Homolog of Pea SGR Controls Stay-Green in Faba Bean (Vicia faba L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Faba Bean Materials and Phenotype Investigation
2.2. DNA Extraction, RNA Extraction, and cDNA Synthesis
2.3. Homology-Based Cloning of VfSGR
2.4. Real-Time PCR
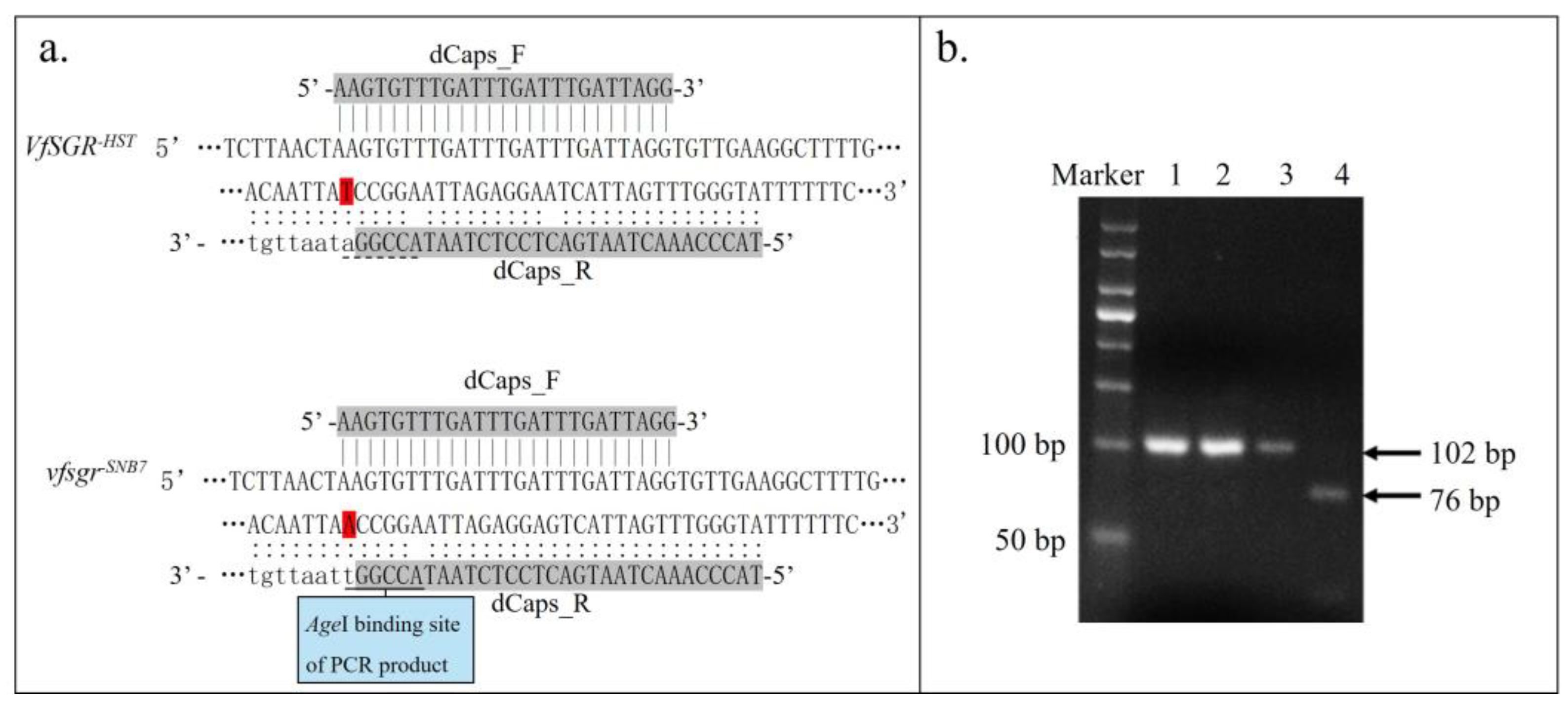
2.5. dCaps Marker Design and Analysis
2.6. Transient Expression
2.7. Measurement of Chlorophyll Content (SPAD)
3. Results
3.1. Homology-Based Searching of VfSGR of Faba Bean
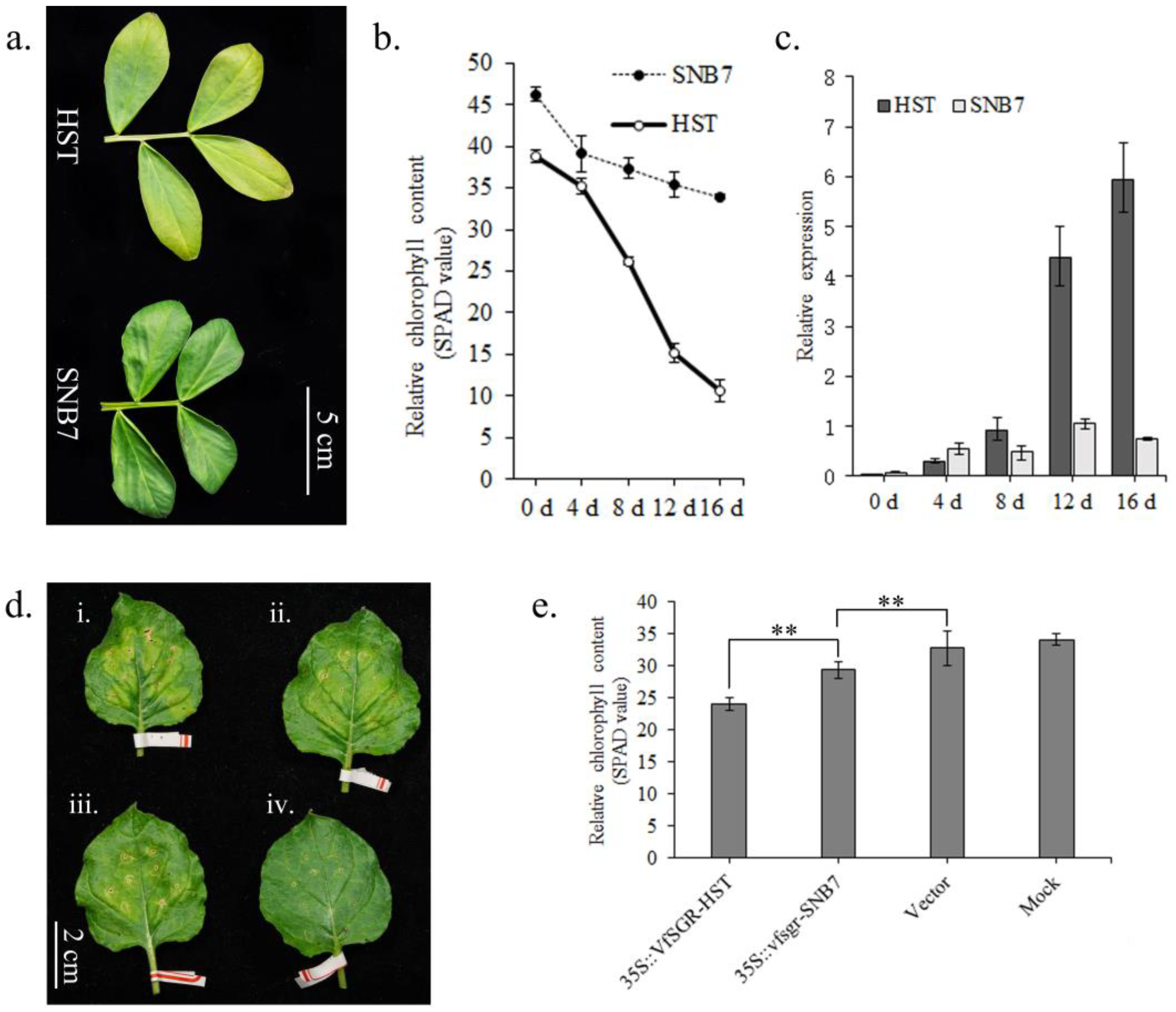
3.2. Expression and Sequence Polymorphism of VfSGR
3.3. Genetic Analysis of Vfsgr and Green Cotyledon of Faba Bean
3.4. The Involvement of VfSGR in Chlorophyll Degradation and Senescence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanno, K.I.; Willcox, G. The origins of cultivation of Cicer arietinum L. and Vicia faba L.: Early finds from Tell el-Kerkh, north-west Syria, late 10th millennium B.P. Veg. Hist. Archaeobot. 2006, 15, 197–204. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/statistics (accessed on 15 December 2022).
