Genetic Predictors of Antipsychotic Efflux Impairment via Blood-Brain Barrier: Role of Transport Proteins
Abstract
1. Introduction
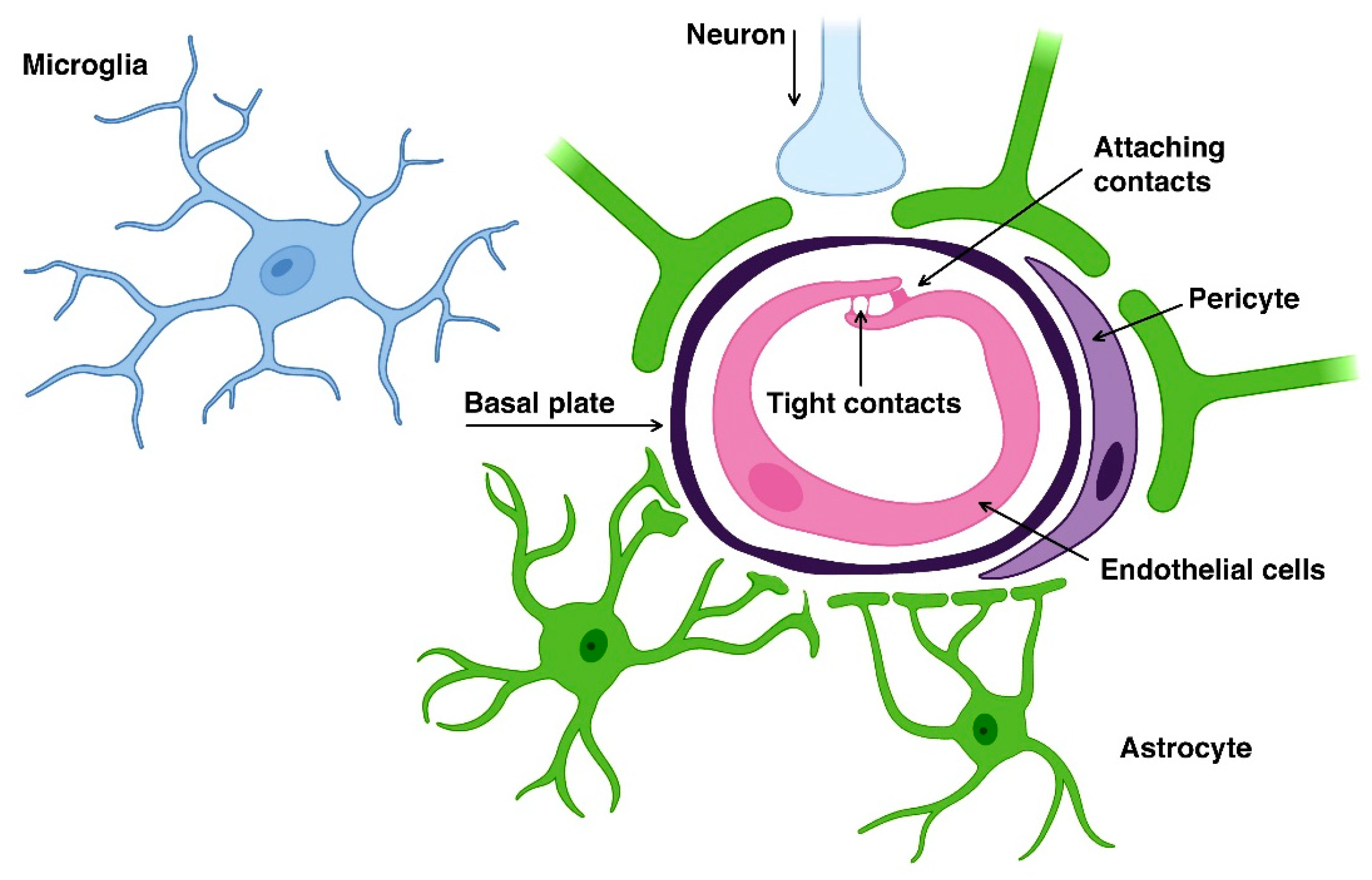
2. Blood-Brain Barrier
3. Transcellular Drug Transport via the Blood-Brain Barrier
- simple diffusion;
- facilitated diffusion;
- endocytosis via receptor-mediated transcytosis;
- efflux transport.
