The First Complete Chloroplast Genome of Cordia monoica: Structure and Comparative Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. Cp-Genome Sequencing, Assembly, and Annotation
2.3. Genome Analysis, Codon Usage, and Tandem Repeats Structures
2.4. Sequence Divergence in Boraginaceae Family and Region Boundaries
2.5. Synonymous (dS) and Non-Synonymous (dN) Substitution Rate Analysis
2.6. Phylogenetic Analyses
3. Results
3.1. Complete Chloroplast Genome Sequence of C. monoica
3.2. Tandem Repeats Sequence
3.3. Codon Usage Bias of C. monoica
3.4. Comparative Analysis of Chloroplast Genome in Boraginaceae Family
3.5. IR Expansion and Contraction
3.6. SNPs, Indels, and Selective Pressure Analysis
3.7. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, K.-S.; Yun, B.-K.; Yoon, Y.-H.; Hong, S.-Y.; Mekapogu, M.; Kim, K.-H.; Yang, T.-J. Complete Chloroplast Genome Sequence of Tartary Buckwheat (Fagopyrum tataricum) and Comparative Analysis with Common Buckwheat (F. esculentum). PLoS ONE 2015, 10, e0125332. [Google Scholar] [CrossRef]
- Howe, C.J.; Barbrook, A.C.; Koumandou, V.L.; Nisbet, R.E.R.; Symington, H.A.; Wightman, T.F. Evolution of the Chloroplast Genome. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Esmail, S.M.; Aboulila, A.A.; El-Moneim, D.A. Variation in several pathogenesis—Related (PR) protein genes in wheat (Triticum aestivum) involved in defense against Puccinia striiformis f.sp. tritici. Physiol. Mol. Plant Pathol. 2020, 112, 101545. [Google Scholar] [CrossRef]
- Neuhaus, H.; Emes, M. Nonphotosynthetic Metabolism in Plastids. Annu. Rev. Plant Biol. 2000, 51, 111. [Google Scholar] [CrossRef]
- Bausher, M.G.; Singh, N.D.; Lee, S.-B.; Jansen, R.K.; Daniell, H. The Complete Chloroplast Genome Sequence of Citrus sinensis (L.) Osbeck Var’Ridge Pineapple’: Organization and Phylogenetic Relationships to Other Angiosperms. BMC Plant Biol. 2006, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Nie, L.; Sun, W.; Xu, Z.; Wang, Y.; Yu, J.; Song, J.; Yao, H. Comparative and Phylogenetic Analyses of Ginger (Zingiber officinale) in the Family Zingiberaceae Based on the Complete Chloroplast Genome. Plants 2019, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.M.; Md Shah, M.U.; Makale, K.; Mohd-Yusuf, Y.; Khalid, N.; Othman, R.Y. Complete Chloroplast Genome Sequence of Musa Balbisiana Corroborates Structural Heterogeneity of Inverted Repeats in Wild Progenitors of Cultivated Bananas and Plantains. Plant Genome 2016, 9, plantgenome2015-09. [Google Scholar] [CrossRef]
