Identification of Differentially Expressed Genes and Molecular Pathways Involved in Osteoclastogenesis Using RNA-seq
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Recruitment and Generation of Osteoclast-like Cells
2.2. Nucleic Acid Extraction
2.3. Data Processing for Gene Expression
2.4. Differential Gene Expression Analysis
2.5. Functional Enrichment Analysis of DEGs
2.6. Protein–Protein Interaction (PPI) Network and Module Analysis
3. Results
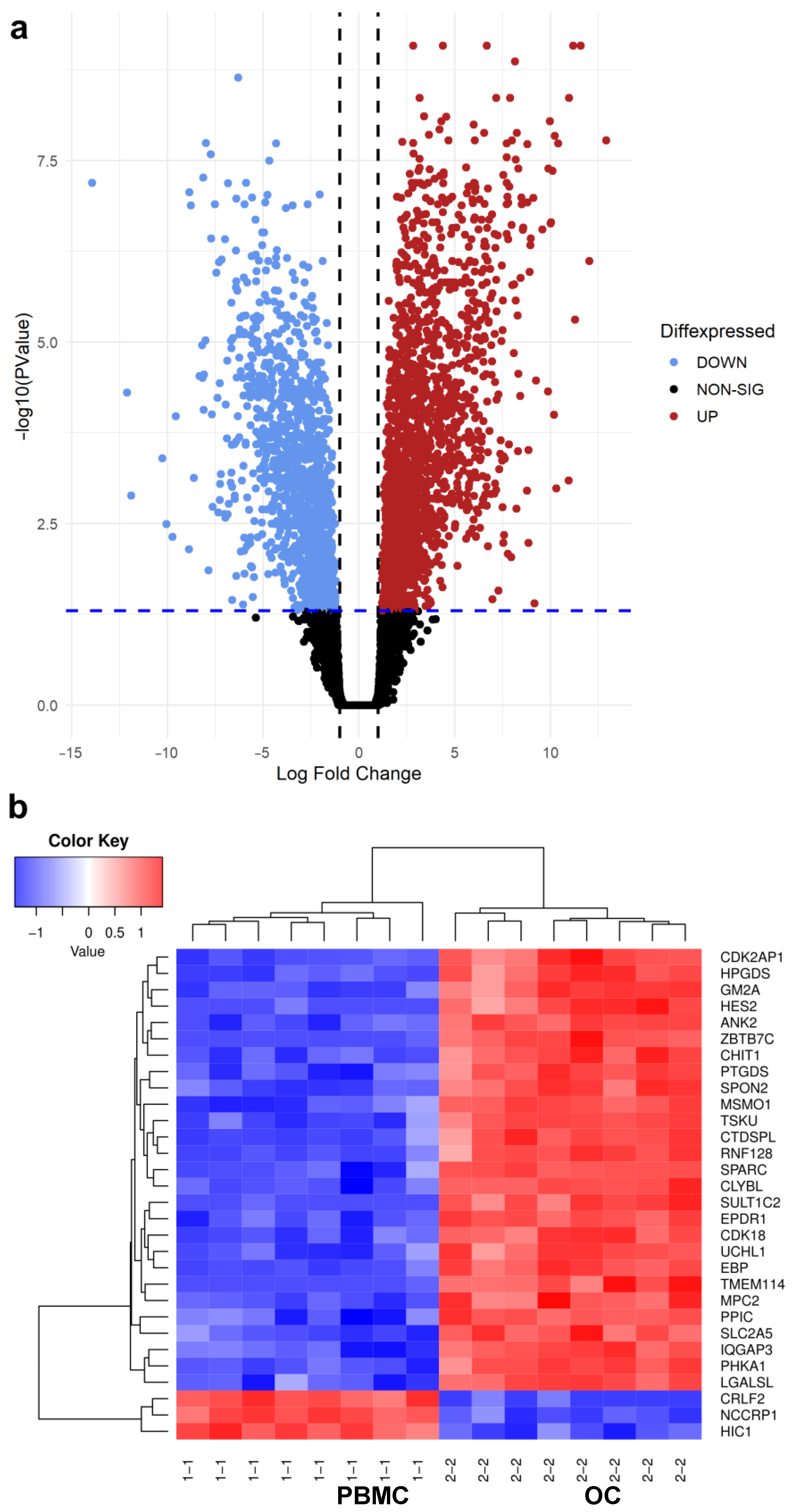
3.1. Differential Gene Expression Analysis between PBMC and OC Groups
3.2. Analysis of Established OC Genes
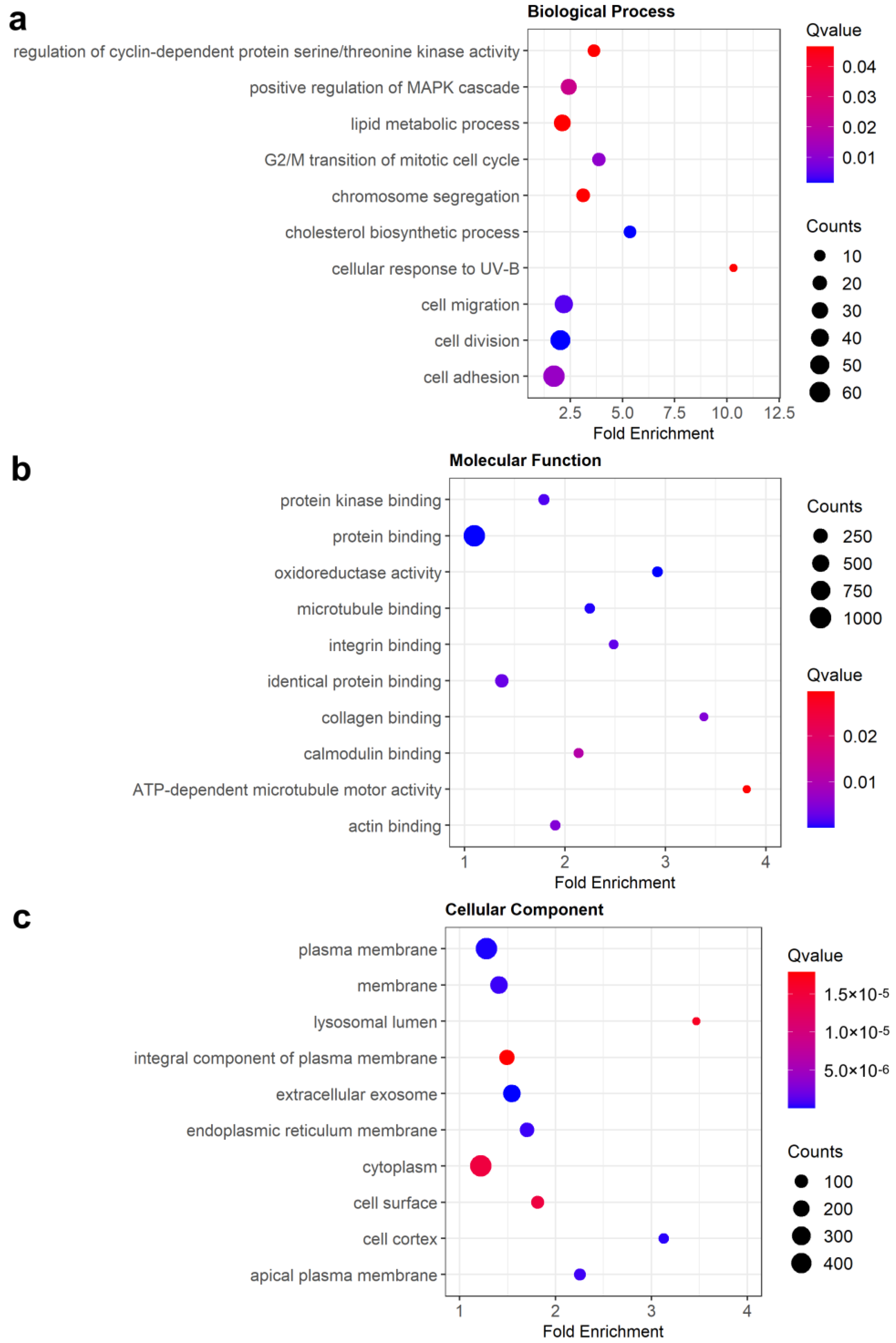
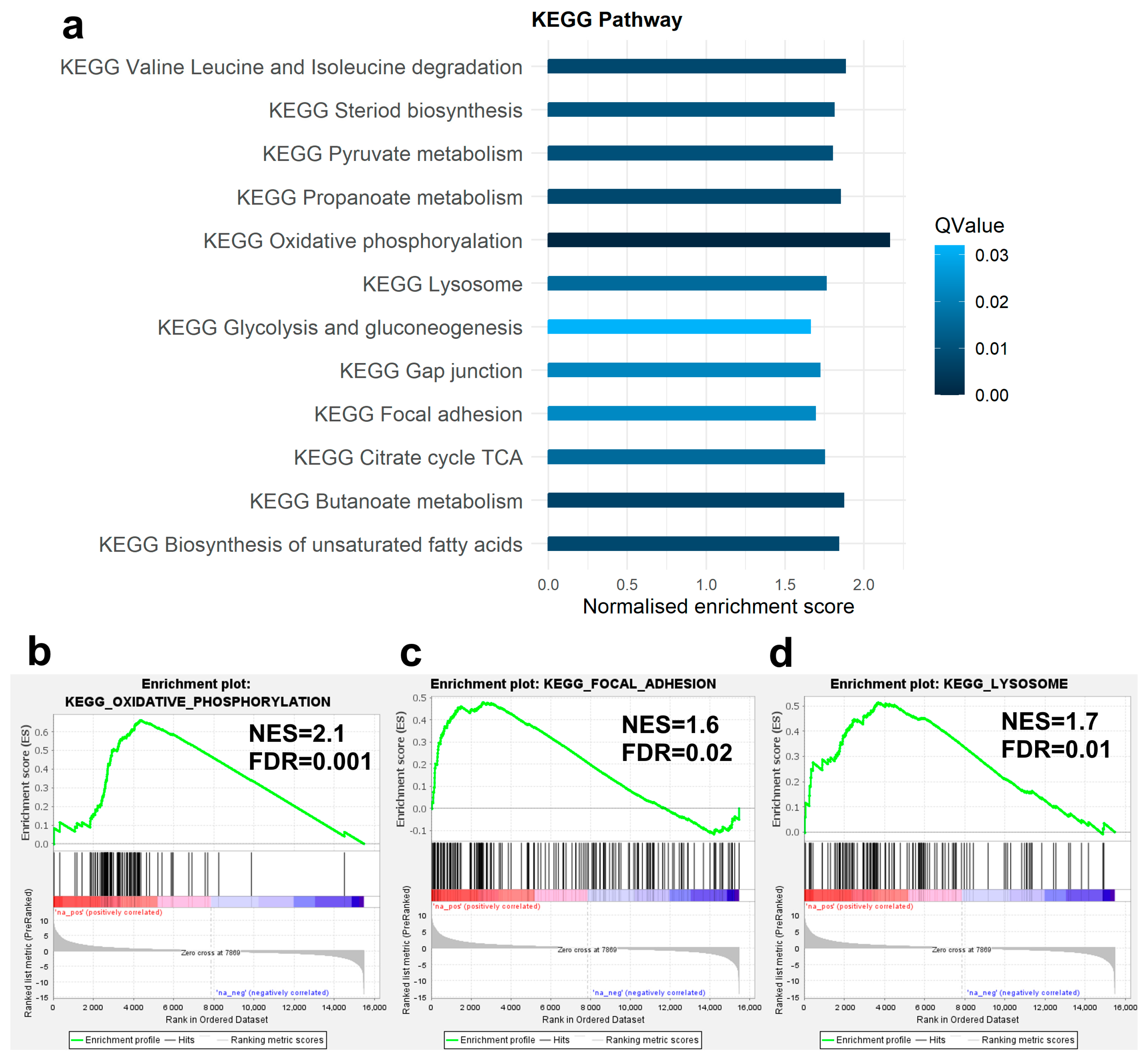
3.3. Enrichment Analysis of DEGs
3.4. PPI Interaction Network Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias, C.F.; Herrero, M.A.; Echeverri, L.F.; Oleaga, G.E.; Lopez, J.M. Bone remodeling: A tissue-level process emerging from cell-level molecular algorithms. PLoS ONE 2018, 13, e0204171. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Faccio, R.; Teitelbaum, S.L.; Takayanagi, H. Osteoclast Biology: Regulation of Formation and Function. In Osteoimmunology; Academic Press: Cambridge, MA, USA, 2016; pp. 41–70. [Google Scholar]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef]