3.1. Simple Diffusion
3.2. Facilitated Diffusion
3.3. Endocytosis via Receptor-Mediated Transcytosis
3.4. Efflux Transport
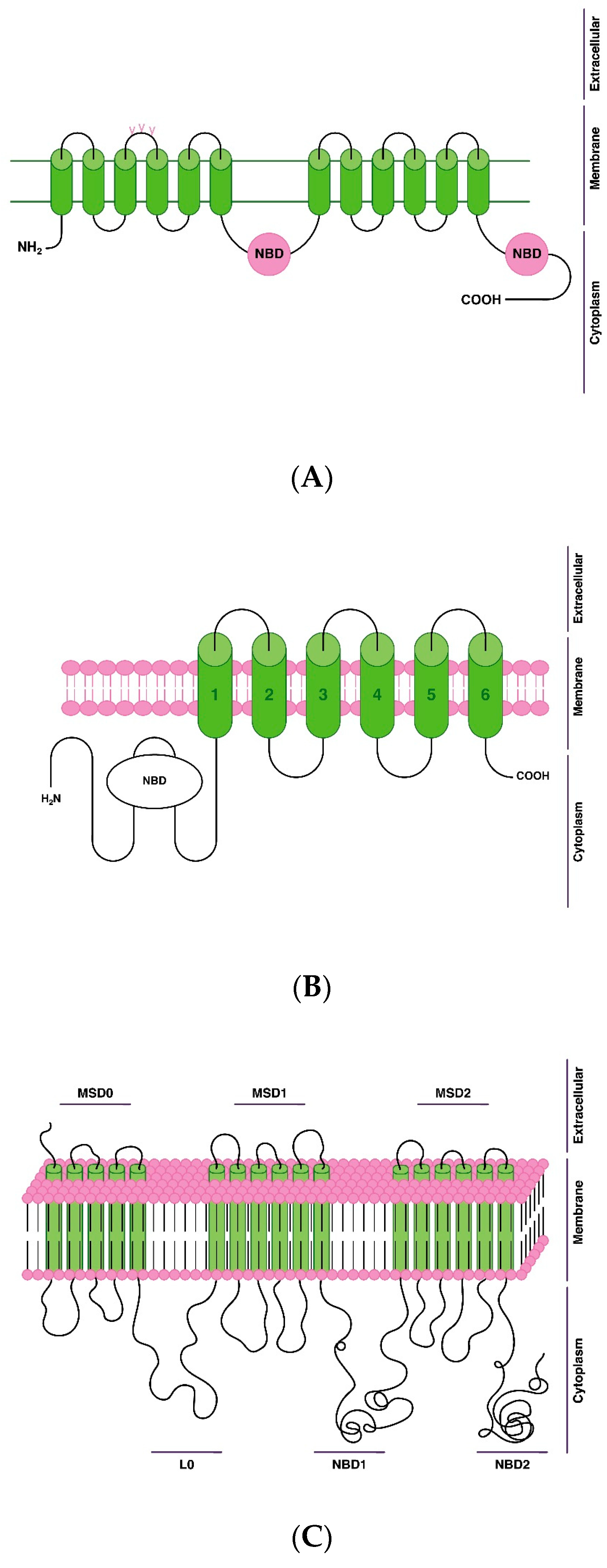
4. Transport Proteins of Antipsychotic Efflux via the Blood-Brain Barrier
- P-gp, or multidrug resistance protein 1 (MDR1);
- breast cancer resistance protein (BCRP);
- multidrug resistance-associated protein (MRP11).
5. Genetic Predisposition to Reduction of Antipsychotic Efflux via the Brain-Blood Barrier
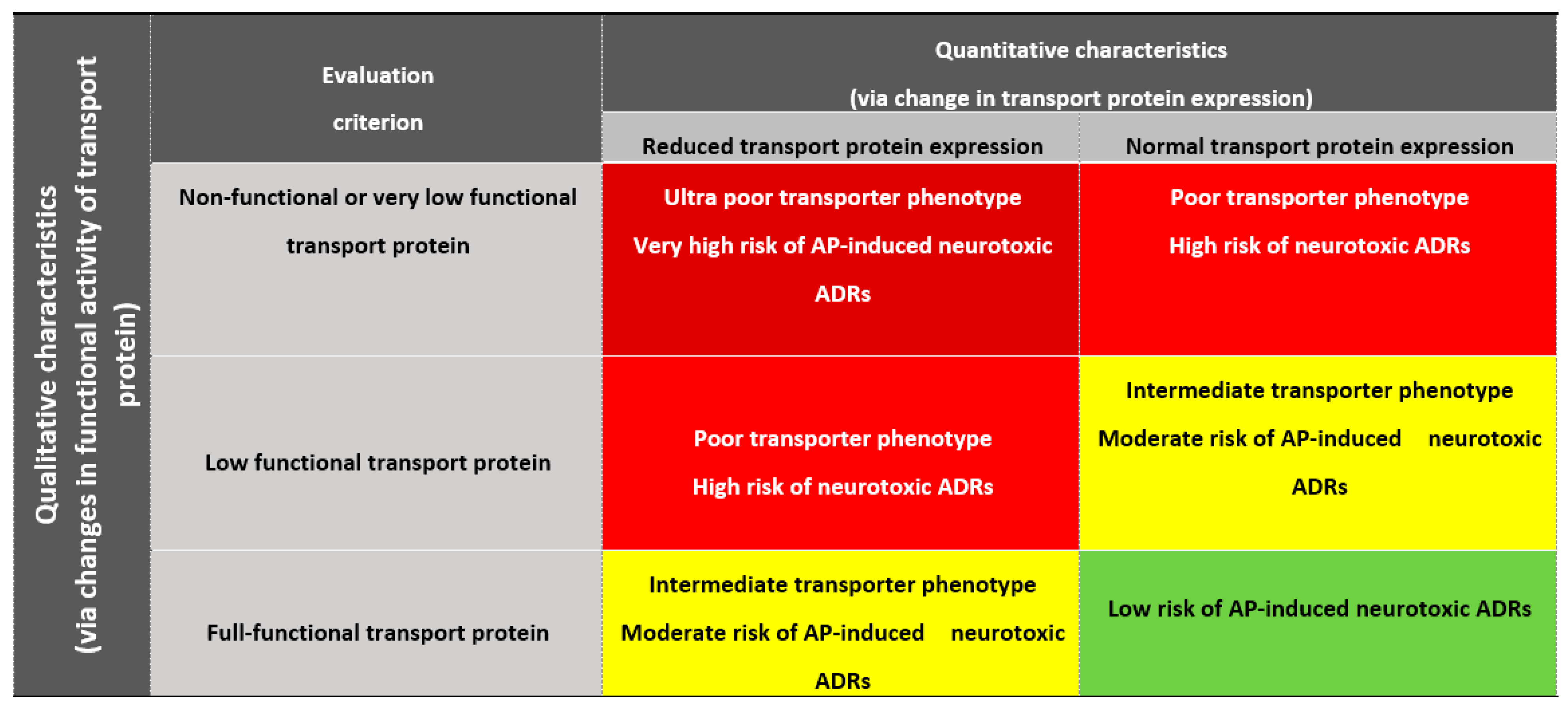
5.1. Phenotyping of Patients Depending on Antipsychotic Efflux Reduction via the Blood-Brain Barrier
5.1.1. Poor Transporter
5.1.2. Intermediate Transporter
5.1.3. Extensive Transporter
5.2. Prediction of a Genetically Determined Decrease of Antipsychotic Efflux via the Blood Brain Barrier
6. Antipsychotics-Substrates of Transport Proteins
7. Discussion
7.1. Perspective Pharmacogenetic Panel
7.2. Riskometer for PTAP-PGx
7.3. PTAP-PGx Decision Algorithm
7.3.1. Very High Risk of Antipsychotic-Induced Neurotoxic Adverse Drug Reactions
- Cancellation of the previously used AP, transported from brain to blood via the BBB mainly by this transport protein, if the patient is already taking this AP.
- Refusal to prescribe an AP transported from brain to blood via the BBB mainly by this transport protein, if the patient has not previously taken this AP.
7.3.2. High Risk of Antipsychotic-Induced Neurotoxic Adverse Drug Reactions
- Reduction by ~50% of single and daily doses of a previously used AP, transported mainly by this transport protein, if the patient is already taking this AP.
- The start of pharmacotherapy with a decrease of ~50% from the average starting dose of a newly prescribed AP, transported from brain to blood via the BBB mainly by this transport protein, if the patient has not previously taken this AP.
- A very slow pace of increasing the dose of AP (reducing the rate of increasing the dose by 2 times: for example, 1 time in 4 weeks instead of 1 time in 2 weeks).