- Wambugu, P.W.; Brozynska, M.; Furtado, A.; Waters, D.L.; Henry, R.J. Relationships of Wild and Domesticated Rices (Oryza AA Genome Species) Based upon Whole Chloroplast Genome Sequences. Sci. Rep. 2015, 5, 13957. [Google Scholar] [CrossRef]
- Wicke, S.; Schneeweiss, G.M.; Depamphilis, C.W.; Müller, K.F.; Quandt, D. The Evolution of the Plastid Chromosome in Land Plants: Gene Content, Gene Order, Gene Function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef]
- Lee, H.-L.; Jansen, R.K.; Chumley, T.W.; Kim, K.-J. Gene Relocations within Chloroplast Genomes of Jasminum and Menodora (Oleaceae) Are Due to Multiple, Overlapping Inversions. Mol. Biol. Evol. 2007, 24, 1161–1180. [Google Scholar] [CrossRef]
- Wolfe, K.H.; Mordent, C.W.; Ems, S.C.; Palmer, J.D. Rapid Evolution of the Plastid Translational Apparatus in a Nonphotosynthetic Plant: Loss or Accelerated Sequence Evolution of TRNA and Ribosomal Protein Genes. J. Mol. Evol. 1992, 35, 304–317. [Google Scholar] [CrossRef]
- Dong, W.; Liu, J.; Yu, J.; Wang, L.; Zhou, S. Highly Variable Chloroplast Markers for Evaluating Plant Phylogeny at Low Taxonomic Levels and for DNA Barcoding. PLoS ONE 2012, 7, e35071. [Google Scholar] [CrossRef]
- Wu, F.-H.; Chan, M.-T.; Liao, D.-C.; Hsu, C.-T.; Lee, Y.-W.; Daniell, H.; Duvall, M.R.; Lin, C.-S. Complete Chloroplast Genome of Oncidium Gower Ramsey and Evaluation of Molecular Markers for Identification and Breeding in Oncidiinae. BMC Plant Biol. 2010, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Iaffaldano, B.J.; Zhuang, X.; Cardina, J.; Cornish, K. Chloroplast Genome Resources and Molecular Markers Differentiate Rubber Dandelion Species from Weedy Relatives. BMC Plant Biol. 2017, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Gao, L.; Liu, B.; Yang, Y.; Kong, S.; Sun, Y.; Yang, Y.; Wu, X. Complete Chloroplast Genome Sequences of Four Allium Species: Comparative and Phylogenetic Analyses. Sci. Rep. 2019, 9, 12250. [Google Scholar] [CrossRef]
- Martin, W.; Deusch, O.; Stawski, N.; Grünheit, N.; Goremykin, V. Chloroplast Genome Phylogenetics: Why We Need Independent Approaches to Plant Molecular Evolution. Trends Plant Sci. 2005, 10, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sanitá Lima, M.; Woods, L.C.; Cartwright, M.W.; Smith, D.R. The (in)Complete Organelle Genome: Exploring the Use and Nonuse of Available Technologies for Characterizing Mitochondrial and Plastid Chromosomes. Mol. Ecol. Resour. 2016, 16, 1279–1286. [Google Scholar] [CrossRef]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology (5 Volume Set); CRC Press: Boca Raton, FL, USA, 2012; ISBN 1-4200-8044-X. [Google Scholar]
- Ramana, K.V.; Trivedi, M.H.; Reddy, P.R.K.; Rao, C.V. Anti ulcer activity of cordia monoica roxb root. Adv. Pharmacol. Toxicol. 2014, 15, 57. [Google Scholar]
