Neuroprotective Peptides and New Strategies for Ischemic Stroke Drug Discoveries
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Peptides Are New Potential Neuroprotective Agents for the Treatment of Acute IS
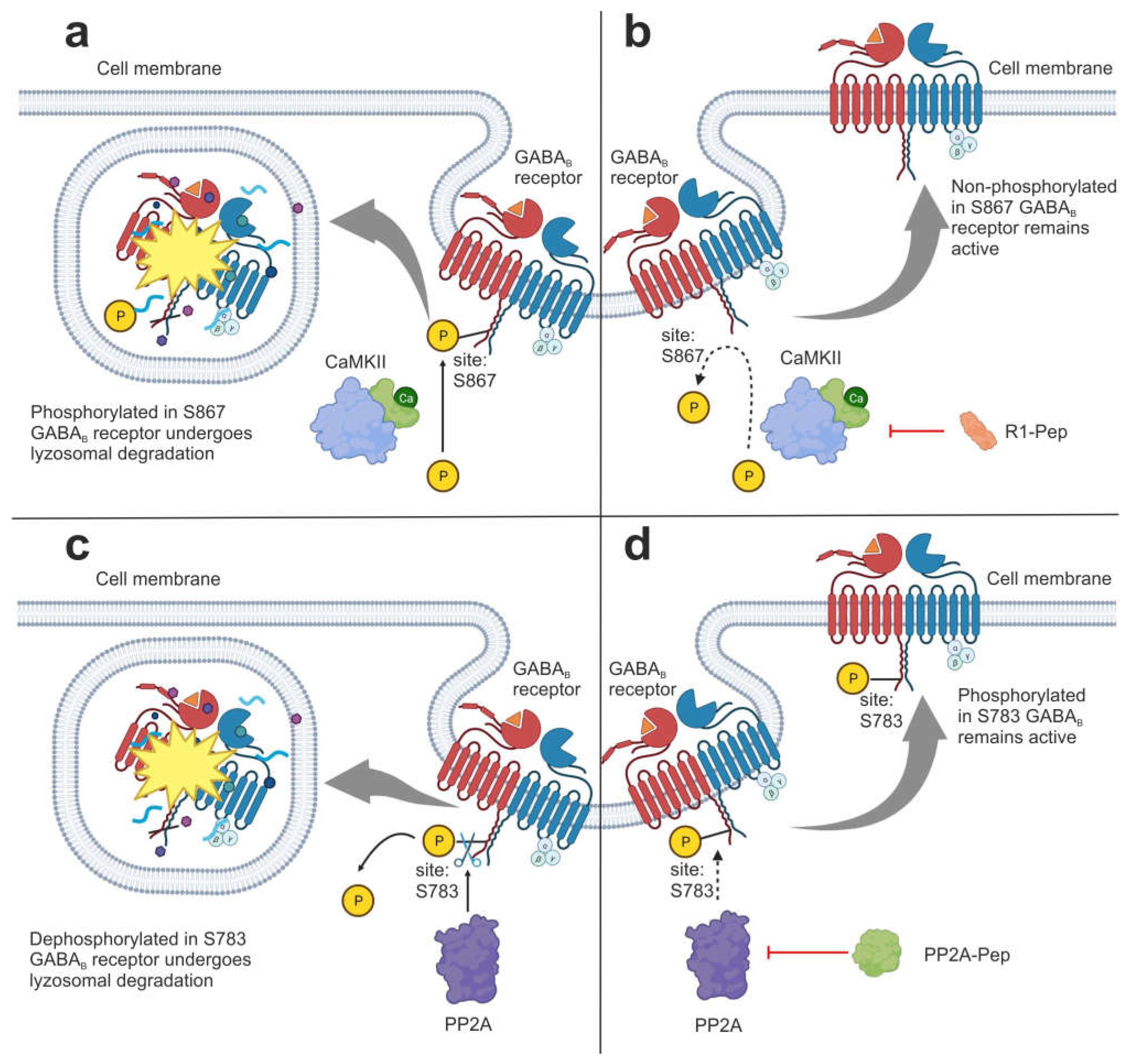
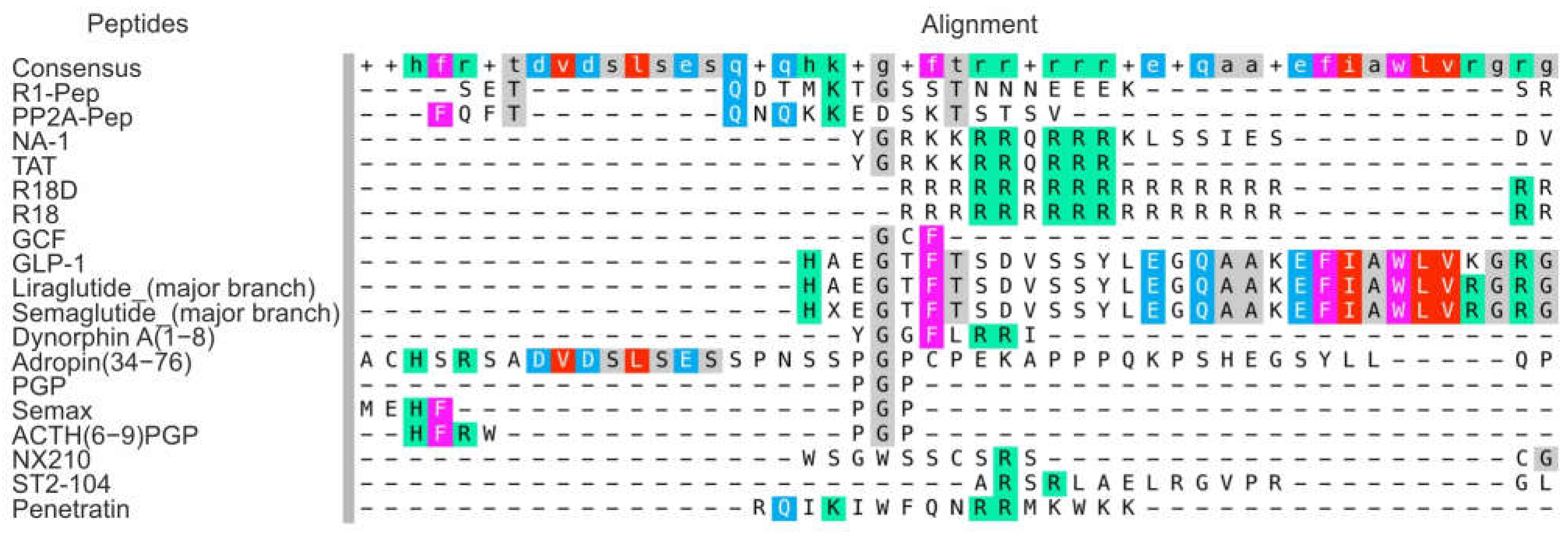
3.1.1. Interfering Peptides
3.1.2. Arginine-Rich Peptides
3.1.3. Shuttle Peptides to Provide Neuroprotection
3.1.4. Peptides That Mimic Natural Regulatory Peptides and Hormones
| Type of Peptide | Peptides | Functions | References |
|---|---|---|---|
| Interfering peptides (IPs) | R1-Pep | It inhibits the interaction of GABAB receptor with CaMKII, preventing receptor phosphorylation. | [46] |
| PP2A-Pep | It inhibits the interaction of GABAB receptor with PP2A, preventing receptor dephosphorylation. | [47] | |
| NA-1 | It attenuates neurotoxic signaling cascades that lead to excessive calcium ion entry into neurons. | [8,51] | |
| Cationic arginine-rich peptides (CARPs) | R18D, R18 | They have high anti-excitotoxic and anti-inflammatory efficiency, and are able to interfere with calcium influx into cells, stabilize mitochondria, inhibit proteolytic enzymes, induce survival signaling, and reduce oxidative stress. Peptides increase the maximum activity of the thrombolysis reaction when co-administered with thrombolytic agents | [54,55,56,57,58] |
| ST2-104 | It effects on apoptosis and autophagy via the CaMKKβ/AMPK/mTOR signaling pathway. | [59] | |
| Shuttle peptides | GCF | It acts as a BBB shuttle and prodrug, delivering the amino acid glycine to the brain to provide neuroprotection. | [65] |
| CPP-SOD | The recombinant CPP-SOD fusion protein can cross the BBB and alleviate severe oxidative damage in various brain tissues by scavenging reactive oxygen species, reducing the expression of inflammatory factors and inhibiting NF-κB/MAPK signaling pathways. | [61] | |
| Peptides that mimic natural regulatory peptides and hormones | Adropin(34–76) | It reduces infarct size by activating eNOS and reducing oxidative damage, maintains the integrity of the BBB and reduces the activity of MMP-9. | [67,69] |
| Dynorphin A(1–8) | It affords neuroprotection through NMDA receptor and κ-opioid receptor channels. | [70] | |
| NX210 | It prevents oxidative stress and neuronal apoptosis in cerebral IR through enhancement of the integrin-β1/PI3K/Akt signaling pathway. | [71] | |
| Liraglutide | Long acting GL1-RA that promotes angiogenesis, reduces neurological deficits, apoptosis, inhibits pyroptosis, an inflammatory form of programmed cell death. | [74,76,77,78,79,80,81,82,83] | |
| Semaglutide | Long acting GL1-RA that reduces ischemic cerebrovascular events in type 2 diabetes | [75,85,86,87,88] | |
| Semax | It has a pronounced nootropic, neuroprotective, and immunomodulatory effects. Peptide initiates a neurotransmitter and anti-inflammatory response. | [27,91,92,96,97] | |
| ACTH(6–9)PGP | It protects neurons from cell death, protected cells from oxidative stress and exhibited proliferative activity. | [94,95] |
3.2. Transcriptomic Analysis as a New Approach to Reveal the Molecular Mechanisms of Ischemic Damage and the Action of Potential Neuroprotectors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, J.M. Recombinant tissue plasminogen activator for the treatment of acute ischemic stroke. Proc. Bayl. Univ. Med. Cent. 2011, 24, 257–259. [Google Scholar] [CrossRef]