- Clinical monitoring of possible neurotoxic ADRs is recommended in monotherapy with these APs and, especially, in polytherapy with APs.
- TDM of the level of this AP in the blood or plasma at least once every 3 months.
- Refusal of polytherapy with the appointment of two or more APs, transported from brain to blood via the BBB mainly by this transport protein.
- With low efficiency of monotherapy with this AP, transported mainly by this transport protein, it is possible to additionally prescribe an AP, transported mainly by another transport protein.
7.3.3. Moderate Risk of Antipsychotic-Induced Neurotoxic Adverse Drug Reactions
- Reduction by ~25% of single and daily doses of a previously used AP, transported mainly by this transport protein, if the patient is already taking this AP.
- The start of pharmacotherapy with a decrease of ~25% from the average starting dose of a newly prescribed AP, transported mainly from brain to blood via the BBB by this transport protein, if the patient has not previously taken this AP.
- Slow rate of increase in the dose of this AP (decrease in the rate of increase in the dose of AP by ~25%: for example, once every 3 weeks instead of once every 2 weeks).
- Clinical monitoring of possible neurotoxic ADRs in AP polytherapy is recommended.
- TDM of the level of this AP in the blood or plasma once every 6 months.
7.3.4. No Risk (or Minimal Risk) of Antipsychotic-Induced Neurotoxic Adverse Drug Reactions
- It is possible to prescribe the average or maximum allowable (according to the current instructions for this AP), according to indications, therapeutic single and daily doses of the previously used AP, transported mainly by this transport protein, if the patient is already taking this AP.
- Start of pharmacotherapy with an average starting dose of a newly prescribed AP, transported mainly by this protein, if the patient has not previously taken this AP.