- Macarulla, M.T.; Medina, C. Effects of the whole seed and a protein isolate of faba bean (Vicia faba) on the cholesterol metabolism of hypercholesterolaemic rats. Br. J. Nutr. 2001, 85, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Ofuya, Z.M.; Akhidue, V. The role of pulses in human nutrition: A review. J. Appl. Sci. Environ. Manag. 2005, 9, 99–104. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M. Accumulation of L-DOPA in various organs of faba bean and influence of drought, nitrogen stress, and processing methods on L-DOPA yield. Crop. J. 2018, 6, 426–434. [Google Scholar] [CrossRef]
- Multari, S.; Stewart, D. Potential of fava bean as future protein supply to partially replace meat intake in the human diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Bennett, M.D.; Leitch, I.J. Nuclear DNA amounts in angiosperms. Ann. Bot. 1995, 76, 113–176. [Google Scholar] [CrossRef]
- Jayakod, M.; Golicz, A.A. The giant diploid faba genome unlocks variation in a global protein crop. Nature 2023, 615, 652–659. [Google Scholar] [CrossRef]
- Webb, A.; Cottage, A. A SNP-based consensus genetic map for synteny-based trait targeting in faba bean (Vicia faba L.). Plant Biotechnol. J. 2016, 14, 177–185. [Google Scholar] [CrossRef]
- Carrillo-Perdomo, E.; Vidal, A. Development of new genetic resources for faba bean (Vicia faba L.) breeding through the discovery of gene-based SNP markers and the construction of a high-density consensus map. Sci. Rep. 2020, 10, 6790. [Google Scholar] [CrossRef]
- Khazaei, H.; O’Sullivan, D.M. Recent advances in faba bean genetic and genomic tools for crop improvement. Legume Sci. 2021, 3, e75. [Google Scholar] [CrossRef]
- Björnsdotter, E.; Nadzieja, M. VC1 catalyses a key step in the biosynthesis of vicine in faba bean. Nat. Plants 2021, 7, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, N.; Avila, C.M. The bHLH transcription factor VfTT8 underlies zt2, the locus determining zero tannin content in faba bean (Vicia faba L.). Sci. Rep. 2020, 10, 14299. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.M.; Nadal, S. Development of a simple PCR-based marker for the determination of growth habit in Vicia faba L. using a candidate gene approach. Mol. Breed. 2006, 17, 185–190. [Google Scholar] [CrossRef]
- Avila, C.M.; Atienza, S.G. Development of a new diagnostic marker for growth habit selection in faba bean (Vicia faba L.) breeding. Theor. Appl. Genet. 2007, 115, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Duc, G.; Moussy, F. Single gene mutation for green cotyledons as a marker for the embryonic genotype in faba bean, Vicia faba. Plant Breed. 1999, 118, 577–578. [Google Scholar] [CrossRef]
- Armstead, I.; Donnison, I. Cross-species identification of Mendel’s I locus. Science 2007, 315, 73. [Google Scholar] [CrossRef]
- Sato, Y.; Morita, R. Mendel’s green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 14169–14174. [Google Scholar] [CrossRef]
- Thomas, H.; Stoddart, J.L. Separation of chlorophyll degradation from other senescence processes in leaves of a mutant genotype of meadow fescue (Festuca pratensis L.). Plant Physiol. 1975, 56, 438–441. [Google Scholar] [CrossRef]
- Thomas, H. Sid: A Mendelian locus controlling thylakoid membrane disassembly in senescing leaves of Festuca pratensis. Theor. Appl. Genet. 1987, 73, 551–555. [Google Scholar] [CrossRef]
- Ren, G.; An, K. Identification of a novel chloroplast protein AtNYE1 regulating chlorophyll degradation during leaf senescence in Arabidopsis. Plant Physiol. 2007, 144, 1429–1441. [Google Scholar] [CrossRef]