- Glover, P.E.; Stewart, J.; Gwynne, M.D. Masai and Kipsigis Notes on East African Plants: Part III—Medicinal Uses of Plants. East Afr. Agric. For. J. 1966, 32, 200–207. [Google Scholar] [CrossRef]
- Oza, M.J.; Kulkarni, Y.A. Traditional Uses, Phytochemistry and Pharmacology of the Medicinal Species of the Genus Cordia (Boraginaceae). J. Pharm. Pharmacol. 2017, 69, 755–789. [Google Scholar] [CrossRef]
- Pradheeps, M. Ethnobotany and Utilization of Plant Resources in Irula Villages (Sigur Plateau, Nilgiri Biosphere Reserve, India). J. Med. Plants Res. 2013, 7, 267–276. [Google Scholar]
- Ruffo, C.K.; Birnie, A.; Tengnäs, B. Edible Wild Plants of Tanzania, RELMA Technical Handbook (TH) Series; Palzer, C., Ed.; Regional Land Management Unit (RELMA), Swedish International Development Cooperation Agency (Sida): Nairobi, Kenya, 2002; TH No. 26; ISBN 9966-896-60-0.
- Magdy, M.; Ou, L.; Yu, H.; Chen, R.; Zhou, Y.; Hassan, H.; Feng, B.; Taitano, N.; van der Knaap, E.; Zou, X.; et al. Pan-Plastome Approach Empowers the Assessment of Genetic Variation in Cultivated Capsicum Species. Hortic. Res. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Magdy, M.; Ouyang, B. The Complete Mitochondrial Genome of the Chiltepin Pepper (Capsicum annuum Var. Glabriusculu), the Wild Progenitor of Capsicum annuum L. Mitochondrial DNA Part B 2020, 5, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq—Versatile and Accurate Annotation of Organelle Genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) Version 1.3.1: Expanded Toolkit for the Graphical Visualization of Organellar Genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef]
- Chan, P.P.; Lin, B.Y.; Mak, A.J.; Lowe, T.M. TRNAscan-SE 2.0: Improved Detection and Functional Classification of Transfer RNA Genes. Nucleic Acids Res. 2021, 49, 9077–9096. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational Tools for Comparative Genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- Nei, M.; Gojobori, T. Simple Methods for Estimating the Numbers of Synonymous and Nonsynonymous Nucleotide Substitutions. Mol. Biol. Evol. 1986, 3, 418–426. [Google Scholar]
- Nielsen, R. Molecular Signatures of Natural Selection. Annu. Rev. Genet. 2005, 39, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, C.L.; Ahmed, I.; Carlsen, M.M.; Zuluaga, A.; Croat, T.B.; McKain, M.R. Evolutionary Dynamics of Chloroplast Genomes in Subfamily Aroideae (Araceae). Genomics 2020, 112, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, F.; Shahzadi, I.; Waseem, S.; Mirza, B.; Ahmed, I.; Waheed, M.T. Chloroplast Genome of Hibiscus Rosa-Sinensis (Malvaceae): Comparative Analyses and Identification of Mutational Hotspots. Genomics 2020, 112, 581–591. [Google Scholar]
