Abstract
Using a whole-genome assembly of Bos taurus, I applied my bioinformatics strategy to locate candidate imprinting control regions (ICRs) genome-wide. In mammals, genomic imprinting plays essential roles in embryogenesis. In my strategy, peaks in plots mark the locations of known, inferred, and candidate ICRs. Genes in the vicinity of candidate ICRs correspond to potential imprinted genes. By displaying my datasets on the UCSC genome browser, one could view peak positions with respect to genomic landmarks. I give two examples of candidate ICRs in loci that influence spermatogenesis in bulls: CNNM1 and CNR1. I also give examples of candidate ICRs in loci that influence muscle development: SIX1 and BCL6. By examining the ENCODE data reported for mice, I deduced regulatory clues about cattle. I focused on DNase I hypersensitive sites (DHSs). Such sites reveal accessibility of chromatin to regulators of gene expression. For inspection, I chose DHSs in chromatin from mouse embryonic stem cells (ESCs) ES-E14, mesoderm, brain, heart, and skeletal muscle. The ENCODE data revealed that the SIX1 promoter was accessible to the transcription initiation apparatus in mouse ESCs, mesoderm, and skeletal muscles. The data also revealed accessibility of BCL6 locus to regulatory proteins in mouse ESCs and examined tissues.
1. Introduction
For many centuries, Bos taurus populations were selected for economically important traits [1,2,3,4]. Examples include milk production [5,6,7,8] and beef quality: i.e., tenderness, juiciness, flavor, color, and fat content [9,10]. Furthermore, emerging novel approaches could expedite trait selection: examples include cloning, somatic cell nuclear transfer (SCNT), and assisted reproduction technologies (ART). However, such practices could produce developmental anomalies known as LOS: Large Offspring Syndrome [11,12,13,14,15,16,17,18]. Mechanistically, the abnormalities are often caused by defects in genomic imprinting—a process that impacts embryonic development in mammals [19,20,21,22,23]. Briefly, genomic imprinting directs repression of a subset of genes in either the maternal or the paternal autosomal chromosomes [20,24,25,26,27,28]. Insight into genomic imprinting emerged from studies on mice [20,26,29,30,31]. Subsequently, it was extended to studies of humans [32,33] and farm animals [3]. As in other mammals, the cattle genome includes imprinted domains, genes, and transcripts [3].
Genomic imprinting involves the orchestrated action of several enzymes and proteins [28]. The imprinting marks are epigenetic and consist of methylated CpGs in ICRs [34]. Also known as KAP1, TRIM28 is associated with several proteins required for imprinted gene repression [28,35,36]. The process is relatively complex. A subset of DNA methyltransferases (DNMTs) write the epigenetic marks in ICRs. ZFP57 binds clusters of methylated hexanucleotides (TGCmCGC) to protect the modified CpGs from demethylation [37,38,39,40]. SETDB1 trimethylates lysine 9 in histone H3 to produce repressive H3K9me3 marks in chromatin [29]. HP1 binds H3K9me3 marks to initiate chromatin condensation [28,41].
Since imprinted genes are important to developmental processes [19,20,21,22,23], it is crucial to innovate novel strategies for their identification. Efforts in that direction include trained statistical models [42]. Another approach applied algorithms to predict imprinting status of genes in the human genome [43]. Another method employed an array of SNPs (Single Nucleotide Polymorphisms) and usual molecular methods to identify epigenetic features that correlated with imprinting status in mice [44]. A different strategy applied weighting methods, machine learning, and data mining to characterize Bos taurus imprinted genes from genomic to amino acid attributes [45]. I have developed a genome-wide strategy that locates known, inferred, and candidate ICRs in mammals. My strategy assumes that genes in the vicinity of candidate ICRs are potential imprinted genes.
To date, I have applied my genome-wide studies to three different mammals: mouse [46], human [47], and Bos taurus [48]. In this report, I focus on demonstrating that my strategy could identify candidate ICRs for potential imprinted genes important to spermatogenesis in bulls, and myogenesis in Bos taurus. I also demonstrate that with Mouse ENCODE data [49], it is possible to obtain regulatory information about cattle.
2. Materials and Methods
A whole-genome assembly of Bos taurus [50] offered the opportunity to perform genome-wide analyses to discover ICRs in domestic cattle [48]. Briefly, from the UCSC genome browser, I downloaded the build bosTau8 assembly. For genome-wide studies, I created two text files. One file contained TGCCGC and its complement. The double-stranded DNA defines sequences that, after CpG methylation, bind the ICRs that depend on ZFP57 to regulate allele-specific gene expression [40]. The other file included the nucleotide sequences of unmethylated ZFBS-morph overlaps and their complementary sequences [51]. For details, see the results section. To produce one dataset, a Perl script determined the genomic positions of ZFBS-morph overlaps [52]. I used the same script to obtain the genomic positions of TGCCGC. To locate known and candidate ICRs, another Perl script scanned a specified chromosomal DNA to count the number of ZFBS-morph overlaps in a sliding window consisting of 850 bases. The script created a density plot by disregarding isolated overlap occurrences. This strategy eliminated background noise. After the steps above, I wrote UNIX subroutines to format the output files for display on the UCSC genome browser [53,54,55,56]. This resource is located at the University of California Santa Cruz (UCSC). It can be accessed at (https://genome.ucsc.edu/cgi-bin/hgGateway (accessed on 29 April 2023)).
3. Results
3.1. Defining the Positions of Known, Inferred, and Predicted ICRs in Bos taurus
To date, my strategy correctly pinpointed the genomic positions of a substantial fraction of fully characterized ICRs/gDMRs in three mammalian species [46,47,48]. The strategy is based on studies demonstrating that in emouse, nearly 90% of ICRs/gDMRs include composite DNA elements consisting of the ZFP57 binding site (ZFBS) overlapping a subset of the MLL1 morphemes [52]. I refer to these elements as ZFBS-morph overlaps. In these overlaps, the morphemes correspond to sites recognized by MLL1/KMT2A and MLL2/KMT2B [57,58]. The key feature of these sites is that they include two or more CpGs. MLL1 (Mixed-Lineage Leukemia 1) is the founding members of a protein family. Their structure contains a domain that contains methylates lysine 4 in histone H3 producing H3K4me3 marks in chromatin [59]. This H3 modification creates chromatin states that support gene expression [59,60]. Although it is not known whether the MLL1/MLL2 binding sites may contribute to allele-specific gene expression, their CpG-richness could be good targets for methylations of ICRs by DNA methyltransferases.
In ICRs, the hexameric ZFP57 binding sites are clustered [40]. It is well known that methylated CpGs tend to mutate to T as a consequence of deamination [61]. This disposition to mutations leads to CpG deficiency. However, although the nonmethylated hexamer (TGCCGC) includes a CpG, it is short and thus occurs frequently along chromosomal DNA (Figure 1). In contrast, ZFBS-morph overlaps include two or more CpGs. Therefore, their frequency is far less than that observed for TGCCGC along chromosomal DNA (Figure 1). Furthermore, in ICRs, the ZFBS-morph overlaps occur as clusters [52]. Therefore, by counting the number of these overlaps in a sliding window, it became possible to create density plots in which peaks identified cluster positions (Figure 1).