- Turc, G.; Bhogal, P.; Fischer, U.; Khatri, P.; Lobotesis, K.; Mazighi, M.; Schellinger, P.D.; Toni, D.; de Vries, J.; White, P.; et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur. Stroke J. 2019, 4, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Turc, G.; Tsivgoulis, G.; Audebert, H.J.; Boogaarts, H.; Bhogal, P.; De Marchis, G.M.; Fonseca, A.C.; Khatri, P.; Mazighi, M.; Pérez de la Ossa, N.; et al. European Stroke Organisation (ESO)-European Society for Minimally Invasive Neurological Therapy (ESMINT) expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischemic stroke and anterior. J. Neurointerv. Surg. 2022, 14, 209. [Google Scholar] [CrossRef]
- Alhadid, K.; Oliveira, L.; Etherton, M.R. Intravenous Thrombolytics in the Treatment of Acute Ischemic Stroke. Curr. Treat. Options Cardiovasc. Med. 2023, 25, 15–28. [Google Scholar] [CrossRef]
- Al-Mufti, F.; Amuluru, K.; Roth, W.; Nuoman, R.; El-Ghanem, M.; Meyers, P.M. Cerebral ischemic reperfusion injury following recanalization of large vessel occlusions. Neurosurgery 2018, 82, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nagai, N.; Umemura, K. A Review of the Mechanisms of Blood-Brain Barrier Permeability by Tissue-Type Plasminogen Activator Treatment for Cerebral Ischemia. Front. Cell. Neurosci. 2016, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Ringleb, P.A.; Bousser, M.G.; Ford, G.; Bath, P.; Brainin, M.; Caso, V.; Cervera, Á.; Chamorro, A.; Cordonnier, C.; Csiba, L.; et al. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc. Dis. 2008, 25, 457–507. [Google Scholar]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke. Stroke 2019, 50, E344–E418. [Google Scholar] [CrossRef]
- Kikuchi, K.; Tanaka, E.; Murai, Y.; Tancharoen, S. Clinical trials in acute ischemic stroke. CNS Drugs 2014, 28, 929–938. [Google Scholar] [CrossRef]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. Vitamin D3 and Ischemic Stroke: A Narrative Review. Antioxidants 2022, 11, 2120. [Google Scholar] [CrossRef]
- Solana-Manrique, C.; Sanz, F.J.; Martínez-Carrión, G.; Paricio, N. Antioxidant and Neuroprotective Effects of Carnosine: Therapeutic Implications in Neurodegenerative Diseases. Antioxidants 2022, 11, 848. [Google Scholar] [CrossRef]
- Marques, E.P.; Wyse, A.T.S. Creatine as a Neuroprotector: An Actor that Can Play Many Parts. Neurotox. Res. 2019, 36, 411–423. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, S.; Nao, J. Kaempferol as a therapeutic agent in Alzheimer’s disease: Evidence from preclinical studies. Ageing Res. Rev. 2023, 87, 101910. [Google Scholar] [CrossRef]
- Isaev, N.K.; Genrikhs, E.E.; Stelmashook, E.V. Antioxidant Thymoquinone and Its Potential in the Treatment of Neurological Diseases. Antioxidants 2023, 12, 433. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Cabri, W.; Cantelmi, P.; Corbisiero, D.; Fantoni, T.; Ferrazzano, L.; Martelli, G.; Mattellone, A.; Tolomelli, A. Therapeutic Peptides Targeting PPI in Clinical Development: Overview, Mechanism of Action and Perspectives. Front. Mol. Biosci. 2021, 8, 697586. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Peptides in clinical development for the treatment of brain tumors. Curr. Opin. Pharmacol. 2019, 47, 102–109. [Google Scholar] [CrossRef]