- Jansen, R.K.; Cai, Z.; Raubeson, L.A.; Daniell, H.; Depamphilis, C.W.; Leebens-Mack, J.; Müller, K.F.; Guisinger-Bellian, M.; Haberle, R.C.; Hansen, A.K. Analysis of 81 Genes from 64 Plastid Genomes Resolves Relationships in Angiosperms and Identifies Genome-Scale Evolutionary Patterns. Proc. Natl. Acad. Sci. USA 2007, 104, 19369–19374. [Google Scholar] [CrossRef]
- Moore, M.J.; Bell, C.D.; Soltis, P.S.; Soltis, D.E. Using Plastid Genome-Scale Data to Resolve Enigmatic Relationships among Basal Angiosperms. Proc. Natl. Acad. Sci. USA 2007, 104, 19363–19368. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, D. The Complete Chloroplast Genome Sequence of Onosma Paniculatum Bur. et Franch. (Boraginaceae), a Medicinal Plant in Yunnan and Its Adjacent Regions. Mitochondrial DNA Part B 2019, 4, 3330–3332. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Lei, J.; Liang, Z. The Complete Chloroplast Genome Sequence of Trigonotis Peduncularis (Boraginaceae). Mitochondrial DNA Part B 2022, 7, 456–457. [Google Scholar] [CrossRef]
- Lei, W.; Ni, D.; Wang, Y.; Shao, J.; Wang, X.; Yang, D.; Wang, J.; Chen, H.; Liu, C. Intraspecific and Heteroplasmic Variations, Gene Losses and Inversions in the Chloroplast Genome of Astragalus Membranaceus. Sci. Rep. 2016, 6, 21669. [Google Scholar] [CrossRef]
- Ruhlman, T.A.; Jansen, R.K. The Plastid Genomes of Flowering Plants. In Chloroplast Biotechnology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–38. [Google Scholar] [CrossRef]
- Park, I.; Song, J.-H.; Yang, S.; Kim, W.J.; Choi, G.; Moon, B.C. Cuscuta Species Identification Based on the Morphology of Reproductive Organs and Complete Chloroplast Genome Sequences. Int. J. Mol. Sci. 2019, 20, 2726. [Google Scholar] [CrossRef] [PubMed]
- Provan, J.; Powell, W.; Hollingsworth, P.M. Chloroplast Microsatellites: New Tools for Studies in Plant Ecology and Evolution. Trends Ecol. Evol. 2001, 16, 142–147. [Google Scholar] [CrossRef]
- Addisalem, A.; Esselink, G.D.; Bongers, F.; Smulders, M. Genomic Sequencing and Microsatellite Marker Development for Boswellia Papyrifera, an Economically Important but Threatened Tree Native to Dry Tropical Forests. AoB Plants 2015, 7, plu086. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Peakall, R. Chloroplast Simple Sequence Repeats (CpSSRs): Technical Resources and Recommendations for Expanding CpSSR Discovery and Applications to a Wide Array of Plant Species. Mol. Ecol. Resour. 2009, 9, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Gao, L.; Wang, B.; Su, Y.-J.; Wang, T. The Complete Chloroplast Genome Sequence of Cephalotaxus Oliveri (Cephalotaxaceae): Evolutionary Comparison of Cephalotaxus Chloroplast DNAs and Insights into the Loss of Inverted Repeat Copies in Gymnosperms. Genome Biol. Evol. 2013, 5, 688–698. [Google Scholar] [CrossRef]