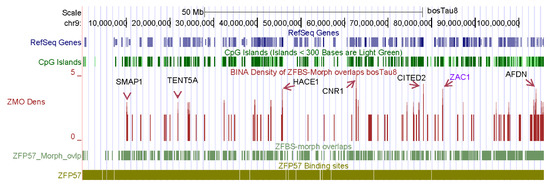
Figure 1.
A snapshot of the density plot created for studies of chromosome 9. In this plot, a robust peak located the known ICR of ZAC1 in nearly 106 Mb long chromosomal DNA. Additional robust peaks point to candidate ICRs near potential imprinted genes including SMAP1, TENT5A, HACE1, CNR1, CITED2, and AFDN.
For ICR detection, I tailored my datasets for uploading on the UCSC genome browser to view peak positions in the context of genomic landmarks, including SNPs, CpG islands, genes, and transcripts. SNPs are important to genetic studies. Examples include accurate prediction of cattle breeds [62], association of body stature with frame size at puberty in cattle [63], and comprehensive characterization of loss of imprinting in LOS induced by assisted reproduction technologies [16]. On the browser, uploading the datasets reveals three custom tracks (Figure 1). With respect to selected genomic landmarks, a track displays the genomic positions of TGCCGC: the hexamer that after methylation binds ZFP57. A second track marks the positions of ZFBS-morph overlaps. A third track shows peak positions in the density plots (Figure 1). Peak positions are markers of known, inferred, and predicted ICRs along chromosomal DNA sequences [46,47,48].
The browser displays genomic data with respect to the underlying DNA sequence [64]. Therefore, the format of the datasets enables viewing results of genome wide analyses in the context of the sequences selected as reference. RefSeq Genes are known cattle genes; Non-Cow RefSeq Genes were deduced from comparative sequence analyses. Additionally, the browser offers built-in tools to display genomic information in dense, pack, or full formats [55,56]. For example, examine the peak positions in the plot obtained for the entire Bos taurus chromosome 9 (Figure 1). In this figure, peaks are shown in full format; dense format gives the location of RefSeq Genes, the CpG islands, ZFBS-morph overlaps, and the TGCCGC hexanucleotide. To identify genes in the vicinity of peaks, one could obtain closeup views. Subsequent sections give a few examples. Note that peak intensities vary and depend on the number of ZFBS-morph overlaps that they encompass. Evaluations have revealed that robust peaks encompass three or more ZFBS-morph overlaps. Peaks that cover two could be true or false positives. I annotated the genes manually. Along chromosome nine, manually annotated genes include ZAC1, SMAP1, TENT5A, HACE1, CNR1, CITED2, and AFDN (Figure 1). ZAC1 is a known imprinted transcript in the PLAGL1 locus in mice [65]. ZAC1 is also an imprinted transcript in cattle [27]. The expression and the methylation pattern of PLAGL1 are conserved in human and cattle [66]. In LOS induced by ART, ZAC1 was biallelically expressed in fetuses [16]. In the PLAGL1 locus in Bos taurus, a peak in plots is within the experimentally defined imprinted DMR [48]. Thus, my strategy correctly located an imprinted DMR in nearly 106 Mb long cattle chromosomal DNA (Figure 1).
Along Bos taurus chromosome 9, additional robust peaks correspond to candidate ICRs for potential imprinted genes (Figure 1). Among the annotated genes, SMAP1 encodes a membrane-associated protein implicated in exerting a stimulatory effect on stroma-supported erythropoiesis [67]. Steady state erythropoiesis produces new erythrocytes [68]. TENT5A is a cytoplasmic poly(A) polymerase that regulates bone mineralization [69]. TENT5A knockout mouse displayed bone fragility and defects in skeletal mineralization [69]. TENT5A also impacts the formation of muscle fibers in adolescent idiopathic scoliosis by preserving production of myogenin [70]. HACE1 is an ankyrin containing E3 ubiquitin ligase [71]. Its deficiency decreased the number of synapses and caused both structural and behavioral neuropathologic features [72]. CITED2 is required for heart morphogenesis and the establishment of left-right axis during mouse development [73]. CITED2-null mice die during gestation with fully penetrant heart defects and partially penetrant laterality defects [73]. CITED2 is a transcription co-factor. It also contributes to sex determination and early gonad development [74]. The adherens junction formation factor (AFDN) is an F-actin scaffold protein with essential roles in the organization of cell-cell junctions of polarized epithelia [75]. In mouse, transcription of AFDN produces two major isoforms [75,76]. Their products were detected in distinguishable regions in the mouse central nervous system [75].
3.2. Examples of Candidate ICRs for Potential Imprinted Genes Important to Bull Fertility
In cattle, fertility is influenced by semen quality, testis size, and efficiency of sperm production [77]. As in other mammals, spermatogenesis in bulls is a highly regulated process that drives multiplication and differentiation of germ cells [78]. Spermatogenesis involves the differentiation of round spermatids into fully mature spermatozoa, in which the histones are replaced with cysteine-rich protamines to fully condense the DNA [79]. With the exception of humans, bulls have a lower efficiency of spermatogenesis than most other species [78]. In Bos taurus genomic DNA, my approach discovered a candidate ICR for the imprinted repression of CNNM1 (Figure 2). In mouse testes, CNNM1 was produced from neonatal to adult stages, and was associated with cell cycle and differentiation of spermatogenic cells [80].
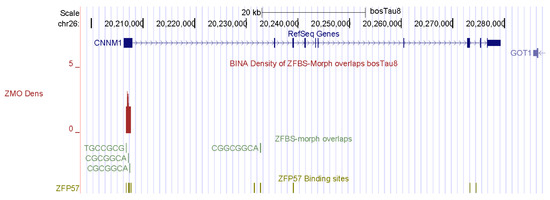
Figure 2.
A candidate ICR at the 5′ end of CNNM1. In mice, Cnnm1 triggered differentiation of spermatogenic cells.
My approach also discovered a candidate ICR for the imprinted repression of CNR1: tetrahydrocannabinol receptor 1 (Figure 3). Endocannabinoids are naturally occurring lipids, and the principal component of marijuana [81]. They regulate a large array of physiological functions and behaviors. Since CNR1 is expressed in elongating spermatids and spermatozoa, it affects male germ cell progression and sperm maturation mediated by endocannabinoids [82]. Although prevailing studies have emphasized the impact of endocannabinoids on the brain, this group of compounds also control reproductive events in males [81,82,83]. In CNR1 knock-out mice, low levels of 17beta-estradiol affected chromatin remodeling in spermatid by interfering with high levels of DNA compaction by protamines [82].
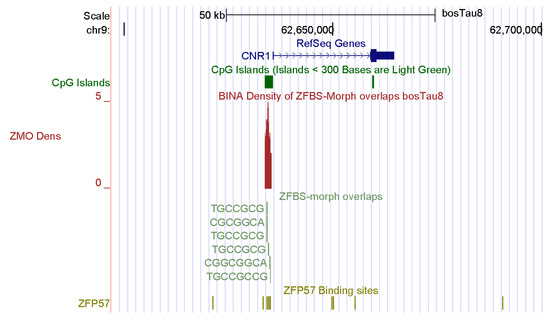
Figure 3.
A candidate ICR at the 5′ end of CNR1. In mice, CNR1 was expressed in elongating spermatids and spermatozoa, impacting male germ cell progression and sperm maturation mediated by the endocannabinoids.