- Cha, K.W.; Lee, Y.J. Isolation, characterization, and mapping of the stay green mutant in rice. Theor. Appl. Genet. 2002, 104, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, M. Molecular cloning and function analysis of the stay green gene in rice. Plant J. 2007, 52, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, S. Natural variations at the Stay-Green gene promoter control lifespan and yield in rice cultivars. Nat. Commun. 2020, 11, 2819. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Goldschmidt, E.E. Altered patterns of senescence and ripening in gf, a stay-green mutant of tomato (Lycopersicon esculentum Mill.). J. Exp. Bot. 1999, 50, 1115–1122. [Google Scholar] [CrossRef]
- Efrati, A.; Eyal, Y. Molecular mapping of the chlorophyll retainer (cl) mutation in pepper (Capsicum spp.) and screening for candidate genes using tomato ESTs homologous to structural genes of the chlorophyll catabolism pathway. Genome 2005, 48, 347–351. [Google Scholar] [CrossRef]
- Borovsky, Y.; Paran, I. Chlorophyll breakdown during pepper fruit ripening in the chlorophyll retainer mutation is impaired at the homolog of the senescence-inducible stay-green gene. Theor. Appl. Genet. 2008, 117, 235–240. [Google Scholar] [CrossRef]
- Liu, Y.; Ou, L. A novel single-base mutation in CaSGR1 confers the stay-green phenotype in pepper (Capsicum annuum L.). Hortic. Plant J. 2022, 9, 293–305. [Google Scholar] [CrossRef]
- Zhou, C.; Han, L. From model to crop: Functional analysis of a STAY-GREEN gene in the model legume Medicago truncatula and effective use of the gene for alfalfa improvement. Plant Physiol. 2011, 157, 1483–1496. [Google Scholar] [CrossRef]
- Shi, S.; Miao, H. GmSGR1, a stay-green gene in soybean (Glycine max L.), plays an important role in regulating early leaf-yellowing phenotype and plant productivity under nitrogen deprivation. Acta Physiol. Plant. 2016, 38, 97. [Google Scholar] [CrossRef]
- Sivasakthi, K.; Marques, E. Functional Dissection of the chickpea (Cicer arietinum L.) stay-green phenotype associated with molecular variation at an ortholog of Mendel’s I gene for cotyledon color: Implications for crop production and carotenoid biofortification. Int. J. Mol. Sci. 2019, 20, 5562. [Google Scholar] [CrossRef]
- Davis, J.; Myers, J.R. Staygreen (sgr), a candidate gene for the persistent color phenotype in common bean. Int. Symp. Mol. Markers Hortic. 2010, 859, 99–102. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y. Defect in Brnym1, a magnesium-dechelatase protein, causes a stay-green phenotype in an EMS-mutagenized Chinese cabbage (Brassica campestris L. ssp. pekinensis) line. Hortic. Res. 2020, 7, 8. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y. Assessment of a stay-green mutant for variety improvement in Pakchoi (Brassica campestris L. ssp. chinensis). Sci. Hortic. 2020, 266, 109261. [Google Scholar] [CrossRef]
- Kuai, B.; Chen, J. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, Y.; Ito, H. Arabidopsis STAY-GREEN, Mendel’s green cotyledon gene, encodes magnesium-dechelatase. Plant Cell 2016, 28, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yu, J.W. The senescence-induced staygreen protein regulates chlorophyll degradation. Plant Cell 2007, 19, 1649–1664. [Google Scholar] [CrossRef]
- Chen, Y.; Yamori, W. Degradation of the photosystem II core complex is independent of chlorophyll degradation mediated by Stay-Green Mg2+ dechelatase in Arabidopsis. Plant Sci. 2021, 307, 110902. [Google Scholar] [CrossRef]
