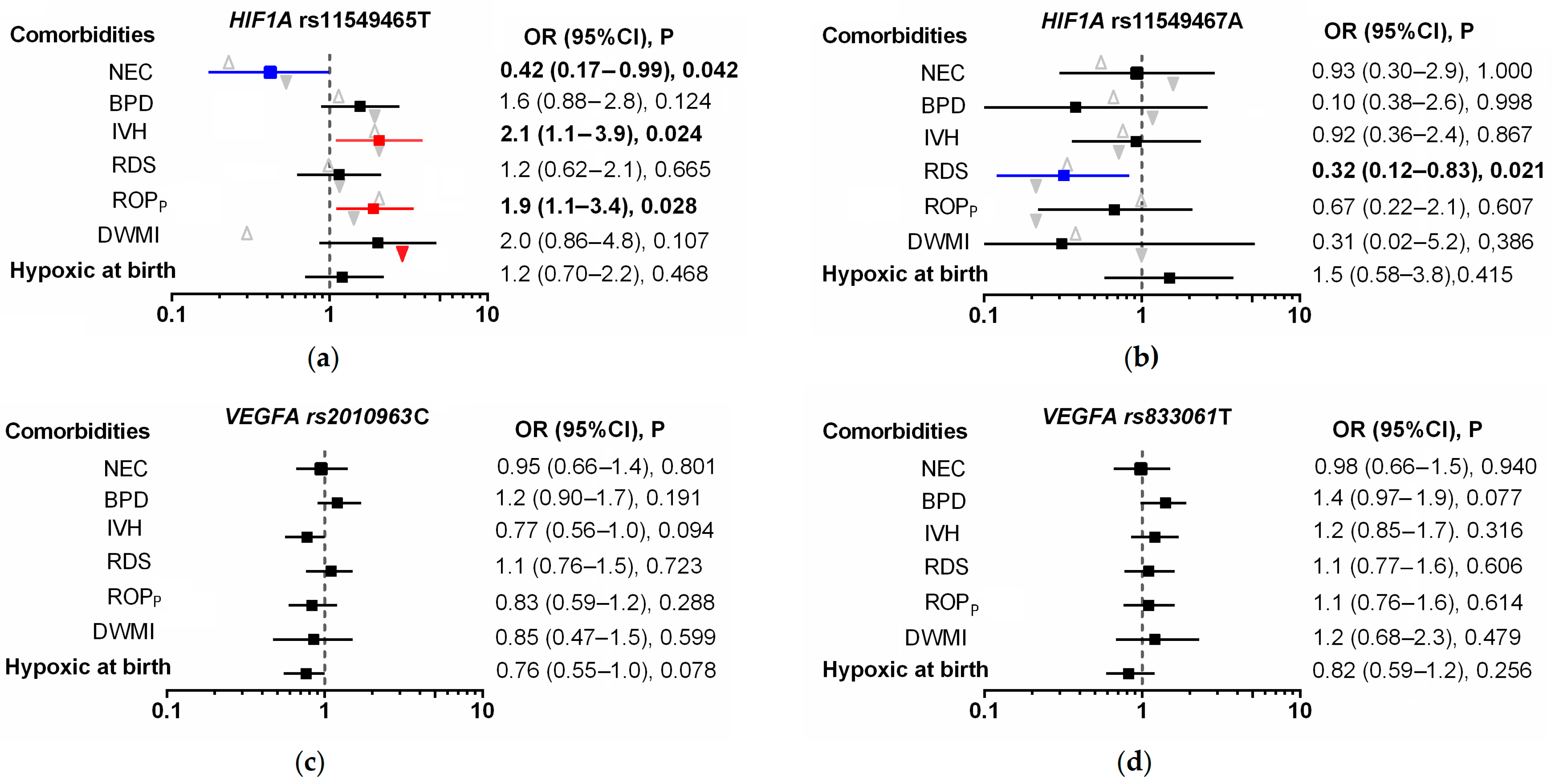
| HIF1A Genotype, n (%) | Premature Infants N = 334 | VEGFA Genotype, n (%) | Premature Infants N = 334 |
|---|---|---|---|
| rs11549465 | rs2010963 | ||
| CC | 285 (85.3) | GG | 164 (49.1) |
| CT | 47 (14.1) | GC | 150 (44.9) |
| TT | 2 (0.6) | CC | 20 (5.9) |
| T allele frequency | 0.076 | C allele frequency | 0.284 |
| pHWE | 0.967 | pHWE | 0.059 |
| rs11549467 | rs833061 | ||
| GG | 316 (94.6) | CC | 129 (38.6) |
| GA | 18 (5.4) | CT | 143 (42.8) |
| AA | 0 (0.0) | TT | 62 (18.6) |
| A allele frequency | 0.027 | T allele frequency | 0.400 |
| pHWE | 0.613 | pHWE | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strauss, E.; Gotz-Więckowska, A.; Sobaniec, A.; Chmielarz-Czarnocińska, A.; Szpecht, D.; Januszkiewicz-Lewandowska, D. Hypoxia-Inducible Pathway Polymorphisms and Their Role in the Complications of Prematurity. Genes 2023, 14, 975. https://doi.org/10.3390/genes14050975
Strauss E, Gotz-Więckowska A, Sobaniec A, Chmielarz-Czarnocińska A, Szpecht D, Januszkiewicz-Lewandowska D. Hypoxia-Inducible Pathway Polymorphisms and Their Role in the Complications of Prematurity. Genes. 2023; 14(5):975. https://doi.org/10.3390/genes14050975
Chicago/Turabian StyleStrauss, Ewa, Anna Gotz-Więckowska, Alicja Sobaniec, Anna Chmielarz-Czarnocińska, Dawid Szpecht, and Danuta Januszkiewicz-Lewandowska. 2023. "Hypoxia-Inducible Pathway Polymorphisms and Their Role in the Complications of Prematurity" Genes 14, no. 5: 975. https://doi.org/10.3390/genes14050975
APA StyleStrauss, E., Gotz-Więckowska, A., Sobaniec, A., Chmielarz-Czarnocińska, A., Szpecht, D., & Januszkiewicz-Lewandowska, D. (2023). Hypoxia-Inducible Pathway Polymorphisms and Their Role in the Complications of Prematurity. Genes, 14(5), 975. https://doi.org/10.3390/genes14050975