3.3. Examples of Candidates ICRs for Genes That Impact Muscle Formation in Bos taurus
Skeletal muscle mass is an important economic trait [4]. In fact, several groups of farm animals are bred and selected to improve meat production [84]. The genesis of skeletal muscle involves complex mechanisms that control myogenesis at all stages of development [85]. Key regulators of myogenesis include SIX1, MYOD, and myogenin [4,86]. My predictive strategy discovered candidate ICRs for potential imprinted genes that impacted formation of muscles in Bos taurus. Examples include SIX1 and BCL6 (Figure 4 and Figure 5). SIX1 is the founding member of a transcription factor family whose structure includes a homeodomain for binding DNA [87]. SIX homeoproteins are produced in several tissues. Furthermore, this group of regulatory proteins impacts diverse differentiation processes [88]. Mice lacking SIX1 died at birth due to malformations of several ribs. The mice also showed extensive muscle hypoplasia affecting most of the body muscles and certain hypaxial muscles [88]. Furthermore, SIX1 controls the expression of MYOD in adult muscle progenitor cells [86]. In cattle, SIX1 was highly expressed in the longissimus thoracis [87]. Biochemical studies revealed that in bovine DNA, the SIX1 promotor interacted with MYOD, PAX7, and CREB [87]. The other potential imprinted gene in cattle (BCL6) encodes another DNA-binding protein that controls gene expression [89,90]. In mice, at gestational day 17, BCL6 was primarily expressed in skeletal muscle, the olfactory epithelium, and the epithelium lining the upper airways and esophagus [90].
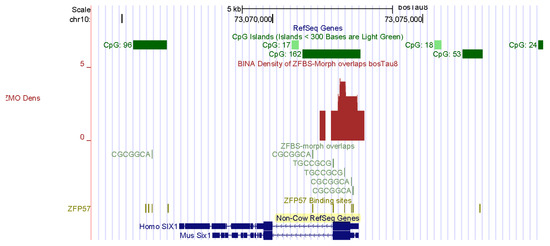
Figure 4.
A candidate ICR at the 5′ end of SIX1. In adult muscle progenitor cells, SIX1 controls the expression of MYOD.
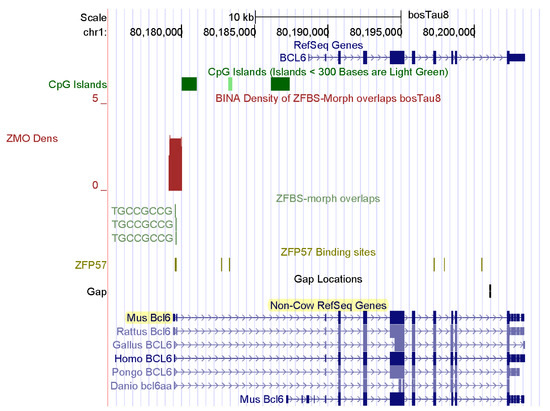
Figure 5.
A candidate ICR at the 5′ end of BCL6 with respect to Non-Cow RefSeq Genes. In mice, at gestational day 17, BCL6 was primarily expressed in skeletal muscle.
3.4. Cell-Type and Tissue-Specific Expression Patterns of SIX1 and BCL6 in Mouse
To further demonstrate the power of my strategy, I also examined my datasets reported for mouse [51]. Two density plots show that as in Bos taurus, the mouse genome includes candidate ICRs for parent-of-origin-specific expressions of SIX1 and BCL6 (Figure 6 and Figure 7). As observed for Bos taurus (Figure 4), the candidate ICR for allele-specific SIX1 repression is in a CpG island in mouse (Figure 6). In plots, a candidate ICR encompasses the longest BCL6 transcriptional isoform in mouse (Figure 7). Next, I explored the effectiveness of ENCODE data for studies of cattle. For functional analyses of human and mouse genomes, the genome browser gives the option of viewing tracks, displaying large-scale experimental data produced by ENCODE: the Encyclopedia of DNA Elements [49,91,92]. The goal of the ENCODE Consortium was to discover and define the functional elements in mouse and human genomes [49,91]. Although the consortium has not applied their technologies to studies of cattle, one could assume that data reported for mice [49] could serve as a model for studies of other mammals. Therefore, I thought it could be informative to examine SIX1 and BCL6 loci in the context of ENCODE data obtained for mouse [49].
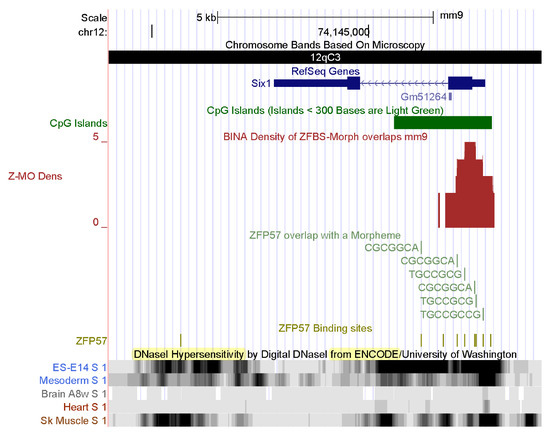
Figure 6.
A candidate ICR at the 5′ end of Six1 in mouse. Vertical bars reveal the positions of DHSs in chromatin prepared from mouse ESCs ES-E14, mesoderm, brain, heart, and skeletal muscle. Note cell-type and tissue-specific patterns of chromatin accessibility to regulatory proteins.
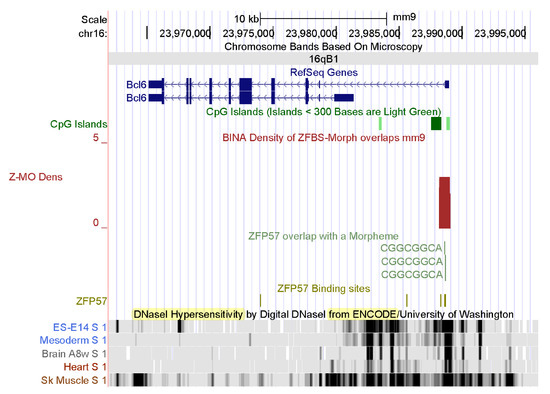
Figure 7.
A candidate at the 5′ end of Bcl6in mouse. Several tracks display the positions of DHSs in chromatin prepared from mouse ESCs ES-E14, mesoderm, and skeletal muscle. Vertical bars mark the positions of DHSs in chromatin prepared from mouse ESCs ES-E14, mesoderm, brain, heart, and skeletal muscle. Note cell-type and tissue-specific patterns of chromatin accessibility to regulatory proteins.