- Lee, A.C.L.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A comprehensive review on current advances in peptide drug development and design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Lubell, W.D. Peptide-Based Drug Development. Biomedicines 2022, 10, 2037. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Deigin, V.I.; Poluektova, E.A.; Beniashvili, A.G.; Kozin, S.A.; Poluektov, Y.M. Development of Peptide Biopharmaceuticals in Russia. Pharmaceutics 2022, 14, 716. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.; Liu, W.W.; Manaenko, A.; Hou, X.; Mei, Q.; Huang, J.L.; Tang, J.; Zhang, J.H.; Yao, H.; et al. Advances in stroke pharmacology. Pharmacol. Ther. 2018, 191, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Savitz, S.I. Pharmacological brain cytoprotection in acute ischaemic stroke—Renewed hope in the reperfusion era. Nat. Rev. Neurol. 2022, 18, 193. [Google Scholar] [CrossRef]
- Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Sudarkina, O.Y.; Dmitrieva, V.G.; Gubsky, L.V.; Myasoedov, N.F.; Limborska, S.A.; et al. Novel insights into the protective properties of acth(4-7)pgp (semax) peptide at the transcriptome level following cerebral ischaemia—Reperfusion in rats. Genes 2020, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Valieva, L.V.; Remizova, J.A.; Mozgovoy, I.V.; Zaytceva, E.I.; Gubsky, L.V.; Limborska, S.A.; Dergunova, L. V Genome-Wide RNA-Sequencing Reveals Massive Circular RNA Expression Changes of the Neurotransmission Genes in the Rat Brain after Ischemia-Reperfusion. Genes 2021, 12, 1870. [Google Scholar] [CrossRef]
- Tian, R.; Deng, A.; Pang, X.; Chen, Y.; Gao, Y.; Liu, H.; Hu, Z. VR-10 polypeptide interacts with CD36 to induce cell apoptosis and autophagy in choroid-retinal endothelial cells: Identification of VR-10 as putative novel therapeutic agent for choroid neovascularization (CNV) treatment. Peptides 2022, 157, 170868. [Google Scholar] [CrossRef] [PubMed]
- Matei, N.; Camara, J.; Zhang, J.H. The Next Step in the Treatment of Stroke. Front. Neurol. 2021, 11, 582605. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Dergunova, L.V.; Filippenkov, I.B.; Limborska, S.A.; Myasoedov, N.F. Pharmacotranscriptomics of peptide drugs with neuroprotective properties. Med. Res. Rev. 2021, 41, 754–769. [Google Scholar] [CrossRef]
- Chiou, L.-C.; Liao, Y.-Y.; Fan, P.-C.; Kuo, P.-H.; Wang, C.-H.; Riemer, C.; Prinssen, E. Nociceptin/Orphanin FQ Peptide Receptors: Pharmacology and Clinical Implications. Curr. Drug Targets 2006, 8, 117–135. [Google Scholar] [CrossRef]
- Plášek, J.; Lazárová, M.; Dodulík, J.; Šulc, P.; Stejskal, D.; Švagera, Z.; Všianský, F.; Václavík, J. Secretoneurin as a Novel Biomarker of Cardiovascular Episodes: Are We There Yet? A Narrative Review. J. Clin. Med. 2022, 11, 7191. [Google Scholar] [CrossRef]
- Yeo, X.Y.; Cunliffe, G.; Ho, R.C.; Lee, S.S.; Jung, S. Potentials of Neuropeptides as Therapeutic Agents for Neurological Diseases. Biomedicines 2022, 10, 35203552. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef]
- BioRender: Scientific Image and Illustration Software. Available online: https://app.biorender.com/ (accessed on 7 April 2023).