- Asaf, S.; Waqas, M.; Khan, A.L.; Khan, M.A.; Kang, S.-M.; Imran, Q.M.; Shahzad, R.; Bilal, S.; Yun, B.-W.; Lee, I.-J. The Complete Chloroplast Genome of Wild Rice (Oryza minuta) and Its Comparison to Related Species. Front. Plant Sci. 2017, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.-Y.; Wu, H.; Wang, Y.-L.; Gao, L.-M.; Zhang, S.-Z.; Lu, L. Complete Chloroplast Genome Sequence of Magnolia Kwangsiensis (Magnoliaceae): Implication for DNA Barcoding and Population Genetics. Genome 2011, 54, 663–673. [Google Scholar] [CrossRef]
- Chen, X.; Li, Q.; Li, Y.; Qian, J.; Han, J. Chloroplast Genome of Aconitum Barbatum Var. Puberulum (Ranunculaceae) Derived from CCS Reads Using the PacBio RS Platform. Front. Plant Sci. 2015, 6, 42. [Google Scholar] [CrossRef]
- Sharp, P.M.; Emery, L.R.; Zeng, K. Forces That Influence the Evolution of Codon Bias. Phil. Trans. R. Soc. B 2010, 365, 1203–1212. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Liu, S.; Zhong, Y.; Wu, Y.; Li, J.; Xu, L.-A.; Xu, M. The Complete Chloroplast Genome of Cinnamomum Camphora and Its Comparison with Related Lauraceae Species. PeerJ 2017, 5, e3820. [Google Scholar] [CrossRef]
- Trofimov, D.; Cadar, D.; Schmidt-Chanasit, J.; Rodrigues de Moraes, P.L.; Rohwer, J.G. A Comparative Analysis of Complete Chloroplast Genomes of Seven Ocotea Species (Lauraceae) Confirms Low Sequence Divergence within the Ocotea Complex. Sci. Rep. 2022, 12, 1120. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, L.; Zhao, W.; Xu, J.; Li, Y.; Zhang, X.; Shen, X.; Wu, M.; Hou, X. Complete Chloroplast Genome Sequence and Phylogenetic Analysis of Paeonia Ostii. Molecules 2018, 23, 246. [Google Scholar] [CrossRef]
- Zhou, J.; Cui, Y.; Chen, X.; Li, Y.; Xu, Z.; Duan, B.; Li, Y.; Song, J.; Yao, H. Complete Chloroplast Genomes of Papaver Rhoeas and Papaver Orientale: Molecular Structures, Comparative Analysis, and Phylogenetic Analysis. Molecules 2018, 23, 437. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, T.; Tsudzuki, J.; Ito, S.; Nakashima, K.; Tsudzuki, T.; Sugiura, M. Loss of All Ndh Genes as Determined by Sequencing the Entire Chloroplast Genome of the Black Pine Pinus Thunbergii. Proc. Natl. Acad. Sci. USA 1994, 91, 9794–9798. [Google Scholar] [CrossRef]