Among the extensive ENCODE data, I chose to display the DHSs in chromatin. Locating DHSs is a powerful tool to determine the accessibility of chromatin to regulators of gene expression [93,94,95]. DHSs mark the positions of regulatory DNA. They facilitate the discovery of all classes of cis-regulatory elements: i.e., promoters, enhancers, insulators, silencers, and locus control regions [96]. On the genome browser, I selected tracks displaying DHSs in chromatin prepared from mouse ESCs ES-E14, mesoderm, brain, heart, and skeletal muscle. The data revealed extensive DHSs across both the Six1 and Bcl6 loci (Figure 6 and Figure 7). In mouse ES-E14, one block of DHSs maps to the 5′ end of SIX1 and extends to Six1 intronic sequences (Figure 6). Thus, in ESCs, the Six1 promoter is accessible to the transcription initiation apparatus. DHSs in intronic sequences and downstream of the gene correspond to DNA segments that upregulate or downregulate Six1 expression in mouse ES-E14 (Figure 6). In the mesoderm, DHSs occur in several regions, indicating that the expression of Six1 is regulated during mouse development (Figure 6). Lack of DHSs in the brain and the heart suggests that the Six1 locus is not expressed in these tissues. Notably, DHSs punctate several regions in chromatin from mouse skeletal muscle (Figure 6). Thus, Six1 is robustly expressed in mouse muscle tissues. Furthermore, the positions of DHSs are cell-type and tissue specific (Figure 6). Next, I examined the positions of DHSs in the Bcl6 locus (Figure 7). These hallmarks of open chromatin structure are spread across the entire Bcl6 locus in mouse ES-E14, mesoderm, brain, heart, and skeletal muscle. Notably, expression of Bcl6 is very robust in skeletal muscle and controlled by numerous regulatory DNA segments (Figure 7).
4. Discussion
Genomic imprinting is an evolutionary novelty restricted to mammals [19,20,21,22,23,97,98]. In domesticated animals, genomic imprinting affects complex traits [99]. Since imprinted genes are expressed from either the maternal or the paternal allele [100,101], genomic imprinting reduces gene dosage from two to one. Since genomic imprinting impacts developmental processes, it is essential to identify the genes expressed in a parent-of-origin-specific manner [97]. Most imprinted genes were discovered based on various experimental criteria [22]. My strategy involved creating plots displaying the density of ZFBS-morph overlaps along chromosomal DNA [46,47,48]. Peaks in plots locate the genomic positions of ICRs that are ZFP57 dependent [46,47,48]. Across chromosome 9, peaks are almost fully resolved (Figure 1). This finding demonstrates that peak occurrences are rare events in genomic DNA. Furthermore, one could expect that peaks covering three or more ZFBS-morph overlaps occur less frequently than those that cover two (Figure 1). Overall, peaks in the density plots correctly located the ICRs in cattle imprinting domains, including H19—IGF2, KCNQ1, IGF2R, and PEG3. Peaks also located include: the essential ICR in the GNAS complex locus; and the ICRs in PLAGL1, MEST, NNAT, MEG8, SNRPN, HERC3-NAP1L5, and INPP5F loci [48]. Thus, it became increasingly plausible that in plots, additional peaks corresponded to candidate ICRs [48].
Previously, my strategy discovered candidate ICRs for several potential imprinted genes in Bos taurus [48]. Examples include HMGA2 and LCORL. These genes affect body size and stature. A non-synonymous mutation in HMGA2 decreased height in Shetland ponies and other small horses [102]. In rabbits, a deletion at the HMGA2 locus caused dwarfism and altered craniofacial development [103]. In wild canids, SNPs in LCORL were associated with stature [104]. Hmga2 knockout mice were impaired in muscle development and had diminished proliferation of myoblasts [105]. In contrast, overexpression of Hmga2 boosted myoblast growth [105]. In fact, extreme size diversification is a hallmark reflecting domestication. For example: the LCORL locus was among the genes displaying strong signatures of selection characteristics of morphological changes in domesticated pigs [106]. Additionally, HMGA2 and LCORL are among a few genes that have played major roles in the rapid evolution of animal size as a consequence of trait selection practiced by farmers and breeders [107].
For this report, I chose examples of candidate ICRs for potential imprinted genes important to farming industry: spermatogenesis in bulls and muscle development in cattle. CNNM1 and CNR1 impact spermatogenesis. A candidate ICR maps to the 5′ end of CNNM1 (Figure 2). In mice, CNNM1 was associated with cell cycles and differentiation of spermatogenic cells [80]. The candidate ICR for CNR1 is in a CpG island (CpG162) that encompasses the gene promotor (Figure 3). In Cnr1 knock-out mice, low levels of 17beta-estradiol interfered with chromatin remodeling in spermatids [82]. CNR1 binds endocannabinoids [81]. They regulate numerous physiological processes including male reproductive events [81,82,83]. CNR1 functions during germ cell progression and sperm maturation mediated by the endocannabinoids [82]. In Bos taurus, two candidate ICRs map to genes that affect the development of muscles, and could therefore impact beef quality: SIX1 and BCL6 (Figure 4 and Figure 5). SIX1 is a transcription factor essential for embryonic myogenesis [86]. In muscle progenitor cells, SIX1 regulates the expression of MyoD [86]. In Six1-deficient mice, myogenesis was altered [88]. In cattle, the SIX1 promotor interacted with several muscle-specific transcription factors [87]. These interactions could play key roles in SIX1-mediated skeletal muscle growth in cattle [87].
To further demonstrate the power of my approach, I showed that I could examine the Six1 and Bcl6 loci in the context of the ENCODE data and their imprinting status in mice (Figure 6 and Figure 7). As in Bos taurus (Figure 4), the candidate ICR for Six1 maps to a CpG island in mouse genomic DNA (Figure 6). As in Bos taurus (Figure 5), the candidate ICR for Bcl6 is at 5′ end of the longest Bcl6 transcript (Figure 7). Furthermore, ENCODE provides experimental data that could be relevant to biochemical and developmental studies of cattle. Specifically, the ENCODE Consortium has mapped DHSs, transcription factor binding regions, chromatin modification patterns, and replication domains throughout the mouse genome in diverse cell and tissue types [49,108]. DHSs are signatures of chromatin accessibility to regulators of gene expression [109,110]. For example, bovine estrogen receptor bind to pre-existing nuclease hypersensitive sites in chromatin [111]. Furthermore, distal DHSs correspond to regulatory elements that upregulate or repress gene expression [109]. To conclude: my strategy locates known and candidate ICRs dispersed along Bos taurus chromosomal DNA sequences. The ENCODE data could reveal regulatory information about cattle.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be downloaded from the Purdue University Research Repository [112,113].
Acknowledgments
I thank Maya Bina Afilalo for editing my manuscript and Arnold Stein for helpful discussions.
Conflicts of Interest
The author declares no conflict of interest.