- Mun, S.H.; Park, P.S.U.; Park-Min, K.H. The M-CSF receptor in osteoclasts and beyond. Exp. Mol. Med. 2020, 52, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Enodcrine Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- McDonald, M.M.; Kim, A.S.; Mulholland, B.S.; Rauner, M. New Insights Into Osteoclast Biology. JBMR Plus 2021, 5, e10539. [Google Scholar] [CrossRef]
- Novack, D.V.; Teitelbaum, S.L. The osteoclast: Friend or foe? Annu. Rev. Pathol. 2008, 3, 457–484. [Google Scholar] [CrossRef]
- Cheng, C.; Wentworth, K.; Shoback, D.M. New Frontiers in Osteoporosis Therapy. Annu. Rev. Med. 2020, 71, 277–288. [Google Scholar] [CrossRef]
- De Paula, F.J.A.; Black, D.M.; Rosen, C.J. Osteoporosis and Bone Biology. In Williams Textbook of Endocrinology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1323–1364. [Google Scholar]
- Boyce, B.F.; Rosenberg, E.; de Papp, A.E.; Duong, L.T. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur. J. Clin. Investig. 2012, 42, 1332–1341. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.L.; Danoy, P.; Kemp, J.P.; Leo, P.J.; McCloskey, E.; Nicholson, G.C.; Eastell, R.; Prince, R.L.; Eisman, J.A.; Jones, G.; et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet. 2011, 7, e1001372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ayturk, U. RNA-seq in Skeletal Biology. Curr. Osteoporos. Rep. 2019, 17, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.L.; Walsh, J.P.; Layfield, R.; Ratajczak, T.; Xu, J. New insights into the role of sequestosome 1/p62 mutant proteins in the pathogenesis of Paget’s disease of bone. Endocr. Rev. 2013, 34, 501–524. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.F.; Ang, E.S.; Yip, K.; Woloszyn, M.; Zheng, M.H.; Tan, R.X. NF-kappaB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev. 2009, 20, 7–17. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, H.W.; Shen, H. Circulating monocytes: An appropriate model for bone-related study. Osteoporos. Int. 2015, 26, 2561–2572. [Google Scholar] [CrossRef]
- Deng, F.Y.; Lei, S.F.; Zhang, Y.; Zhang, Y.L.; Zheng, Y.P.; Zhang, L.S.; Pan, R.; Wang, L.; Tian, Q.; Shen, H.; et al. Peripheral blood monocyte-expressed ANXA2 gene is involved in pathogenesis of osteoporosis in humans. Mol. Cell. Proteom. 2011, 10, M111.011700. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Dvornyk, V.; Lu, Y.; Shen, H.; Lappe, J.M.; Recker, R.R.; Deng, H.W. A novel pathophysiological mechanism for osteoporosis suggested by an in vivo gene expression study of circulating monocytes. J. Biol. Chem. 2005, 280, 29011–29016. [Google Scholar] [CrossRef]
- Mullin, B.H.; Tickner, J.; Zhu, K.; Kenny, J.; Mullin, S.; Brown, S.J.; Dudbridge, F.; Pavlos, N.J.; Mocarski, E.S.; Walsh, J.P.; et al. Characterisation of genetic regulatory effects for osteoporosis risk variants in human osteoclasts. Genome Biol. 2020, 21, 80. [Google Scholar] [CrossRef]