- Stumpf, M.P.H.; Thorne, T.; De Silva, E.; Stewart, R.; Hyeong, J.A.; Lappe, M.; Wiuf, C. Estimating the size of the human interactome. Proc. Natl. Acad. Sci. USA 2008, 105, 6959–6964. [Google Scholar] [CrossRef] [PubMed]
- Bano, D.; Ankarcrona, M. Beyond the critical point: An overview of excitotoxicity, calcium overload and the downstream consequences. Neurosci. Lett. 2018, 663, 79–85. [Google Scholar] [CrossRef]
- Kostandy, B.B. The role of glutamate in neuronal ischemic injury: The role of spark in fire. Neurol. Sci. 2012, 33, 223–237. [Google Scholar] [CrossRef]
- Ludhiadch, A.; Sharma, R.; Muriki, A.; Munshi, A. Role of Calcium Homeostasis in Ischemic Stroke: A Review. CNS Neurol. Disord. Drug Targets 2021, 21, 52–61. [Google Scholar] [CrossRef]
- Shen, Z.; Xiang, M.; Chen, C.; Ding, F.; Wang, Y.; Shang, C.; Xin, L.; Zhang, Y.; Cui, X. Glutamate excitotoxicity: Potential therapeutic target for ischemic stroke. Biomed. Pharmacother. 2022, 151, 113125. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Poleszak, K.; Kaminska, B. Short peptides interfering with signaling pathways as new therapeutic tools for cancer treatment. Future Med. Chem. 2017, 9, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.I.; Clemmensen, L.S.; Bredt, D.S.; Bettler, B.; Strømgaard, K. Targeting receptor complexes: A new dimension in drug discovery. Nat. Rev. Drug Discov. 2020, 19, 884–901. [Google Scholar] [CrossRef]
- Ugalde-Triviño, L.; Díaz-Guerra, M. PSD-95: An Effective Target for Stroke Therapy Using Neuroprotective Peptides. Int. J. Mol. Sci. 2021, 22, 12585. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Hleihil, M.; Bhat, M.A.; Ganley, R.P.; Vaas, M.; Klohs, J.; Zeilhofer, H.U.; Benke, D. Targeting the interaction of GABAB receptors with CaMKII with an interfering peptide restores receptor expression after cerebral ischemia and inhibits progressive neuronal death in mouse brain cells and slices. Brain Pathol. 2022, 33, e13099. [Google Scholar]
- Hleihil, M.; Balakrishnan, K.; Benke, D. Protein phosphatase 2A regulation of GABA B receptors normalizes ischemia-induced aberrant receptor trafficking and provides neuroprotection. Front. Mol. Neurosci. 2022, 15, 1015906. [Google Scholar] [CrossRef]
- Zemoura, K.; Balakrishnan, K.; Grampp, T.; Benke, D. Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) β-Dependent Phosphorylation of GABAB1 Triggers Lysosomal Degradation of GABAB Receptors via Mind Bomb-2 (MIB2)-Mediated Lys-63-Linked Ubiquitination. Mol. Neurobiol. 2019, 56, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Terunuma, M.; Vargas, K.J.; Wilkins, M.E.; Ramírez, O.A.; Jaureguiberry-Bravo, M.; Pangalos, M.N.; Smart, T.G.; Moss, S.J.; Couve, A. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 13918–13923. [Google Scholar] [CrossRef]
- Zemoura, K.; Trümpler, C.; Benke, D. Lys-63-linked Ubiquitination of γ-Aminobutyric Acid (GABA), Type B1, at Multiple Sites by the E3 Ligase Mind Bomb-2 Targets GABAB Receptors to Lysosomal Degradation. J. Biol. Chem. 2016, 291, 21682–21693. [Google Scholar] [CrossRef]
- Ballarin, B.; Tymianski, M. Discovery and development of NA-1 for the treatment of acute ischemic stroke. Acta Pharmacol. Sin. 2018, 39, 661–668. [Google Scholar] [CrossRef]
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet 2020, 395, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Mishra, A.; Lai, G.H.; Wong, G.C.L. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010, 584, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Meloni, B.P.; Milani, D.; Edwards, A.B.; Anderton, R.S.; O’Hare Doig, R.L.; Fitzgerald, M.; Palmer, T.N.; Knuckey, N.W. Neuroprotective peptides fused to arginine-rich cell penetrating peptides: Neuroprotective mechanism likely mediated by peptide endocytic properties. Pharmacol. Ther. 2015, 153, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.S.; Anderton, R.S.; Knuckey, N.W.; Meloni, B.P. The neuroprotective potential of arginine-rich peptides for the acute treatment of traumatic brain injury. Expert Rev. Neurother. 2016, 16, 361–363. [Google Scholar] [CrossRef]
- Meloni, B.P.; Milani, D.; Cross, J.L.; Clark, V.W.; Edwards, A.B.; Anderton, R.S.; Blacker, D.J.; Knuckey, N.W. Assessment of the Neuroprotective Effects of Arginine-Rich Protamine Peptides, Poly-Arginine Peptides (R12-Cyclic, R22) and Arginine-Tryptophan-Containing Peptides Following In Vitro Excitotoxicity and/or Permanent Middle Cerebral Artery Occlusion in Rats. Neuromolecular Med. 2017, 19, 271–285. [Google Scholar] [CrossRef]
- Meloni, B.P.; Mastaglia, F.L.; Knuckey, N.W. Cationic Arginine-Rich Peptides (CARPs): A Novel Class of Neuroprotective Agents With a Multimodal Mechanism of Action. Front. Neurol. 2020, 11, 108. [Google Scholar] [CrossRef]
- Meloni, B.P.; Blacker, D.J.; Edwards, A.B.; Knuckey, N.W. Impact of poly-arginine peptides R18D and R18 on alteplase and tenecteplase thrombolysis in vitro, and neuroprotective stability to proteolysis. J. Thromb. Thrombolysis 2022, 54, 172–182. [Google Scholar] [CrossRef]