References
- Lawson, H.A.; Cheverud, J.M.; Wolf, J.B. Genomic imprinting and parent-of-origin effects on complex traits. Nat. Rev. Genet. 2013, 14, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Magee, D.A.; Spillane, C.; Berkowicz, E.W.; Sikora, K.M.; MacHugh, D.E. Imprinted loci in domestic livestock species as epigenomic targets for artificial selection of complex traits. Anim. Genet. 2014, 45 (Suppl. 1), 25–39. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.C. Genomic imprinting in farm animals. Annu. Rev. Anim. Biosci. 2014, 2, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key Genes Regulating Skeletal Muscle Development and Growth in Farm Animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef]
- Plante, Y.; Gibson, J.P.; Nadesalingam, J.; Mehrabani-Yeganeh, H.; Lefebvre, S.; Vandervoort, G.; Jansen, G.B. Detection of quantitative trait loci affecting milk production traits on 10 chromosomes in Holstein cattle. J. Dairy Sci. 2001, 84, 1516–1524. [Google Scholar] [CrossRef]
- Ashwell, M.S.; Heyen, D.W.; Sonstegard, T.S.; Van Tassell, C.P.; Da, Y.; VanRaden, P.M.; Ron, M.; Weller, J.I.; Lewin, H.A. Detection of quantitative trait loci affecting milk production, health, and reproductive traits in Holstein cattle. J. Dairy Sci. 2004, 87, 468–475. [Google Scholar] [CrossRef]
- Mosig, M.O.; Lipkin, E.; Khutoreskaya, G.; Tchourzyna, E.; Soller, M.; Friedmann, A. A whole genome scan for quantitative trait loci affecting milk protein percentage in Israeli-Holstein cattle, by means of selective milk DNA pooling in a daughter design, using an adjusted false discovery rate criterion. Genetics 2001, 157, 1683–1698. [Google Scholar] [CrossRef]
- Sanchez, M.P.; Govignon-Gion, A.; Ferrand, M.; Gele, M.; Pourchet, D.; Amigues, Y.; Fritz, S.; Boussaha, M.; Capitan, A.; Rocha, D.; et al. Whole-genome scan to detect quantitative trait loci associated with milk protein composition in 3 French dairy cattle breeds. J. Dairy Sci. 2016, 99, 8203–8215. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Nguyen, O.C.; Malau-Aduli, A.E.O. Main regulatory factors of marbling level in beef cattle. Vet. Anim. Sci. 2021, 14, 100219. [Google Scholar] [CrossRef]
- Seideman, S.C. Muscle Fiber Studies Comparing Bos Indicus and Bos Taurus. The U.S. Department of Agriculture, Agricultural Research Service: Lincoln, NE, USA, 1985. [Google Scholar]
- Liu, J.H.; Yin, S.; Xiong, B.; Hou, Y.; Chen, D.Y.; Sun, Q.Y. Aberrant DNA methylation imprints in aborted bovine clones. Mol. Reprod. Dev. 2008, 75, 598–607. [Google Scholar] [CrossRef]
- Smith, L.C.; Suzuki, J., Jr.; Goff, A.K.; Filion, F.; Therrien, J.; Murphy, B.D.; Kohan-Ghadr, H.R.; Lefebvre, R.; Brisville, A.C.; Buczinski, S.; et al. Developmental and epigenetic anomalies in cloned cattle. Reprod. Domest. Anim. Zuchthyg. 2012, 47 (Suppl. 4), 107–114. [Google Scholar] [CrossRef] [PubMed]
- Couldrey, C.; Lee, R.S. DNA methylation patterns in tissues from mid-gestation bovine foetuses produced by somatic cell nuclear transfer show subtle abnormalities in nuclear reprogramming. BMC Dev. Biol. 2010, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Nagai, M.; Hirayama, M.; Hirai, T.; Matsuda, K.; Hayashi, M.; Tanaka, T.; Ozawa, T.; Horike, S. Aberrant CpG methylation of the imprinting control region KvDMR1 detected in assisted reproductive technology-produced calves and pathogenesis of large offspring syndrome. Anim. Reprod. Sci. 2010, 122, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Urrego, R.; Rodriguez-Osorio, N.; Niemann, H. Epigenetic disorders and altered gene expression after use of Assisted Reproductive Technologies in domestic cattle. Epigenetics 2014, 9, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hagen, D.E.; Elsik, C.G.; Ji, T.; Morris, C.J.; Moon, L.E.; Rivera, R.M. Characterization of global loss of imprinting in fetal overgrowth syndrome induced by assisted reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 4618–4623. [Google Scholar] [CrossRef]
- O’Doherty, A.M.; McGettigan, P.; Irwin, R.E.; Magee, D.A.; Gagne, D.; Fournier, E.; Al-Naib, A.; Sirard, M.A.; Walsh, C.P.; Robert, C.; et al. Intragenic sequences in the trophectoderm harbour the greatest proportion of methylation errors in day 17 bovine conceptuses generated using assisted reproductive technologies. BMC Genom. 2018, 19, 438. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Donnelly, C.G.; Rivera, R.M. Overgrowth Syndrome. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, M.S.; Tilghman, S.M. Genomic imprinting in mammals. Annu. Rev. Genet. 1997, 31, 493–525. [Google Scholar] [CrossRef]
- Ideraabdullah, F.Y.; Vigneau, S.; Bartolomei, M.S. Genomic imprinting mechanisms in mammals. Mutat. Res. 2008, 647, 77–85. [Google Scholar] [CrossRef]
- Proudhon, C.; Bourc’his, D. Evolution of genomic imprinting in mammals: What a zoo! Med. Sci. 2010, 26, 497–503. [Google Scholar]
- Tucci, V.; Isles, A.R.; Kelsey, G.; Ferguson-Smith, A.C.; Erice Imprinting, G. Genomic Imprinting and Physiological Processes in Mammals. Cell 2019, 176, 952–965. [Google Scholar] [CrossRef]
- Barlow, D.P.; Bartolomei, M.S. Genomic imprinting in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a018382. [Google Scholar] [CrossRef] [PubMed]
- Delaval, K.; Feil, R. Epigenetic regulation of mammalian genomic imprinting. Curr. Opin. Genet. Dev. 2004, 14, 188–195. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, S.T.; Ferguson-Smith, A.C. Genomic imprinting. Curr. Biol. 2004, 14, R646–R649. [Google Scholar] [CrossRef]
- Ferguson-Smith, A.C. Genomic imprinting: The emergence of an epigenetic paradigm. Nat. Rev. Genet. 2011, 12, 565–575. [Google Scholar] [CrossRef]
- O’Doherty, A.M.; O’Shea, L.C.; Fair, T. Bovine DNA methylation imprints are established in an oocyte size-specific manner, which are coordinated with the expression of the DNMT3 family proteins. Biol. Reprod. 2012, 86, 67. [Google Scholar] [CrossRef] [PubMed]
- Strogantsev, R.; Ferguson-Smith, A.C. Proteins involved in establishment and maintenance of imprinted methylation marks. Brief Funct Genom. 2012, 11, 227–239. [Google Scholar] [CrossRef]
- Arnaud, P. Genomic imprinting in germ cells: Imprints are under control. Reproduction 2010, 140, 411–423. [Google Scholar] [CrossRef]
- Ferguson-Smith, A.C.; Bourc’his, D. The discovery and importance of genomic imprinting. Elife 2018, 7, e42368. [Google Scholar] [CrossRef]
- Fedoriw, A.M.; Engel, N.I.; Bartolomei, M.S. Genomic imprinting: Antagonistic mechanisms in the germ line and early embryo. Cold Spring Harb. Symp. Quant. Biol. 2004, 69, 39–45. [Google Scholar] [CrossRef]