- Tang, J.; Xia, H.; Cao, M.; Zhang, X.; Zeng, W.; Hu, S.; Tong, W.; Wang, J.; Wang, J.; Yu, J. A Comparison of Rice Chloroplast Genomes. Plant Physiol. 2004, 135, 412–420. [Google Scholar] [CrossRef]
- Wicke, S.; Naumann, J. Molecular Evolution of Plastid Genomes in Parasitic Flowering Plants. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 85, pp. 315–347. ISBN 0065-2296. [Google Scholar] [CrossRef]
- Kode, V.; Mudd, E.A.; Iamtham, S.; Day, A. The Tobacco Plastid AccD Gene Is Essential and Is Required for Leaf Development. Plant J. 2005, 44, 237–244. [Google Scholar] [CrossRef]
- Raubeson, L.A.; Peery, R.; Chumley, T.W.; Dziubek, C.; Fourcade, H.M.; Boore, J.L.; Jansen, R.K. Comparative Chloroplast Genomics: Analyses Including New Sequences from the Angiosperms Nuphar Advena and Ranunculus Macranthus. BMC Genom. 2007, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-J.; Cheng, C.-L.; Chang, C.-C.; Wu, C.-L.; Su, T.-M.; Chaw, S.-M. Dynamics and Evolution of the Inverted Repeat-Large Single Copy Junctions in the Chloroplast Genomes of Monocots. BMC Evol. Biol. 2008, 8, 36. [Google Scholar] [CrossRef]
- Yao, X.; Tang, P.; Li, Z.; Li, D.; Liu, Y.; Huang, H. The First Complete Chloroplast Genome Sequences in Actinidiaceae: Genome Structure and Comparative Analysis. PLoS ONE 2015, 10, e0129347. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, N.; Barnaud, A.; Eiserhardt, W.; Treier, U.A.; Seveno, M.; d’Anfray, A.; Vigouroux, Y.; Pintaud, J.-C. A Set of 100 Chloroplast DNA Primer Pairs to Study Population Genetics and Phylogeny in Monocotyledons. PLoS ONE 2011, 6, e19954. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.; Cherney, B.W.; Merlin, E. The Role of Insertions/Deletions in the Evolution of the Intergenic Region BetweenpsbA AndtrnH in the Chloroplast Genome. Curr. Genet. 1988, 14, 137–146. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Henry, R.J.; Rossetto, M.; Wang, Y.; Chen, S. Plant DNA Barcoding: From Gene to Genome. Biol. Rev. 2015, 90, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yang, M.; Mo, C.; Xie, W.; Liu, C.; Wu, B.; Ma, X. Complete Chloroplast Genomes of Two Siraitia Merrill Species: Comparative Analysis, Positive Selection and Novel Molecular Marker Development. PLoS ONE 2019, 14, e0226865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, T.; Yang, J.; Sun, J.; Ju, M.; Zhao, Y.; Zhao, G. Comparative Analyses of Chloroplast Genomes of Cucurbitaceae Species: Lights into Selective Pressures and Phylogenetic Relationships. Molecules 2018, 23, 2165. [Google Scholar] [CrossRef]