- Yao, Y.; Ji, Y.; Ren, J.; Liu, H.; Khanna, R.; Sun, L. Inhibition of autophagy by CRMP2-derived peptide ST2-104 (R9-CBD3) via a CaMKKβ/AMPK/mTOR pathway contributes to ischemic postconditioning-induced neuroprotection against cerebral ischemia-reperfusion injury. Mol. Brain 2021, 14, 1–20. [Google Scholar] [CrossRef]
- Sánchez-Navarro, M.; Giralt, E. Peptide Shuttles for Blood-Brain Barrier Drug Delivery. Pharmaceutics 2022, 14, 1874. [Google Scholar] [CrossRef]
- Wang, X.-L.; Jiang, R.-W. Therapeutic Potential of Superoxide Dismutase Fused with Cell- Penetrating Peptides in Oxidative Stress-Related Diseases. Mini Rev. Med. Chem. 2022, 22, 2287–2298. [Google Scholar]
- Zhou, X.; Smith, Q.R.; Liu, X. Brain penetrating peptides and peptide–drug conjugates to overcome the blood–brain barrier and target CNS diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1695. [Google Scholar] [CrossRef]
- Pawar, B.; Vasdev, N.; Gupta, T.; Mhatre, M.; More, A.; Anup, N.; Tekade, R.K. Current Update on Transcellular Brain Drug Delivery. Pharmaceutics 2022, 14, 2719. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhuang, Y.; Zhang, Y.; Liao, H.; Liu, R.; Cheng, J.; Zhang, Z.; Sun, J.; Gao, J.; Wang, X.; et al. A synthetic BBB-permeable tripeptide GCF confers neuroprotection by increasing glycine in the ischemic brain. Front. Pharmacol. 2022, 13, 950376. [Google Scholar] [CrossRef] [PubMed]
- Reigado, G.R.; Adriani, P.P.; dos Santos, J.F.; Freitas, B.L.; Fernandes, M.T.P.; Chambergo Alcalde, F.S.; Leo, P.; Nunes, V.A. Delivery of superoxide dismutase by TAT and abalone peptides for the protection of skin cells against oxidative stress. Biotechnol. Appl. Biochem. 2022, 69, 2673–2685. [Google Scholar] [CrossRef]
- Yang, C.; Lavayen, B.P.; Liu, L.; Sanz, B.D.; DeMars, K.M.; Larochelle, J.; Pompilus, M.; Febo, M.; Sun, Y.Y.; Kuo, Y.M.; et al. Neurovascular protection by adropin in experimental ischemic stroke through an endothelial nitric oxide synthase-dependent mechanism. Redox Biol. 2021, 48, 102197. [Google Scholar] [CrossRef]
- Jasaszwili, M.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin as a fat-burning hormone with multiple functions—Review of a decade of research. Molecules 2020, 25, 549. [Google Scholar] [CrossRef]
- Yang, C.; Liu, L.; Lavayen, B.P.; Larochelle, J.; Gunraj, R.E.; Butler, A.A.; Candelario-Jalil, E. Therapeutic Benefits of Adropin in Aged Mice After Transient Ischemic Stroke via Reduction of Blood-Brain Barrier Damage. Stroke 2023, 54, 234–244. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Fan, J.; Sun, H.; Yao, Q.; Shi, J.; Qu, H.; Du, S.; Cheng, Y.; Ma, S.; et al. Dynorphin A (1–8) inhibits oxidative stress and apoptosis in MCAO rats, affording neuroprotection through NMDA receptor and κ-opioid receptor channels. Neuropeptides 2021, 89, 34298371. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, J.; Yang, L.; Gan, Y.; Hu, D.; Zhao, J.; Zhao, Y. SCO-spondin-derived peptide NX210 rescues neurons from cerebral ischemia/reperfusion injury through modulating the Integrin-β1 mediated PI3K/Akt pathway. Int. Immunopharmacol. 2022, 111, 35930911. [Google Scholar] [CrossRef]
- Meierhans, R.; Béchir, M.; Ludwig, S.; Sommerfeld, J.; Brandi, G.; Haberthür, C.; Stocker, R.; Stover, J.F. Brain metabolism is significantly impaired at blood glucose below 6 mM and brain glucose below 1 mM in patients with severe traumatic brain injury. Crit. Care 2010, 14, 20141631. [Google Scholar] [CrossRef]
- Trujillo, J.M.; Nuffer, W.; Smith, B.A. GLP-1 receptor agonists: An updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2021, 12, 33767808. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T. Liraglutide: A new treatment for type 2 Diabetes. Drugs Today 2009, 45, 101–113. [Google Scholar] [CrossRef]
- Hall, S.; Isaacs, D.; Clements, J.N. Pharmacokinetics and Clinical Implications of Semaglutide: A New Glucagon-Like Peptide (GLP)-1 Receptor Agonist. Clin. Pharmacokinet. 2018, 57, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; He, J.; Xie, Y.; Yang, Y. Delayed Administration of the Glucagon-Like Peptide 1 Analog Liraglutide Promoting Angiogenesis after Focal Cerebral Ischemia in Mice. J. Stroke Cerebrovasc. Dis. 2018, 27, 1318–1325. [Google Scholar] [CrossRef]
- Briyal, S.; Shah, S.; Gulati, A. Neuroprotective and anti-apoptotic effects of liraglutide in the rat brain following focal cerebral ischemia. Neuroscience 2014, 281, 269–281. [Google Scholar] [CrossRef]
- Sato, K.; Kameda, M.; Yasuhara, T.; Agari, T.; Baba, T.; Wang, F.; Shinko, A.; Wakamori, T.; Toyoshima, A.; Takeuchi, H.; et al. Neuroprotective effects of liraglutide for stroke model of rats. Int. J. Mol. Sci. 2013, 14, 21513–21524. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Miao, Y.; Chen, A.; Cheng, M.; Ye, X.; Song, F.; Zheng, G. Delayed administration of the GLP-1 receptor agonist liraglutide improves metabolic and functional recovery after cerebral ischemia in rats. Neurosci. Lett. 2017, 641, 1–7. [Google Scholar] [CrossRef]