- Ishida, M.; Moore, G.E. The role of imprinted genes in humans. Mol. Asp. Med. 2013, 34, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G. Genomic imprinting disorders in humans: A mini-review. J. Assist. Reprod. Genet. 2009, 26, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Beard, C.; Jaenisch, R. Role for DNA methylation in genomic imprinting. Nature 1993, 366, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Wang, X.; Shibata, M.; Clark, A.G.; Garcia-Garcia, M.J. TRIM28 Controls Genomic Imprinting through Distinct Mechanisms during and after Early Genome-wide Reprogramming. Cell Rep. 2015, 13, 1194–1205. [Google Scholar] [CrossRef]
- Lechner, M.S.; Begg, G.E.; Speicher, D.W.; Rauscher, F.J., 3rd. Molecular determinants for targeting heterochromatin protein 1-mediated gene silencing: Direct chromoshadow domain-KAP-1 corepressor interaction is essential. Mol. Cell. Biol. 2000, 20, 6449–6465. [Google Scholar] [CrossRef]
- Riso, V.; Cammisa, M.; Kukreja, H.; Anvar, Z.; Verde, G.; Sparago, A.; Acurzio, B.; Lad, S.; Lonardo, E.; Sankar, A.; et al. ZFP57 maintains the parent-of-origin-specific expression of the imprinted genes and differentially affects non-imprinted targets in mouse embryonic stem cells. Nucleic Acids Res. 2016, 44, 8165–8178. [Google Scholar] [CrossRef]
- Strogantsev, R.; Krueger, F.; Yamazawa, K.; Shi, H.; Gould, P.; Goldman-Roberts, M.; McEwen, K.; Sun, B.; Pedersen, R.; Ferguson-Smith, A.C. Allele-specific binding of ZFP57 in the epigenetic regulation of imprinted and non-imprinted monoallelic expression. Genome Biol. 2015, 16, 112. [Google Scholar] [CrossRef]
- Quenneville, S.; Turelli, P.; Bojkowska, K.; Raclot, C.; Offner, S.; Kapopoulou, A.; Trono, D. The KRAB-ZFP/KAP1 system contributes to the early embryonic establishment of site-specific DNA methylation patterns maintained during development. Cell Rep. 2012, 2, 766–773. [Google Scholar] [CrossRef]
- Quenneville, S.; Verde, G.; Corsinotti, A.; Kapopoulou, A.; Jakobsson, J.; Offner, S.; Baglivo, I.; Pedone, P.V.; Grimaldi, G.; Riccio, A.; et al. In embryonic stem cells, ZFP57/KAP1 recognize a methylated hexanucleotide to affect chromatin and DNA methylation of imprinting control regions. Mol. Cell 2011, 44, 361–372. [Google Scholar] [CrossRef]
- Schultz, D.C.; Ayyanathan, K.; Negorev, D.; Maul, G.G.; Rauscher, F.J., 3rd. SETDB1: A novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 2002, 16, 919–932. [Google Scholar] [CrossRef]
- Luedi, P.P.; Hartemink, A.J.; Jirtle, R.L. Genome-wide prediction of imprinted murine genes. Genome Res 2005, 15, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Luedi, P.P.; Dietrich, F.S.; Weidman, J.R.; Bosko, J.M.; Jirtle, R.L.; Hartemink, A.J. Computational and experimental identification of novel human imprinted genes. Genome Res. 2007, 17, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Brideau, C.M.; Eilertson, K.E.; Hagarman, J.A.; Bustamante, C.D.; Soloway, P.D. Successful computational prediction of novel imprinted genes from epigenomic features. Mol. Cell. Biol. 2010, 30, 3357–3370. [Google Scholar] [CrossRef] [PubMed]
- Karami, K.; Zerehdaran, S.; Javadmanesh, A.; Shariati, M.M.; Fallahi, H. Characterization of bovine (Bos taurus) imprinted genes from genomic to amino acid attributes by data mining approaches. PLoS ONE 2019, 14, e0217813. [Google Scholar] [CrossRef] [PubMed]
- Bina, M.; Wyss, P. Simultaneous discovery of candidate imprinted genes and Imprinting Control Regions in the mouse genome. bioRxiv 2019. [Google Scholar] [CrossRef]
- Bina, M. Discovering candidate imprinted genes and imprinting control regions in the human genome. BMC Genom. 2020, 21, 378. [Google Scholar] [CrossRef]
- Wyss, P.; Song, C.; Bina, M. Along the Bos taurus genome, uncover candidate imprinting control regions. BMC Genom. 2022, 23, 478. [Google Scholar] [CrossRef]
- Stamatoyannopoulos, J.A.; Snyder, M.; Hardison, R.; Ren, B.; Gingeras, T.; Gilbert, D.M.; Groudine, M.; Bender, M.; Kaul, R.; Canfield, T.; et al. An encyclopedia of mouse DNA elements (Mouse ENCODE). Genome Biol. 2012, 13, 418. [Google Scholar]
- Zimin, A.V.; Delcher, A.L.; Florea, L.; Kelley, D.R.; Schatz, M.C.; Puiu, D.; Hanrahan, F.; Pertea, G.; Van Tassell, C.P.; Sonstegard, T.S.; et al. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009, 10, R42. [Google Scholar] [CrossRef]
- Bina, M.; Wyss, P.; Song, X.C. Datasets on the genomic positions of the MLL1 morphemes, the ZFP57 binding site, and ZFBS-Morph overlaps in the build mm9 of the mouse genome. Data Brief 2017, 13, 202–207. [Google Scholar] [CrossRef]
- Bina, M. Imprinted control regions include composite DNA elements consisting of the ZFP57 binding site overlapping MLL1 morphemes. Genomics 2017, 109, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Barber, G.P.; Casper, J.; Clawson, H.; Diekhans, M.; Gonzalez, J.N.; Hinrichs, A.S.; Lee, B.T.; Nassar, L.R.; Powell, C.C.; et al. UCSC Genome Browser enters 20th year. Nucleic Acids Res. 2020, 48, D756–D761. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.T.; Barber, G.P.; Benet-Pages, A.; Casper, J.; Clawson, H.; Diekhans, M.; Fischer, C.; Gonzalez, J.N.; Hinrichs, A.S.; Lee, C.M.; et al. The UCSC Genome Browser database: 2022 update. Nucleic Acids Res. 2022, 50, D1115–D1122. [Google Scholar] [CrossRef] [PubMed]
- Zweig, A.S.; Karolchik, D.; Kuhn, R.M.; Haussler, D.; Kent, W.J. UCSC genome browser tutorial. Genomics 2008, 92, 75–84. [Google Scholar] [CrossRef]
- Bina, M. The genome browser at UCSC for locating genes, and much more! Mol. Biotechnol. 2008, 38, 269–275. [Google Scholar] [CrossRef]
- Bina, M.; Wyss, P.; Novorolsky, E.; Zulkelfi, N.; Xue, J.; Price, R.; Fay, M.; Gutmann, Z.; Fogler, B.; Wang, D. Discovery of MLL1 binding units, their localization to CpG Islands, and their potential function in mitotic chromatin. BMC Genom. 2013, 14, 927. [Google Scholar] [CrossRef]
- Bina, M.; Wyss, P. Impact of the MLL1 morphemes on codon utilization and preservation in CpG Islands. Biopolymers 2015, 103, 480–490. [Google Scholar] [CrossRef]
- Ruthenburg, A.J.; Allis, C.D.; Wysocka, J. Methylation of lysine 4 on histone H3: Intricacy of writing and reading a single epigenetic mark. Mol. Cell 2007, 25, 15–30. [Google Scholar] [CrossRef]
- Zhou, V.W.; Goren, A.; Bernstein, B.E. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 2011, 12, 7–18. [Google Scholar] [CrossRef]
- Bird, A.P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980, 8, 1499–1504. [Google Scholar] [CrossRef]