- Xu, J.-H.; Liu, Q.; Hu, W.; Wang, T.; Xue, Q.; Messing, J. Dynamics of Chloroplast Genomes in Green Plants. Genomics 2015, 106, 221–231. [Google Scholar] [CrossRef] [PubMed]
- de Souza, U.J.B.; Nunes, R.; Targueta, C.P.; Diniz-Filho, J.A.F.; Telles, M.P.D.C. The Complete Chloroplast Genome of Stryphnodendron Adstringens (Leguminosae-caesalpinioideae): Comparative Analysis with Related Mimosoid Species. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Hertel, S.; Zoschke, R.; Neumann, L.; Qu, Y.; Axmann, I.M.; Schmitz-Linneweber, C. Multiple Checkpoints for the Expression of the Chloroplast-Encoded Splicing Factor MatK. Plant Physiol. 2013, 163, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhu, H.; Wang, J.; Xu, Y.; Xu, F.; Wang, X. Complete Chloroplast Genome Sequence Determination of Rheum Species and Comparative Chloroplast Genomics for the Members of Rumiceae. Plant Cell Rep. 2020, 39, 811–824. [Google Scholar] [CrossRef]
- Tyagi, S.; Jung, J.-A.; Kim, J.S.; Won, S.Y. A Comparative Analysis of the Complete Chloroplast Genomes of Three Chrysanthemum Boreale Strains. PeerJ 2020, 8, e9448. [Google Scholar] [CrossRef]
- Gao, C.; Deng, Y.; Wang, J. The Complete Chloroplast Genomes of Echinacanthus Species (Acanthaceae): Phylogenetic Relationships, Adaptive Evolution, and Screening of Molecular Markers. Front. Plant Sci. 2019, 9, 1989. [Google Scholar] [CrossRef]
- Kofer, W.; Koop, H.-U.; Wanner, G.; Steinmüller, K. Mutagenesis of the Genes Encoding Subunits A, C, H, I, J and K of the Plastid NAD (P) H-Plastoquinone-Oxidoreductase in Tobacco by Polyethylene Glycol-Mediated Plastome Transformation. Mol. Gen. Genet. MGG 1998, 258, 166–173. [Google Scholar] [CrossRef]
- Drescher, A.; Ruf, S.; Calsa, T., Jr.; Carrer, H.; Bock, R. The Two Largest Chloroplast Genome-encoded Open Reading Frames of Higher Plants Are Essential Genes. Plant J. 2000, 22, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Bédard, J.; Hirano, M.; Hirabayashi, Y.; Oishi, M.; Imai, M.; Takase, M.; Ide, T.; Nakai, M. Uncovering the Protein Translocon at the Chloroplast Inner Envelope Membrane. Science 2013, 339, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, D.; He, X.; Yang, Y.; Li, X. Comparative Analysis of the Complete Chloroplast Genomes in Allium Section Bromatorrhiza Species (Amaryllidaceae): Phylogenetic Relationship and Adaptive Evolution. Genes 2022, 13, 1279. [Google Scholar] [CrossRef]
- Särkinen, T.; George, M. Predicting Plastid Marker Variation: Can Complete Plastid Genomes from Closely Related Species Help? PLoS ONE 2013, 8, e82266. [Google Scholar] [CrossRef]
- Yang, Z.; Rannala, B. Molecular Phylogenetics: Principles and Practice. Nat. Rev. Genet. 2012, 13, 303–314. [Google Scholar] [CrossRef] [PubMed]