- He, W.; Wang, H.; Zhao, C.; Tian, X.; Li, L.; Wang, H. Role of liraglutide in brain repair promotion through Sirt1-mediated mitochondrial improvement in stroke. J. Cell. Physiol. 2020, 235, 2986–3001. [Google Scholar] [CrossRef]
- Deng, C.; Cao, J.; Han, J.; Li, J.; Li, Z.; Shi, N.; He, J. Liraglutide Activates the Nrf2/HO-1 Antioxidant Pathway and Protects Brain Nerve Cells against Cerebral Ischemia in Diabetic Rats. Comput. Intell. Neurosci. 2018, 2018, 29623090. [Google Scholar] [CrossRef]
- Bai, B.; Li, D.; Xue, G.; Feng, P.; Wang, M.; Han, Y.; Wang, Y.; Hölscher, C. The novel GLP-1/GIP dual agonist DA3-CH is more effective than liraglutide in reducing endoplasmic reticulum stress in diabetic rats with cerebral ischemia-reperfusion injury. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 333–343. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, J.; Shi, G.; Zhang, C.; Du, Y.; Chen, L.; Qiao, H.; Chen, R.; Zhang, X. Liraglutide Ameliorates Cerebral Ischemia in Mice via Antipyroptotic Pathways. Neurochem. Res. 2022, 47, 1904–1916. [Google Scholar] [CrossRef]
- Lau, J.; Bloch, P.; Schäffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef] [PubMed]
- Anam, M.; Maharjan, S.; Amjad, Z.; Abaza, A.; Vasavada, A.M.; Sadhu, A.; Valencia, C.; Fatima, H.; Nwankwo, I. Efficacy of Semaglutide in Treating Obesity: A Systematic Review of Randomized Controlled Trials (RCTs). Cureus 2022, 14, 36654602. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Pal, R.; Mukhopadhyay, S.; Nair, K. GLP-1 Receptor Agonists and Risk of Adverse Cerebrovascular Outcomes in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2023, 2023, 36800286. [Google Scholar] [CrossRef]
- Lingvay, I.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide for cardiovascular event reduction in people with overweight or obesity: SELECT study baseline characteristics. Obesity 2023, 31, 111–122. [Google Scholar] [CrossRef]
- Mares, A.C.; Chatterjee, S.; Mukherjee, D. Semaglutide for weight loss and cardiometabolic risk reduction in overweight/obesity. Curr. Opin. Cardiol. 2022, 37, 350–355. [Google Scholar] [CrossRef]
- Gusev, E.I.; Martynov, M.Y.; Kostenko, E.V.; Petrova, L.V.; Bobyreva, S.N. The efficacy of semax in the tretament of patients at different stages of ischemic stroke. Zhurnal Nevrol. I Psikhiatr. Im. SS Korsakova 2018, 118, 61–68. [Google Scholar] [CrossRef]
- EI Gusev, V.S.E.C. Semax in prevention of disease progress and development of exacerbations in patients with cerebrovascular insufficiency. Zhurnal Nevrol. I Psikhiatr. Im. SS Korsakova 2005, 105, 35–40. [Google Scholar]
- Kolomin, T.; Shadrina, M.; Slominsky, P.; Limborska, S.; Myasoedov, N.; Kolomin, T.; Shadrina, M.; Slominsky, P.; Limborska, S.; Myasoedov, N. A New Generation of Drugs: Synthetic Peptides Based on Natural Regulatory Peptides. Neurosci. Med. 2013, 4, 223–252. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Dmitrieva, V.G.; Povarova, O.V.; Limborska, S.A.; Skvortsova, V.I.; Myasoedov, N.F.; Dergunova, L. V The peptide semax affects the expression of genes related to the immune and vascular systems in rat brain focal ischemia: Genome-wide transcriptional analysis. BMC Genom. 2014, 15, 228. [Google Scholar] [CrossRef]
- Yasenyavskaya, A.; Samotrueva, M.; Tsibizova, A.; Bashkina, O.; Andreeva, L.; Myasoedov, N. Effects of melanocortinson the behavior of ratsin thetest ofelevated cruciform maze and experimentally induced ofsocials. Exp. Clin. Pharmacol. 2020, 10, 35–38. [Google Scholar] [CrossRef]
- Bakaeva, Z.V.; Surin, A.M.; Lizunova, N.V.; Zgodova, A.E.; Krasilnikova, I.A.; Fisenko, A.P.; Frolov, D.A.; Andreeva, L.A.; Myasoedov, N.F.; Pinelis, V.G. Neuroprotective Potential of Peptides HFRWPGP (ACTH 6-9 PGP), KKRRPGP, and PyrRP in Cultured Cortical Neurons at Glutamate Excitotoxicity. Dokl. Biochem. Biophys. 2020, 491, 62–66. [Google Scholar] [CrossRef]
- Akimov, M.G.; Fomina-Ageeva, E.V.; Dudina, P.V.; Andreeva, L.A.; Myasoyedov, N.F.; Bezuglov, V.V. ACTH(6–9)PGP Peptide Protects SH-SY5Y Cells from H2O2, tert-Butyl Hydroperoxide, and Cyanide Cytotoxicity via Stimulation of Proliferation and Induction of Prosurvival-Related Genes. Molecules 2021, 26, 1878. [Google Scholar] [CrossRef]
- Dergunova, L.V.; Dmitrieva, V.G.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Valieva, L.V.; Sudarkina, O.Y.; Gubsky, L.V.; et al. The Peptide Drug ACTH(4-7)PGP (Semax) Suppresses mRNA Transcripts Encoding Proinflammatory Mediators Induced by Reversible Ischemia of the Rat Brain. Mol. Biol. 2021, 55, 402–411. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Dmitrieva, V.G.; Limborska, S.A.; Myasoedov, N.F.; Dergunova, L.V. Semax, an analog of ACTH(4-7), regulates expression of immune response genes during ischemic brain injury in rats. Mol. Genet. Genom. 2017, 292, 635–653. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Lau, J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019, 10, 31031702. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, T.; Han, Y.; Zuo, C.; Wang, G. E3 ubiquitin ligase COP1 confers neuroprotection in cerebral ischemia/reperfusion injury via regulation of transcription factor C/EBPβ in microglia. Int. J. Biol. Macromol. 2022, 222, 1789–1800. [Google Scholar] [CrossRef]