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Bot. 2000, 51 (Suppl. 1), 329–337. [Google Scholar] [CrossRef]
- Lodhi, M.A.; Ye, G.N. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol. Biol. Report. 1994, 12, 6–13. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D. NGPhylogeny. fr: New generation phylogenetic services for non-specialists. Nucl. Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Neff, M.M.; Turk, E. Web-based primer design for single nucleotide polymorphism analysis. Trends Genet. 2002, 18, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Ohmiya, A.; Hirashima, M. Identification of genes associated with chlorophyll accumulation in flower petals. PLoS ONE 2014, 9, e113738. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, A.; Sasaki, K. Transcriptome analysis in petals and leaves of chrysanthemums with different chlorophyll levels. BMC Plant Biol. 2017, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.M.; Gan, S. A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol. Biol. 1998, 37, 455–469. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci. 2009, 14, 155–162. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, S. The C-terminal cysteine-rich motif of NYE1/SGR1 is indispensable for its function in chlorophyll degradation in Arabidopsis. Plant Mol. Biol. 2019, 101, 257–268. [Google Scholar] [CrossRef]
- Vidal-Valverde, C.; Frias, J. Assessment of nutritional compounds and antinutritional factors in pea (Pisum sativum) seeds. J. Sci. Food Agric. 2003, 83, 298–306. [Google Scholar] [CrossRef]
- Bangar, P.; Glahn, R.P. Iron bioavailability in field pea seeds: Correlations with iron, phytate, and carotenoids. Crop Sci. 2017, 57, 891–902. [Google Scholar] [CrossRef]
- Sha, W. Study on SSR Markers of Cotyledon Colour Traits in Faba Bean. Master’s Thesis, Qinghai University, Xining, China, 3 June 2017. [Google Scholar]
- Zhang, J.; Li, H. Staygreen-mediated chlorophyll a catabolism is critical for photosystem stability during heat-induced leaf senescence in perennial ryegrass. Plant Cell Environ. 2022, 45, 1412–1427. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, S. NYEs/SGRs-mediated chlorophyll degradation is critical for detoxification during seed maturation in Arabidopsis. Plant J. 2017, 92, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Cirak, M.; Myers, J.R. Cosmetic stay-green trait in snap bean and the event cascade that reduces seed germination and emergence. J. Am. Soc. Hortic. Sci. 2021, 146, 329–338. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhou, H.; Yuan, X.; He, Y.; Yan, Q.; Lin, Y.; Wu, R.; Liu, J.; Xue, C.; Chen, X. Homolog of Pea SGR Controls Stay-Green in Faba Bean (Vicia faba L.). Genes 2023, 14, 1030. https://doi.org/10.3390/genes14051030
Chen J, Zhou H, Yuan X, He Y, Yan Q, Lin Y, Wu R, Liu J, Xue C, Chen X. Homolog of Pea SGR Controls Stay-Green in Faba Bean (Vicia faba L.). Genes. 2023; 14(5):1030. https://doi.org/10.3390/genes14051030
Chicago/Turabian StyleChen, Jingbin, Huimin Zhou, Xingxing Yuan, Yaming He, Qiang Yan, Yun Lin, Ranran Wu, Jinyang Liu, Chenchen Xue, and Xin Chen. 2023. "Homolog of Pea SGR Controls Stay-Green in Faba Bean (Vicia faba L.)" Genes 14, no. 5: 1030. https://doi.org/10.3390/genes14051030
APA StyleChen, J., Zhou, H., Yuan, X., He, Y., Yan, Q., Lin, Y., Wu, R., Liu, J., Xue, C., & Chen, X. (2023). Homolog of Pea SGR Controls Stay-Green in Faba Bean (Vicia faba L.). Genes, 14(5), 1030. https://doi.org/10.3390/genes14051030