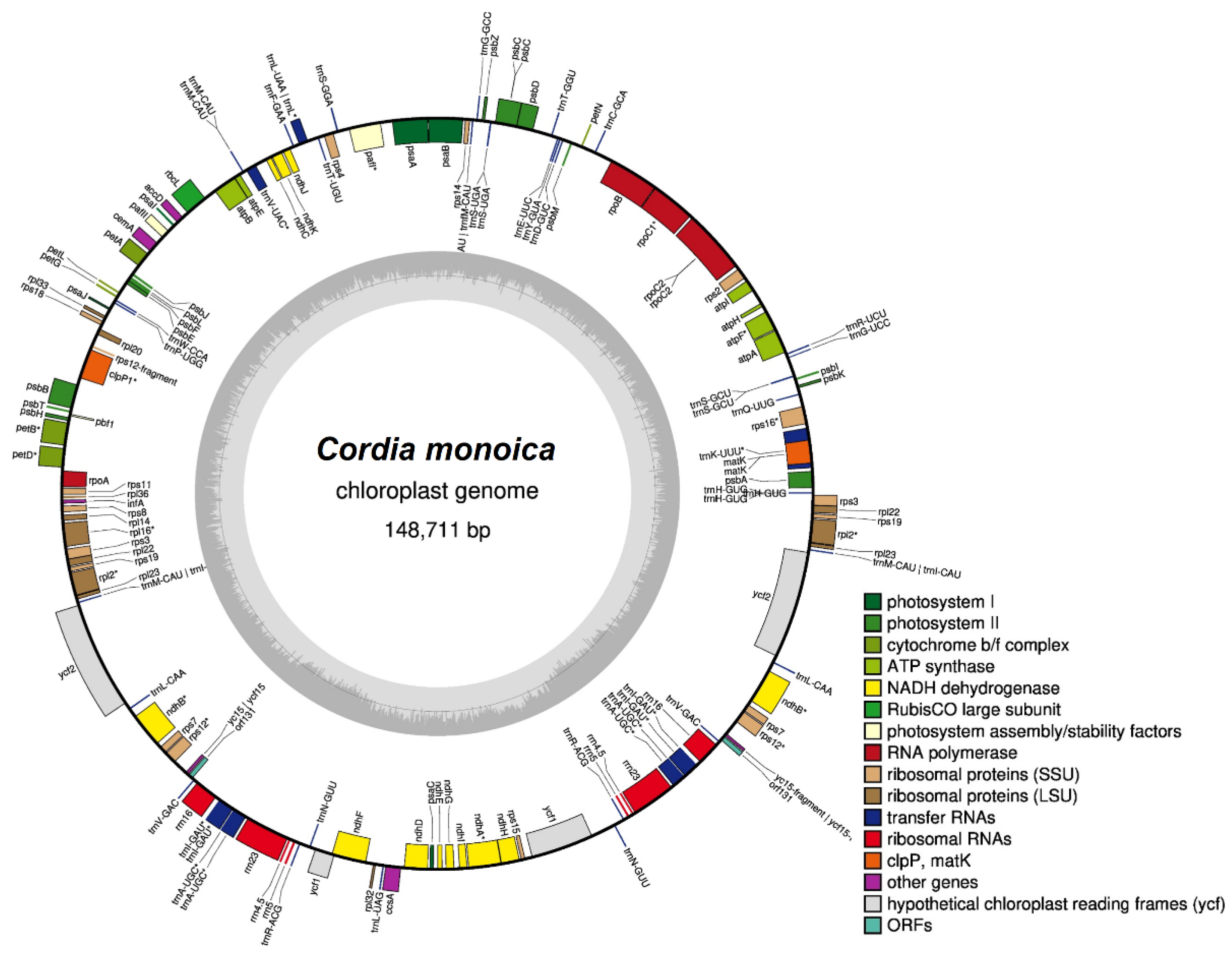
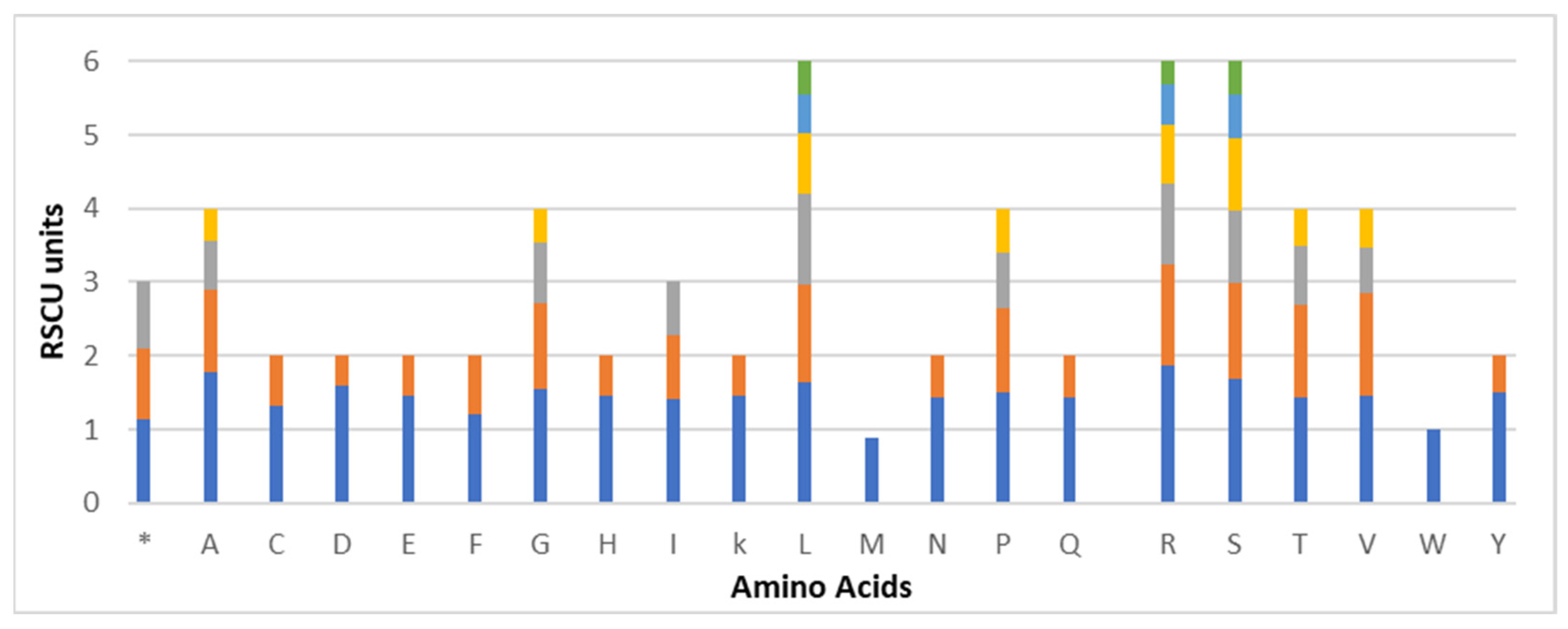

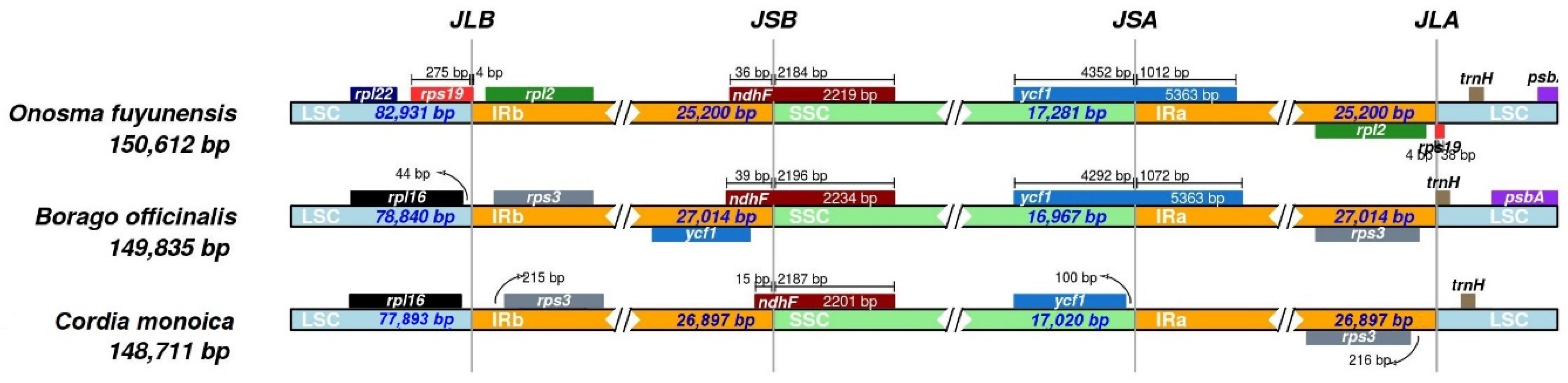
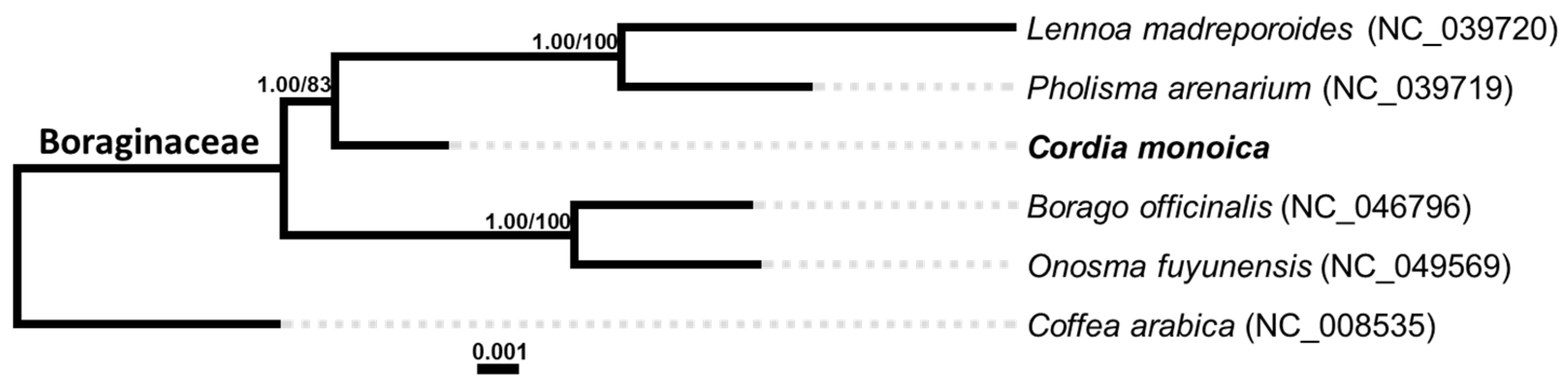
| Feature | C. monoica | Feature | C. monoica |
|---|---|---|---|
| Total cp DNA size (bp) | 148,711 | Intergenic sequences (%) | 39.0% |
| LSC size (bp) | 77,893 | Number of genes | 133 |
| SSC size (bp) | 17,020 | Number of different protein-coding genes | 90 |
| IR size (bp) | 26,897 | Number of different tRNA genes | 37 |
| Protein-coding regions (%) | 52.98% | Number of different rRNA genes | 8 |
| rRNA and tRNA (%) | 7.94% | Number of different duplicated genes | 21 |
| Introns size (% total) | 13.58% | GC content | 38.2% |
| Repeat Class | Repeat Abundances | Abundance (%) |
|---|---|---|
| Dinucleotide | 110 | 8% |
| Trinucleotide | 194 | 14% |
| Tetranucleotide | 241 | 17% |
| Pentanucleotide | 281 | 20% |
| Hexanucleotide | 388 | 28% |
| 7-nucleotide | 77 | 6% |
| 8-nucleotide | 47 | 3% |
| 9-nucleotide | 30 | 2% |
| 10-nucleotide | 13 | 1% |
| Total | 1387 | 100.00 |
| Species | Total Length | LSC bp | SSC bp | IR bp | Similarity% | Accession No. |
|---|---|---|---|---|---|---|
| Cordia monoica | 148,711 | 77,893 | 17,020 | 26,897 | 100% | - |
| Borago officinalis | 149,835 | 78,840 | 16,967 | 27,014 | 88% | NC_046796 |
| Onosma fuyunensis | 150,612 | 82,931 | 17,281 | 25,200 | 84.8% | NC_049569 |
| Pholisma arenarium | 81,198 | 30,262 | 6454 | 22,241 | 39.8% | NC_039719 |
| Lennoa madreporoides | 83,675 | 30,881 | 6830 | 22,982 | 40% | NC_039720 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshegaihi, R.M.; Mansour, H.; Alrobaish, S.A.; Al Shaye, N.A.; Abd El-Moneim, D. The First Complete Chloroplast Genome of Cordia monoica: Structure and Comparative Analysis. Genes 2023, 14, 976. https://doi.org/10.3390/genes14050976
Alshegaihi RM, Mansour H, Alrobaish SA, Al Shaye NA, Abd El-Moneim D. The First Complete Chloroplast Genome of Cordia monoica: Structure and Comparative Analysis. Genes. 2023; 14(5):976. https://doi.org/10.3390/genes14050976
Chicago/Turabian StyleAlshegaihi, Rana M., Hassan Mansour, Shouaa A. Alrobaish, Najla A. Al Shaye, and Diaa Abd El-Moneim. 2023. "The First Complete Chloroplast Genome of Cordia monoica: Structure and Comparative Analysis" Genes 14, no. 5: 976. https://doi.org/10.3390/genes14050976
APA StyleAlshegaihi, R. M., Mansour, H., Alrobaish, S. A., Al Shaye, N. A., & Abd El-Moneim, D. (2023). The First Complete Chloroplast Genome of Cordia monoica: Structure and Comparative Analysis. Genes, 14(5), 976. https://doi.org/10.3390/genes14050976