- Li, W.; Liu, D.; Xu, J.; Zha, J.; Wang, C.; An, J.; Xie, Z.; Qiao, S. Astrocyte-Derived TNF-α-Activated Platelets Promote Cerebral Ischemia/Reperfusion Injury by Regulating the RIP1/RIP3/AKT Signaling Pathway. Mol. Neurobiol. 2022, 59, 5734–5749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, C.; Shi, R.; Zhou, S.; Shan, H.; Deng, L.; Chen, T.; Guo, Y.; Zhang, Z.; Yang, G.Y.; et al. Blocking C3d+/GFAP+ A1 Astrocyte Conversion with Semaglutide Attenuates Blood-Brain Barrier Disruption in Mice after Ischemic Stroke. Aging Dis. 2022, 13, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, F.; Zhou, R.; Xiang, C.; Zhang, Y.; Gao, J.; Cao, G.; Yang, H. Proteomics and transcriptome reveal the key transcription factors mediating the protection of Panax notoginseng saponins (PNS) against cerebral ischemia/reperfusion injury. Phytomedicine 2021, 92, 153613. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, R.; Cao, G.; Zhang, Y.; Xu, H.; Yang, H. Guhong Injection Prevents Ischemic Stroke-Induced Neuro-Inflammation and Neuron Loss Through Regulation of C5ar1. Front. Pharmacol. 2022, 13, 818245. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Shuang, L.; Liu, G.; Zhao, S.; Yuan, Z.; Cai, H.; Jiang, L.; Huang, Z. Insight into the Neuroprotective Effect of Genistein-3’-Sodium Sulfonate Against Neonatal Hypoxic-Ischaemic Brain Injury in Rats by Bioinformatics. Mol. Neurobiol. 2022, 60, 807–819. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Q.; Jiang, J.; Wang, T.; Qiu, L.; Liu, A. Protective Effects of Nuciferine in Middle Cerebral Artery Occlusion Rats Based on Transcriptomics. Brain Sci. 2022, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, S.; Xiong, L.; Zhang, L.; Li, X.; Cao, X.; Xue, J.; Li, L.; Huang, C.; Huang, Z. Genistein-3′-sodium sulfonate attenuates neuroinflammation in stroke rats by down-regulating microglial m1 polarization through α7nachr-nf-κb signaling pathway. Int. J. Biol. Sci. 2021, 17, 1088–1100. [Google Scholar] [CrossRef]
- Wang, C.-M.; Pan, Y.-Y.; Liu, M.-H.; Cheng, B.-H.; Bai, B.; Chen, J. RNA-seq expression profiling of rat MCAO model following reperfusion Orexin-A. Oncotarget 2017, 8, 113066–113081. [Google Scholar] [CrossRef]
- Dmitrieva, V.G.; Povarova, O.V.; Skvortsova, V.I.; Limborska, S.A.; Myasoedov, N.F.; Dergunova, L.V. Semax and Pro-Gly-Pro activate the transcription of neurotrophins and their receptor genes after cerebral ischemia. Cell. Mol. Neurobiol. 2010, 30, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Stavchansky, V.V.; Yuzhakov, V.V.; Botsina, A.Y.; Skvortsova, V.I.; Bondurko, L.N.; Tsyganova, M.G.; Limborska, S.A.; Myasoedov, N.F.; Dergunova, L.V. The effect of Semax and its C-end peptide PGP on the morphology and proliferative activity of rat brain cells during experimental ischemia: A pilot study. J. Mol. Neurosci. 2011, 45, 177–185. [Google Scholar] [CrossRef]
- Dergunova, L.V.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Mozerov, S.A.; Gubsky, L.V.; Limborska, S.A. Genome-wide transcriptome analysis using RNA-Seq reveals a large number of differentially expressed genes in a transient MCAO rat model. BMC Genomics 2018, 19, 655. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Dmitrieva, V.G.; Povarova, O.V.; Limborskaia, S.A.; Skvortsova, V.I.; Miasoedov, N.F.; Dergunova, L.V. Effect of tripeptide Pro-Gly-Pro on rat brain transcriptome in focal ischemia. Mol. Biol. 2014, 48, 277–287. [Google Scholar] [CrossRef]
- Fleishman, M.Y.; Tolstenok, I.V.; Lebed’ko, O.A.; Andreeva, L.A.; Myasoedov, N.F.; Timoshin, S.S. Effects of Glyprolines on DNA Synthesis and Free Radical Oxidation in Mouse Gastric Mucosa Under Physiological Conditions and During Therapy with Oral Non-Steroid Anti-Inflammatory Drugs. Bull. Exp. Biol. Med. 2015, 159, 502–504. [Google Scholar] [CrossRef]
- Edeeva, S.E.; Kopylova, G.N.; Bakaeva, Z.V.; Samonina, G.E.; Umarova, B.A.; Guseva, A.A. Protective and therapeutic effects of glyprolines in psychoemotional stress induced by cholecystokinin-4 injection. Bull. Exp. Biol. Med. 2008, 145, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, K.V.; Nagaev, I.Y.; Andreeva, L.A.; Shevchenko, V.P.; Myasoedov, N.F. Stability of prolin-containing peptides in biological media. Biomed. Khimiya 2019, 65, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Misiura, M.; Miltyk, W. Proline-containing peptides-New insight and implications: A Review. Biofactors 2019, 45, 857–866. [Google Scholar] [CrossRef]
- Myasoedov, N.F.; Lyapina, L.A.; Andreeva, L.A.; Grigorieva, M.E.; Obergan, T.Y.; Shubina, T.A. The modern view on the role of glyprolines by metabolic syndrome. Med. Res. Rev. 2021, 41, 2823–2840. [Google Scholar] [CrossRef] [PubMed]