- The average rate of increasing the dose of AP (according to the current instructions for this AP).
- Dynamic observation is recommended during long-term use of this AP in monotherapy, or when it is prescribed in high (or maximum allowable) doses, or in polytherapy with APs transported from brain to blood via the BBB with the participation of this transport protein.
- TDM of the blood (or plasma) level of this AP and/or its active metabolites according to indications (when prescribing this AP in high or maximum allowable doses or in polytherapy) or TDM of the level of this AP in the blood or plasma at least once every 12 months.
8. Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCutcheon, R.A.; Marques, R.T.; Howes, O.D. Schizophrenia—An overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Orrico-Sánchez, A.; López-Lacort, M.; Muñoz-Quiles, C.; Sanfélix-Gimeno, G.; Díez-Domingo, J. Epidemiology of schizophrenia and its management over 8-years period using real-world data in Spain. BMC Psychiatry 2020, 20, 149. [Google Scholar] [CrossRef]
- Katona, L.; Bitter, I.; Czobor, P. A meta-analysis of effectiveness of real-world studies of antipsychotics in schizophrenia: Are the results consistent with the findings of randomized controlled trials. Transl. Psychiatry 2021, 11, 510. [Google Scholar] [CrossRef]
- Neznanov, N.G.; Nasyrova, R.F.; Shnayder, N.A. From classical to personalized psychoneurology. Pers. Psychiatry Neurol. 2022, 2, 1–3. [Google Scholar] [CrossRef]
- Bahta, M.; Ogbaghebriel, A.; Russom, M.; Tesfamariam, E.H.; Berhe, T. Impact of adverse reactions to first-generation antipsychotics on treatment adherence in outpatients with schizophrenia: A cross-sectional study. Ann. Gen. Psychiatry 2021, 20, 27. [Google Scholar] [CrossRef]
- Kaar, S.J.; Natesan, S.; McCutcheon, R.; Howes, J.D. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology 2020, 172, 107704. [Google Scholar] [CrossRef] [PubMed]
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Nasyrova, R.F.; Neznanov, N.G. (Eds.) Clinical Psychopharmacologenetics: Monography; DEAN Publishing House: St. Petersburg, Russia, 2019; pp. 1–405. ISBN 978-5-6043573-7-8. [Google Scholar]
- Neznanov, N.G. A paradigm shift to treat psychoneurological disorders. Pers. Psychiatry Neurol. 2021, 1, 1–2. [Google Scholar]
- Nasyrova, R.F.; Ivanov, M.V.; Neznanov, N.G. Introduction to Psychopharmacogenetics: Monography; DEAN Publishing House: St. Petersburg, Russia, 2015; pp. 1–272. ISBN 978-5-7452-0020-5. [Google Scholar]
- Xu, Q.; Wu, X.; Xiong, Y.; Xing, Q.; He, L.; Qin, S. Pharmacogenomics can improve antipsychotic treatment in schizophrenia. Front. Med. 2013, 7, 180–190. [Google Scholar] [CrossRef]
- Shnayder, N.A.; Khasanova, A.K.; Strelnik, A.I.; Al-Zamil, M.; Otmakhov, A.P.; Neznanov, N.G.; Shipulin, G.A.; Petrova, M.M.; Garganeeva, N.P.; Nasyrova, R.F. Cytokine imbalance as a biomarker of treatment-resistant schizophrenia. Int. J. Mol. Sci. 2022, 23, 11324. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, D.; Melisie, G.; Taye, K.; Tadesse, E. The role of ABC efflux transporter in treatment of pharmaco-resistant schizophrenia: A review article. Clin. Pharmacol. Biopharm. 2019, 8, 189. [Google Scholar]
- Luptáková, D.; Vallianatou, T.; Nilsson, A.; Shariatgorji, R.; Hammarlund-Udenaes, M.; Loryan, I.; Andrén, P.E. Neuropharmacokinetic visualization of regional and subregional unbound antipsychotic drug transport across the blood–brain barrier. Mol. Psychiatry 2021, 26, 7732–7745. [Google Scholar] [CrossRef] [PubMed]
- Eng, M.E.; Imperio, G.E.; Bloise, E.; Matthews, S.G. ATP-binding cassette (ABC) drug transporters in the developing blood–brain barrier: Role in fetal brain protection. Cell. Mol. Life Sci. 2022, 79, 415. [Google Scholar] [CrossRef]
- Osipova, S.M.; Shnayder, N.A. Pharmacogenetic testing of antipsychotic transporter proteins: A case report in a 32-year-old woman with treatment-resistant schizophrenia. Pers. Psychiatry Neurol. 2022, 2, 98–106. [Google Scholar] [CrossRef]
- Gupta, M.; Lee, H.J.; Barden, C.J.; Weaver, D.F. The blood–brain barrier (BBB) Score. J. Med. Chem. 2019, 62, 9824–9836. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Liebner, S. Structure and function of the blood–brain barrier (BBB). In Physiology, Pharmacology and Pathology of the Blood-Brain Barrier: Handbook of Experimental Pharmacology; Cader, Z., Neuhaus, W., Eds.; Springer: Cham, Switzerland, 2020; pp. 12–23. [Google Scholar]