- Reverter, A.; Hudson, N.J.; McWilliam, S.; Alexandre, P.A.; Li, Y.; Barlow, R.; Welti, N.; Daetwyler, H.; Porto-Neto, L.R.; Dominik, S. A low-density SNP genotyping panel for the accurate prediction of cattle breeds. J. Anim. Sci. 2020, 98, skaa337. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, K.; Watanabe, T.; Weikard, R.; Albrecht, E.; Kuhn, C.; Kinoshita, A.; Sugimoto, Y.; Takasuga, A. The SNP c.1326T>G in the non-SMC condensin I complex, subunit G (NCAPG) gene encoding a p.Ile442Met variant is associated with an increase in body frame size at puberty in cattle. Anim. Genet. 2011, 42, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Nassar, L.R.; Barber, G.P.; Benet-Pages, A.; Casper, J.; Clawson, H.; Diekhans, M.; Fischer, C.; Gonzalez, J.N.; Hinrichs, A.S.; Lee, B.T.; et al. The UCSC Genome Browser database: 2023 update. Nucleic Acids Res. 2022, 51, D1188–D1195. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Arnaud, P.; Konfortova, G.; Dean, W.L.; Beechey, C.V.; Kelsey, G. The mouse Zac1 locus: Basis for imprinting and comparison with human ZAC. Gene 2002, 292, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.M.; Chen, Z.; Wells, K.D.; Rivera, R.M. Expression of KCNQ1OT1, CDKN1C, H19, and PLAGL1 and the methylation patterns at the KvDMR1 and H19/IGF2 imprinting control regions is conserved between human and bovine. J. Biomed. Sci. 2012, 19, 95. [Google Scholar] [CrossRef]
- Sato, Y.; Hong, H.N.; Yanai, N.; Obinata, M. Involvement of stromal membrane-associated protein (SMAP-1) in erythropoietic microenvironment. J. Biochem. 1998, 124, 209–216. [Google Scholar] [CrossRef]
- Ruan, B.; Paulson, R.F. Metabolic regulation of stress erythropoiesis, outstanding questions, and possible paradigms. Front. Physiol. 2022, 13, 1063294. [Google Scholar] [CrossRef]
- Gewartowska, O.; Aranaz-Novaliches, G.; Krawczyk, P.S.; Mroczek, S.; Kusio-Kobialka, M.; Tarkowski, B.; Spoutil, F.; Benada, O.; Kofronova, O.; Szwedziak, P.; et al. Cytoplasmic polyadenylation by TENT5A is required for proper bone formation. Cell Rep. 2021, 35, 109015. [Google Scholar] [CrossRef]
- Luo, M.; Yang, H.; Wu, D.; You, X.; Huang, S.; Song, Y. Tent5a modulates muscle fiber formation in adolescent idiopathic scoliosis via maintenance of myogenin expression. Cell Prolif. 2022, 55, e13183. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Evdokimova, V.; Melnyk, N.; Zhang, L.; Fernandez, C.V.; Grundy, P.E.; Leach, S.; Marra, M.A.; Brooks-Wilson, A.R.; Penninger, J.; et al. Differential expression of a novel ankyrin containing E3 ubiquitin-protein ligase, Hace1, in sporadic Wilms’ tumor versus normal kidney. Hum. Mol. Genet. 2004, 13, 2061–2074. [Google Scholar] [CrossRef]
- Nagy, V.; Hollstein, R.; Pai, T.P.; Herde, M.K.; Buphamalai, P.; Moeseneder, P.; Lenartowicz, E.; Kavirayani, A.; Korenke, G.C.; Kozieradzki, I.; et al. HACE1 deficiency leads to structural and functional neurodevelopmental defects. Neurol. Genet. 2019, 5, e330. [Google Scholar] [CrossRef] [PubMed]
- Lopes Floro, K.; Artap, S.T.; Preis, J.I.; Fatkin, D.; Chapman, G.; Furtado, M.B.; Harvey, R.P.; Hamada, H.; Sparrow, D.B.; Dunwoodie, S.L. Loss of Cited2 causes congenital heart disease by perturbing left-right patterning of the body axis. Hum. Mol. Genet. 2011, 20, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Buaas, F.W.; Val, P.; Swain, A. The transcription co-factor CITED2 functions during sex determination and early gonad development. Hum. Mol. Genet. 2009, 18, 2989–3001. [Google Scholar] [CrossRef]
- Ohama, D.; Matsuda, T.; Oinuma, I. Differential regional and subcellular localization patterns of afadin splice variants in the mouse central nervous system. Brain Res. 2018, 1692, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Sakakibara, S.; Miyata, M.; Itoh, Y.; Kurita, S.; Mandai, K.; Sasaki, T.; Takai, Y. Involvement of l-afadin, but not s-afadin, in the formation of puncta adherentia junctions of hippocampal synapses. Mol Cell Neurosci 2018, 92, 40–49. [Google Scholar] [CrossRef] [PubMed]
- McCool, C.J. Spermatogenesis in Bali cattle (Bos sondaicus) and hybrids with Bos indicus and Bos taurus. Res Vet Sci 1990, 48, 288–294. [Google Scholar] [CrossRef]
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Animal 2018, 12, s27–s35. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Brinkworth, M.; Iles, D. Paternal DNA packaging in spermatozoa: More than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 2010, 139, 287–301. [Google Scholar] [CrossRef]
- Chandran, U.; Indu, S.; Kumar, A.T.; Devi, A.N.; Khan, I.; Srivastava, D.; Kumar, P.G. Expression of Cnnm1 and Its Association with Stemness, Cell Cycle, and Differentiation in Spermatogenic Cells in Mouse Testis. Biol. Reprod. 2016, 95, 7. [Google Scholar] [CrossRef]
- Battista, N.; Meccariello, R.; Cobellis, G.; Fasano, S.; Di Tommaso, M.; Pirazzi, V.; Konje, J.C.; Pierantoni, R.; Maccarrone, M. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol. Cell. Endocrinol. 2012, 355, 1–14. [Google Scholar] [CrossRef]
- Cacciola, G.; Chioccarelli, T.; Ricci, G.; Meccariello, R.; Fasano, S.; Pierantoni, R.; Cobellis, G. The endocannabinoid system in vertebrate male reproduction: A comparative overview. Mol. Cell. Endocrinol. 2008, 286 (Suppl. 1), S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.F.; Schnabel, R.D.; Sutovsky, P. Identification of genomic variants causing sperm abnormalities and reduced male fertility. Anim. Reprod. Sci. 2018, 194, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, X.; Radunz, A.E.; Khatib, H. Maternal nutrition during pregnancy is associated with differential expression of imprinted genes and DNA methyltranfereases in muscle of beef cattle offspring. J. Anim. Sci. 2015, 93, 35–40. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chakroun, I.; Yang, D.; Horner, E.; Liang, J.; Aziz, A.; Chu, A.; De Repentigny, Y.; Dilworth, F.J.; Kothary, R.; et al. Six1 regulates MyoD expression in adult muscle progenitor cells. PLoS ONE 2013, 8, e67762. [Google Scholar] [CrossRef]
- Wei, D.W.; Ma, X.Y.; Zhang, S.; Hong, J.Y.; Gui, L.S.; Mei, C.G.; Guo, H.F.; Wang, L.; Ning, Y.; Zan, L.S. Characterization of the promoter region of the bovine SIX1 gene: Roles of MyoD, PAX7, CREB and MyoG. Sci. Rep. 2017, 7, 12599. [Google Scholar] [CrossRef]
- Laclef, C.; Hamard, G.; Demignon, J.; Souil, E.; Houbron, C.; Maire, P. Altered myogenesis in Six1-deficient mice. Development 2003, 130, 2239–2252. [Google Scholar] [CrossRef]
- Baron, B.W.; Stanger, R.R.; Hume, E.; Sadhu, A.; Mick, R.; Kerckaert, J.P.; Deweindt, C.; Bastard, C.; Nucifora, G.; Zeleznik-Le, N.; et al. BCL6 encodes a sequence-specific DNA-binding protein. Genes Chromosom. Cancer 1995, 13, 221–224. [Google Scholar] [CrossRef]
- Bajalica-Lagercrantz, S.; Piehl, F.; Farnebo, F.; Larsson, C.; Lagercrantz, J. Expression of the BCL6 gene in the pre- and postnatal mouse. Biochem. Biophys. Res. Commun. 1998, 247, 357–360. [Google Scholar] [CrossRef]
- ENCODE: A user’s guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol. 2011, 9, e1001046.