- Stavchansky, V.V.; Filippenkov, I.B.; Remizova, J.A.; Denisova, A.E.; Mozgovoy, I.V.; Gubsky, L.V.; Myasoedov, N.F.; Andreeva, L.A.; Limborska, S.A.; Dergunova, L.V. Insight into Glyproline Peptides’ Activity through the Modulation of the Inflammatory and Neurosignaling Genetic Response Following Cerebral Ischemia–Reperfusion. Genes 2022, 13, 2380. [Google Scholar] [CrossRef]
- Aggarwal, R.; Vaduganathan, M.; Chiu, N.; Bhatt, D.L. Potential implications of the FDA approval of semaglutide for overweight and obese adults in the United States. Prog. Cardiovasc. Dis. 2021, 68, 97–98. [Google Scholar] [CrossRef]
- Vyunova, T.V.; Andreeva, L.A.; Shevchenko, K.V.; Myasoedov, N.F. An integrated approach to study the molecular aspects of regulatory peptides biological mechanism. J. Label. Comp. Radiopharm. 2019, 62, 812–822. [Google Scholar] [CrossRef]
- Forte, M.; Madonna, M.; Schiavon, S.; Valenti, V.; Versaci, F.; Zoccai, G.B.; Frati, G.; Sciarretta, S. Cardiovascular pleiotropic effects of natriuretic peptides. Int. J. Mol. Sci. 2019, 20, 3874. [Google Scholar] [CrossRef]
- Mannes, M.; Martin, C.; Menet, C.; Ballet, S. Wandering beyond small molecules: Peptides as allosteric protein modulators. Trends Pharmacol. Sci. 2022, 43, 406–423. [Google Scholar] [CrossRef]
- Filippenkov, I.B.; Remizova, J.A.; Denisova, A.E.; Stavchansky, V.V.; Golovina, K.D.; Gubsky, L.V.; Limborska, S.A.; Dergunova, L.V. Differential gene expression in the contralateral hemisphere of the rat brain after focal ischemia. Sci. Rep. 2023, 13, 573. [Google Scholar] [CrossRef]
- Han, R.; Zhang, P.; Li, H.; Chen, Y.; Hao, Y.; Guo, Q.; Zhang, A.; Li, D. Differential Expression and Correlation Analysis of Global Transcriptome for Hemorrhagic Transformation After Acute Ischemic Stroke. Front. Neurosci. 2022, 16, 889689. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Aswendt, M.; Lee, A.G.; Ishizaka, S.; Cao, Z.; Wang, E.H.; Levy, S.L.; Smerin, D.L.; McNab, J.A.; Zeineh, M.; et al. RNA-Sequencing Analysis Revealed a Distinct Motor Cortex Transcriptome in Spontaneously Recovered Mice After Stroke. Stroke 2018, 49, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Hay, D.L.; Pioszak, A.A. Calcitonin and Amylin Receptor Peptide Interaction Mechanisms. J. Biol. Chem. 2016, 291, 8686–8700. [Google Scholar] [CrossRef]
- Vyunova, T.V.; Andreeva, L.; Shevchenko, K.; Myasoedov, N. Peptide-based Anxiolytics: The Molecular Aspects of Heptapeptide Selank Biological Activity. Protein Pept. Lett. 2018, 25, 914–923. [Google Scholar] [CrossRef]
- Dona, M.S.I.; Hsu, I.; Meuth, A.I.; Brown, S.M.; Bailey, C.; Aragonez, C.G.; Chandrasekar, B.; Martinez-Lemus, L.A.; DeMarco, V.G.; Grisanti, L.A.; et al. Multi-omic analysis of the cardiac cellulome defines a vascular contribution to cardiac diastolic dysfunction in obese female mice. bioRxiv 2022, 128, 11. [Google Scholar] [CrossRef]
- Fedulova, L.; Vasilevskaya, E.; Tikhonova, O.; Kazieva, L.; Tolmacheva, G.; Makarenko, A. Proteomic Markers in the Muscles and Brain of Pigs Recovered from Hemorrhagic Stroke. Genes 2022, 13, 2204. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, G.A.; Lees-Gayed, N.; Banoub, J.; Abbatielo, S.E.; Robertson, C.; Haskins, W.E.; Yost, R.A.; Wang, K.K.W. Generation and Release of Neurogranin, Vimentin, and MBP Proteolytic Peptides, Following Traumatic Brain Injury. Mol. Neurobiol. 2022, 59, 731–747. [Google Scholar] [CrossRef]
- Hu, W.; Li, P.; Zeng, N.; Tan, S. Exploring the hub mechanisms of ischemic stroke based on protein-protein interaction networks related to ischemic stroke and inflammatory bowel disease. Sci. Rep. 2023, 13, 36720935. [Google Scholar] [CrossRef]
- Qiu, M.; Zong, J.B.; He, Q.W.; Liu, Y.X.; Wan, Y.; Li, M.; Zhou, Y.F.; Wu, J.H.; Hu, B. Cell Heterogeneity Uncovered by Single-Cell RNA Sequencing Offers Potential Therapeutic Targets for Ischemic Stroke. Aging Dis. 2022, 13, 1436–1454. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dergunova, L.V.; Filippenkov, I.B.; Limborska, S.A.; Myasoedov, N.F. Neuroprotective Peptides and New Strategies for Ischemic Stroke Drug Discoveries. Genes 2023, 14, 953. https://doi.org/10.3390/genes14050953
Dergunova LV, Filippenkov IB, Limborska SA, Myasoedov NF. Neuroprotective Peptides and New Strategies for Ischemic Stroke Drug Discoveries. Genes. 2023; 14(5):953. https://doi.org/10.3390/genes14050953
Chicago/Turabian StyleDergunova, Lyudmila V., Ivan B. Filippenkov, Svetlana A. Limborska, and Nikolay F. Myasoedov. 2023. "Neuroprotective Peptides and New Strategies for Ischemic Stroke Drug Discoveries" Genes 14, no. 5: 953. https://doi.org/10.3390/genes14050953
APA StyleDergunova, L. V., Filippenkov, I. B., Limborska, S. A., & Myasoedov, N. F. (2023). Neuroprotective Peptides and New Strategies for Ischemic Stroke Drug Discoveries. Genes, 14(5), 953. https://doi.org/10.3390/genes14050953