- Villabona-Rueda, A.; Erice, C.; Pardo, C.A.; Stins, M.F. The evolving concept of the blood brain barrier (BBB): From a single static barrier to a heterogeneous and dynamic relay center. Front. Cell. Neurosci. 2019, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Gorbachev, V.I.; Bragina, N.V. Blood-brain barrier from the point of view of anesthesiologist. Review. Part 1. Ann. Crit. Care 2020, 3, 35–45. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood–brain barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Rafi, M.A.; Pourseif, M.M.; Omidi, Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. Bioimpacts 2016, 6, 225–248. [Google Scholar] [CrossRef] [PubMed]
- Al Rihani, S.B.; Darakjian, L.I.; Deodhar, M.; Dow, P.; Turgeon, J.; Michaud, V. Disease-induced modulation of drug transporters at the blood–brain barrier level. Int. J. Mol. Sci. 2021, 22, 3742. [Google Scholar] [PubMed]
- Schäfer, A.M.; Meyer zu Schwabedissen, H.E.; Grube, M. Expression and function of organic anion transporting polypeptides in the human brain: Physiological and pharmacological implications. Pharmaceutics 2021, 13, 834. [Google Scholar] [CrossRef]
- Girardin, F. Membrane transporter proteins: A challenge for CNS drug development. Dialogues Clin. Neurosci. 2006, 8, 311–321. [Google Scholar] [CrossRef]
- Hu, C.; Tao, L.; Cao, X.; Chen, L. The solute carrier transporters and the brain: Physiological and pharmacological implications. Asian J. Pharm. Sci. 2020, 15, 131–144. [Google Scholar]
- Pulgar, V.M. Transcytosis to cross the blood brain barrier, new advancements and challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [PubMed]
- Zhang, W.; Liu, Q.Y.; Haqqani, A.S.; Leclerc, S.; Liu, Z.; Fauteux, F.; Baumann, E.; Delaney, C.E.; Ly, D.; Star, A.T.; et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS 2020, 17, 47. [Google Scholar] [PubMed]
- Erickson, M.A.; Banks, W.A. Transcellular routes of blood-brain barrier disruption. Exp. Biol. Med. 2022, 247, 788–796. [Google Scholar] [CrossRef]
- Azarmi, M.; Maleki, H.; Nikkam, N.; Malekinejad, H. Transcellular brain drug delivery: A review on recent advancements. Int. J. Pharm. 2020, 586, 119582. [Google Scholar] [CrossRef]
- Mahringer, A.; Ott, M.; Reimold, I.; Reichel, V.; Fricker, G. The ABC of the blood-brain barrier–regulation of drug efflux pumps. Curr. Pharm. Des. 2011, 17, 2762–2770. [Google Scholar] [CrossRef]
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.-M.; Decleves, X.; Menet, M.-C. ABC Transporters at the blood–brain interfaces, their study models, and drug delivery implications in gliomas. Pharmaceutics 2020, 12, 20. [Google Scholar] [CrossRef]
- Thomas, C.; Tampé, R. Structural and mechanistic principles of ABC transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Miller, D.S.; Pasinelli, P.; Trotti, D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015, 1628, 298–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Manautou, J.E.; Rasmussen, T.P.; Zhong, X. Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm. Sin. 2019, 9, 659–674. [Google Scholar] [CrossRef]
- Sarkadi, B.; Homolya, L.; Hegedűs, T. The ABCG2/BCRP transporter and its variants–from structure to pathology. FEBS Lett. 2020, 594, 4012–4034. [Google Scholar] [CrossRef]
- Bruckmueller, H.; Cascorbi, I. ABCB1, ABCG2, ABCC1, ABCC2, and ABCC3 drug transporter polymorphisms and their impact on drug bioavailability: What is our current understanding. Expert Opin. Drug Metab. Toxicol. 2021, 17, 369–396. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S. Regulation of ABC transporters blood-brain barrier: The good, the bad, and the ugly. Adv. Cancer Res. 2015, 125, 43–70. [Google Scholar]
- Ahmadzai, H.; Tee, L.B.; Crowe, A. Pharmacological role of efflux transporters: Clinical implications for medication use during breastfeeding. World J. Pharmacol. 2014, 3, 153–161. [Google Scholar] [CrossRef]
- Szakács, G.; Homolya, L.; Sarkadi, B.; Váradi, A. MDR-ABC transporters. In Encyclopedia of Molecular Pharmacology; Offermanns, S., Rosenthal, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 748–752. [Google Scholar]
- OMIM: Online Mendelian Inheritance in Man. Available online: https://www.omim.org/ (accessed on 15 August 2022).