- ENCODE: An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [CrossRef]
- Sabo, P.J.; Kuehn, M.S.; Thurman, R.; Johnson, B.E.; Johnson, E.M.; Cao, H.; Yu, M.; Rosenzweig, E.; Goldy, J.; Haydock, A.; et al. Genome-scale mapping of DNase I sensitivity in vivo using tiling DNA microarrays. Nat. Methods 2006, 3, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Crawford, G.E.; Holt, I.E.; Whittle, J.; Webb, B.D.; Tai, D.; Davis, S.; Margulies, E.H.; Chen, Y.; Bernat, J.A.; Ginsburg, D.; et al. Genome-wide mapping of DNase hypersensitive sites using massively parallel signature sequencing (MPSS). Genome Res. 2006, 16, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.; Waxman, D.J. Isolation of nuclei for use in genome-wide DNase hypersensitivity assays to probe chromatin structure. Methods Mol. Biol. 2013, 977, 13–19. [Google Scholar] [PubMed]
- Thurman, R.E.; Rynes, E.; Humbert, R.; Vierstra, J.; Maurano, M.T.; Haugen, E.; Sheffield, N.C.; Stergachis, A.B.; Wang, H.; Vernot, B.; et al. The accessible chromatin landscape of the human genome. Nature 2012, 489, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Plasschaert, R.N.; Bartolomei, M.S. Genomic imprinting in development, growth, behavior and stem cells. Development 2014, 141, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Radford, E.J.; Ferron, S.R.; Ferguson-Smith, A.C. Genomic imprinting as an adaptative model of developmental plasticity. FEBS Lett. 2011, 585, 2059–2066. [Google Scholar] [CrossRef]
- O’Doherty, A.M.; MacHugh, D.E.; Spillane, C.; Magee, D.A. Genomic imprinting effects on complex traits in domesticated animal species. Front. Genet. 2015, 6, 156. [Google Scholar] [CrossRef]
- Oakey, R.J.; Beechey, C.V. Imprinted genes: Identification by chromosome rearrangements and post-genomic strategies. Trends Genet. TIG 2002, 18, 359–366. [Google Scholar] [CrossRef]
- Reik, W.; Walter, J. Genomic imprinting: Parental influence on the genome. Nat. Rev. Genet. 2001, 2, 21–32. [Google Scholar] [CrossRef]
- Frischknecht, M.; Jagannathan, V.; Plattet, P.; Neuditschko, M.; Signer-Hasler, H.; Bachmann, I.; Pacholewska, A.; Drogemuller, C.; Dietschi, E.; Flury, C.; et al. A Non-Synonymous HMGA2 Variant Decreases Height in Shetland Ponies and Other Small Horses. PLoS ONE 2015, 10, e0140749. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.; Hu, D.; Archer, J.; Feng, C.; Afonso, S.; Chen, C.; Blanco-Aguiar, J.A.; Garreau, H.; Boucher, S.; Ferreira, P.G.; et al. Dwarfism and Altered Craniofacial Development in Rabbits Is Caused by a 12.1 kb Deletion at the HMGA2 Locus. Genetics 2017, 205, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Plassais, J.; Kim, J.; Davis, B.W.; Karyadi, D.M.; Hogan, A.N.; Harris, A.C.; Decker, B.; Parker, H.G.; Ostrander, E.A. Whole genome sequencing of canids reveals genomic regions under selection and variants influencing morphology. Nat. Commun. 2019, 10, 1489. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gilbert, J.A.; Zhang, Y.; Zhang, M.; Qiu, Q.; Ramanujan, K.; Shavlakadze, T.; Eash, J.K.; Scaramozza, A.; Goddeeris, M.M.; et al. An HMGA2-IGF2BP2 axis regulates myoblast proliferation and myogenesis. Dev. Cell 2012, 23, 1176–1188. [Google Scholar] [CrossRef]
- Rubin, C.J.; Megens, H.J.; Martinez Barrio, A.; Maqbool, K.; Sayyab, S.; Schwochow, D.; Wang, C.; Carlborg, O.; Jern, P.; Jorgensen, C.B.; et al. Strong signatures of selection in the domestic pig genome. Proc. Natl. Acad. Sci. USA 2012, 109, 19529–19536. [Google Scholar] [CrossRef]
- Makvandi-Nejad, S.; Hoffman, G.E.; Allen, J.J.; Chu, E.; Gu, E.; Chandler, A.M.; Loredo, A.I.; Bellone, R.R.; Mezey, J.G.; Brooks, S.A.; et al. Four loci explain 83% of size variation in the horse. PLoS ONE 2012, 7, e39929. [Google Scholar] [CrossRef]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Ma, Z.; Davis, C.; Pope, B.D.; et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 2014, 515, 355–364. [Google Scholar] [CrossRef]
- Heintzman, N.D.; Stuart, R.K.; Hon, G.; Fu, Y.; Ching, C.W.; Hawkins, R.D.; Barrera, L.O.; Van Calcar, S.; Qu, C.; Ching, K.A.; et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 2007, 39, 311–318. [Google Scholar] [CrossRef]
- Gross, D.S.; Garrard, W.T. Nuclease hypersensitive sites in chromatin. Annu. Rev. Biochem. 1988, 57, 159–197. [Google Scholar] [CrossRef]
- Pratt, K.; Wierowski, J.V.; Hilf, R.; Bambara, R.A. Bovine estrogen receptor binds chromatin at pre-existing nuclease hypersensitive sites. Mol. Cell. Endocrinol. 1984, 35, 205–214. [Google Scholar] [CrossRef]
- Bina, M.; Wyss, P.; Song, X. The Positions of ZFBS and ZFBS-Morph Overlaps in the Build bosTau8 of the Bos Taurus Genome; Purdue University Research Repository: West Lafayette, IN, USA, 2019. [Google Scholar]
- Bina, M.; Wyss, P.; Song, X. Density of ZFBS-Morph Overlaps in the Build bosTau8 of the Bos Taurus Genome; Purdue University Research Repository: West Lafayette, IN, USA, 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).