- GeneCards: The Human Gene Database. Available online: https://www.genecards.org/ (accessed on 15 August 2022).
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 15 August 2022).
- Daood, M.; Tsai, C.; Ahdab-Barmada, M.; Watchko, J.F. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics 2008, 39, 211–218. [Google Scholar] [CrossRef]
- Wolking, S.; Schaeffeler, E.; Lerche, H.; Schwab, M.; Nies, A.T. Impact of genetic polymorphisms of ABCB1 (MDR1, P-glycoprotein) on drug disposition and potential clinical implications: Update of the literature. Clin. Pharmacokinet. 2015, 54, 709–735. [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, S.; Mittal, R.; Mittal, B. The effect of ABCB1 polymorphisms on the outcome of breast cancer treatment. Pharmgenomics Pers. Med. 2016, 9, 47–58. [Google Scholar] [PubMed]
- SNPedia. Available online: https://www.snpedia.com/ (accessed on 15 August 2022).
- The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 15 August 2022).
- PharmGKB. Available online: https://www.pharmgkb.org/ (accessed on 15 August 2022).
- Mo, W.; Zhang, J.T. Human ABCG2: Structure, function, and its role in multidrug resistance. Int. J. Biochem. Mol. Biol. 2012, 3, 1–27. [Google Scholar]
- Jani, M.; Ambrus, C.; Magnan, R.; Jakab, K.T.; Beéry, E.; Zolnerciks, J.K.; Krajcsi, P. Structure and function of BCRP, a broad specificity transporter of xenobiotics and endobiotics. Arch. Toxicol. 2014, 88, 1205–1248. [Google Scholar] [CrossRef] [PubMed]
- Schwabedissen, H.E.; Kroemer, H.K. In vitro and in vivo evidence for the importance of breast cancer resistance protein transporters (BCRP/MXR/ABCP/ABCG2). Handb. Exp. Pharmacol. 2011, 201, 325–371. [Google Scholar]
- Cole, S.P. Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. J. Biol. Chem. 2014, 289, 30880–30888. [Google Scholar] [CrossRef] [PubMed]
- Hipfner, D.R.; Deeley, R.G.; Cole, S.P. Structural, mechanistic and clinical aspects of MRP1. Biochim. Biophys. Acta (BBA)–Biomembr. 1999, 1461, 359–376. [Google Scholar] [CrossRef]
- Wei, M.; Jing-Yuan, L.; Jian-Ting, Z. Biochemistry and pharmacology of human ABCC1/MRP1 and its role in detoxification and in multidrug resistance of cancer chemotherapy. Recent Adv. Cancer Res. Ther. 2012, 12, 371–404. [Google Scholar]
- Abdyrakhmanova, A.K.; Shnayder, N.A.; Nasyrova, R.F. A clinical case of late pharmacogenetic diagnosis of adverse reactions during psychopharmacotherapy in a patient with recurrent depressive disorder. Pharm. Pharm. 2021, 2, 21–23. [Google Scholar]
- Sychev, D.A. Clinical and pharmacological technologies of personalized medicine: What now and what will happen? V.M. Bekhterev Rev. Psychiatry Med. Psychol. 2019, 4, 24–25. [Google Scholar] [CrossRef]
- Dobrodeeva, V.S.; Shnayder, N.A.; Nasyrova, R.F. Pharmacogenetic aspects of the efficacy and safety of trazodone therapy. Pharm. Pharm. 2019, 1, 25–28. [Google Scholar]
- Malla, S.; Muskiewicz, D.E.; Hussein, N.A.; Hall, F.S.; Tiwari, A.K. The role of ABC transporters in the actions of drugs of abuse. In Handbook of Substance Misuse and Addictions; Patel, V.B., Preedy, V.R., Eds.; Springer: Cham, Switzerland, 2022; pp. 1–18. [Google Scholar]
- The Clinical Pharmacogenetics Implementation Consortium (CPIC®). Available online: https://cpicpgx.org/ (accessed on 15 August 2022).
- The Canadian Pharmacogenomics Network for Drug Safety. Available online: https://cpnds.ubc.ca/ (accessed on 15 August 2022).
- Morningstar. Available online: https://www.morningstar.com/funds/xnas/rnpgx/quote (accessed on 15 August 2022).
- The Russian Society of Pharmacogenetics, Pharmacokinetics and Personalized Therapy (SPPPT). Available online: https://xn--80aaalkavmenpjw0bt.xn--p1ai/ (accessed on 15 August 2022).
- Abdullah-Koolmees, H.; van Keulen, A.M.; Nijenhuis, M.; Deneer, V.H.M. Pharmacogenetics guidelines: Overview and comparison of the DPWG, CPIC, CPNDS, and RNPGx guidelines. Front. Pharmacol. 2021, 11, 595219. [Google Scholar] [CrossRef]
- The European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en (accessed on 15 August 2022).
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/ (accessed on 15 August 2022).
- Barbarino, J.M.; Whirl-Carrillo, M.; Altman, R.B.; Klein, T.E. PharmGKB: A worldwide resource for pharmacogenomic information. WIREs Syst. Biol. Med. 2018, 10, e1417. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Roll, S.C. The influence of pharmacogenetics on the clinical relevance of pharmacokinetic drug–drug interactions: Drug–gene, drug–gene–gene and drug–drug–gene interactions. Pharmaceuticals 2021, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Bousman, C.A.; Dunlop, B.W. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. Pharm. J. 2018, 18, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.G.; Shams, T.A.; Tiwari, A.K.; Müller, D.J. Pharmacogenetics and outcome with antipsychotic drugs. Dialogues Clin. Neurosci. 2014, 16, 555–566. [Google Scholar] [CrossRef]
- Bousman, C.A.; Bengesser, S.A.; Aitchison, K.J.; Amare, A.T.; Aschauer, H.; Baune, B.T.; Asl, B.B.; Bishop, J.R.; Burmeister, M.; Chaumette, B.; et al. Review and consensus on pharmacogenomic testing in psychiatry. Pharmacopsychiatry 2021, 54, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Bousman, C.; Maruf, A.A.; Müller, D.J. Towards the integration of pharmacogenetics in psychiatry: A minimum, evidence-based genetic testing panel. Curr. Opin. Psychiatry 2019, 32, 7–15. [Google Scholar] [CrossRef]
- Zhang, J.P.; Malhotra, A.K. Recent progress in pharmacogenomics of antipsychotic drug response. Curr. Psychiatry Rep. 2018, 20, 24. [Google Scholar] [CrossRef]
- The GeneSight®. Available online: https://genesight.com/ (accessed on 15 August 2022).
- Bond, T. Third clinical trial reinforces the use of the GeneSight® pharmacogenomic test. Pharmacogenomics 2014, 15, 257. [Google Scholar] [PubMed]
- Nasyrova, R.F.; Dobrodeeva, V.S.; Skopin, S.D.; Shnayder, N.A.; Neznanov, N.G. Problems and prospects for the implementation of pharmacogenetic testing in real clinical practice in the Russian Federation. Bull. Neurol. Psychiatry Neurosurg. 2020, 3, 2–7. [Google Scholar] [CrossRef]
- Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Gründer, G.; Haen, E.; Baumann, P.; Bergemann, N.; et al. TDM in psychiatry and neurology: A comprehensive summary of the consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology, update 2017; a tool for clinicians. World J. Biol. Psychiatry 2018, 19, 162–174. [Google Scholar] [CrossRef] [PubMed]

| Gene: OMIM * | Chromosomal Location ** | Protein ** | Amino Acid Content *** |
|---|---|---|---|
| ABCB1: 171050 | chr7: 87,503,017-87,713,323 (GRCh38/hg38) | Multidrug Resistance Protein 1 (MDR1) P-Glycoprotein 1 (P-gp) | 1280 |
| ABCB2: 170260 | chr6: 32,845,209-32,853,816 (GRCh38/hg38) | Transporter 1 (TAP1) | 808 |
| ABCB3: 170261 | chr6: 32,821,831-32,838,770 (GRCh38/hg38) | Transporter 2 (TAP2) | 653 |
| ABCB4: 171060 | chr7: 87,398,988-87,480,435 (GRCh38/hg38) | Multidrug Resistance Protein 3 (MDR2) P-Glycoprotein 3 | 1279 |
| ABCB11: 603201 | chr2: 168,915,468-169,031,396 (GRCh38/hg38) | Bile Salt Export Pump (BSEP) | 1321 |
| ABCC1: 158343 | chr16: 15,949,138-16,143,257 (GRCh38/hg38) | Multidrug Resistance-Associated Protein 1 (MRP1) | 1531 |
| ABCC2: 601107 | chr10: 99,782,602-99,853,741 (GRCh38/hg38) | Multidrug Resistance-Associated Protein 2 (MRP2) | 1545 |
| ABCC3: 604323 | chr17: 50,634,777-50,692,253 (GRCh38/hg38) | Multidrug Resistance-Associated Protein 3 (MRP3) | 1527 |
| ABCC4: 605250 | chr13: 95,019,835-95,301,475 (GRCh38/hg38) | Multidrug Resistance-Associated Protein 4 (MRP4) | 1325 |
| ABCC5: 605251 | chr3: 183,919,934-184,018,010 (GRCh38/hg38) | Multidrug Resistance-Associated Protein 5 (MRP5) | 1437 |
| ABCC6: 603234 | chr16: 16,149,565-16,223,617 (GRCh38/hg38) | Multidrug Resistance-Associated Protein 6 (MRP6) | 1503 |
| ABCC10: 612509 | chr6: 43,427,366-43,451,994 (GRCh38/hg38) | Multidrug Resistance-Associated Protein 7 (MRP7) | 1464 |
| ABCC11: 607040 | chr16: 48,164,842-48,249,973 (GRCh38/hg38) | Multidrug Resistance-Associated Protein 8 (MRP8) | 1382 |
| ABCC12: 607041 | chr16: 48,080,882-48,156,018 (GRCh38/hg38) | Multidrug Resistance-Associated Protein 9 (MRP9) | 1359 |
| ABCG2: 603756 | chr4: 88,090,150-88,231,628 (GRCh38/hg38) | Breast Cancer Resistance Protein (BCRP) | 655 |
| Transport Protein | Substrates | Inhibitors |
|---|---|---|
| P-glycoprotein (P-gp) | Amisulpride Aripiprazole Azenapine Chlorpromazine Chlorprothixene Clozapine Fluphenazine Flupentixol Olanzapine Paliperidone Periciazine Quetiapine Risperidone Sertindole Sulpiride Trifluoperazine Ziprasidone Zuclopenthixol | Amiodarone Atorvastatin Azithromycin Bromocriptine Captopril Carvedilol Chlorpromazine Clarithromycin Cyclosporine Diltiazem Dipyridamole Erythromycin Fluoxetine Hydrocortisone Itraconazole Ketoconazole Loratadine Nifedipine Propafenone Propranolol Quinidine Ranolazine Reserpine Ritonavir Sertraline Simvastatin Spironolactone Ticagrelor Verapamil Vinblastine Vincristine Warfarin |
| Breast Cancer Resistance Protein (BCRP) | Aripiprazole Chlorpromazine Clozapine Haloperidol Olanzapine Paliperidone Quetiapine Risperidone Sulpiride | Boceprevir Cyclosporine Dipyridamole Fluconazole Gefitinib Itraconazole Imatinib Ketoconazole Nicardipine Nifedipine Omeprazole Pantoprazole Reserpine Ritonavir Tamoxifen Telaprevir |
| Multidrug Resistance-Associated Protein 1 (MRP1) | Clozapine | |
| Clotrimazole Cyclosporine Disulfiram Flavonoids Verapamil |
| P-gp | BCRP | MRP1 |
|---|---|---|
| Amisulpride Aripiprazole Azenapine Chlorpromazine Chlorprothixene Clozapine Fluphenazine Flupentixol Olanzapine Paliperidone Periciazine Quetiapine Risperidone Sertindole Sulpiride Trifluoperazine Ziprasidone Zuclopenthixol | Aripiprazole Chlorpromazine Clozapine Haloperidol Olanzapine Paliperidone Quetiapine Risperidone Sulpiride | Clozapine |
| ABCB1 | ABCG2 | ABCC1 |
|---|---|---|
| rs1045642 rs1128503 rs2032582 rs2235048 | rs2231142 rs2231137 rs72552713 rs3116448 rs758900849 rs192169063 rs34264773 rs199753603 rs12721643 rs41282401 rs1061017 rs1061018 rs3201997 rs750568956 rs753759474 rs752626614 rs372192400 rs200894058 rs199854112 rs769734146 rs34783571 rs45605536 rs58818712 rs750568956 rs2622604 | rs212090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasyrova, R.F.; Shnayder, N.A.; Osipova, S.M.; Khasanova, A.K.; Efremov, I.S.; Al-Zamil, M.; Petrova, M.M.; Narodova, E.A.; Garganeeva, N.P.; Shipulin, G.A. Genetic Predictors of Antipsychotic Efflux Impairment via Blood-Brain Barrier: Role of Transport Proteins. Genes 2023, 14, 1085. https://doi.org/10.3390/genes14051085
Nasyrova RF, Shnayder NA, Osipova SM, Khasanova AK, Efremov IS, Al-Zamil M, Petrova MM, Narodova EA, Garganeeva NP, Shipulin GA. Genetic Predictors of Antipsychotic Efflux Impairment via Blood-Brain Barrier: Role of Transport Proteins. Genes. 2023; 14(5):1085. https://doi.org/10.3390/genes14051085
Chicago/Turabian StyleNasyrova, Regina F., Natalia A. Shnayder, Sofia M. Osipova, Aiperi K. Khasanova, Ilya S. Efremov, Mustafa Al-Zamil, Marina M. Petrova, Ekaterina A. Narodova, Natalia P. Garganeeva, and German A. Shipulin. 2023. "Genetic Predictors of Antipsychotic Efflux Impairment via Blood-Brain Barrier: Role of Transport Proteins" Genes 14, no. 5: 1085. https://doi.org/10.3390/genes14051085
APA StyleNasyrova, R. F., Shnayder, N. A., Osipova, S. M., Khasanova, A. K., Efremov, I. S., Al-Zamil, M., Petrova, M. M., Narodova, E. A., Garganeeva, N. P., & Shipulin, G. A. (2023). Genetic Predictors of Antipsychotic Efflux Impairment via Blood-Brain Barrier: Role of Transport Proteins. Genes, 14(5), 1085. https://doi.org/10.3390/genes14051085