- Mullin, B.H.; Mamotte, C.; Prince, R.L.; Wilson, S.G. Influence of ARHGEF3 and RHOA knockdown on ACTA2 and other genes in osteoblasts and osteoclasts. PLoS ONE 2014, 9, e98116. [Google Scholar] [CrossRef] [PubMed]
- Mullin, B.H.; Zhu, K.; Xu, J.; Brown, S.J.; Mullin, S.; Tickner, J.; Pavlos, N.J.; Dudbridge, F.; Walsh, J.P.; Wilson, S.G. Expression Quantitative Trait Locus Study of Bone Mineral Density GWAS Variants in Human Osteoclasts. J. Bone Miner. Res. 2018, 33, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Mullin, B.H.; Zhu, K.; Brown, S.J.; Mullin, S.; Tickner, J.; Pavlos, N.J.; Dudbridge, F.; Xu, J.; Walsh, J.P.; Wilson, S.G. Genetic regulatory mechanisms in human osteoclasts suggest a role for the STMP1 and DCSTAMP genes in Paget’s disease of bone. Sci. Rep. 2019, 9, 1052. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sun, Y.; Zhou, Q.; He, Q.; Qian, H. Identification of key genes and pathways by bioinformatics analysis with TCGA RNA sequencing data in hepatocellular carcinoma. Mol. Clin. Oncol. 2018, 9, 597–606. [Google Scholar] [CrossRef]
- Rothzerg, E.; Xu, J.; Wood, D.; Koks, S. 12 Survival-related differentially expressed genes based on the TARGET-osteosarcoma database. Exp. Biol. Med. 2021, 246, 2072–2081. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Lv, M.; Cui, C.; Chen, P.; Li, Z. Identification of osteoporosis markers through bioinformatic functional analysis of serum proteome. Medicine 2020, 99, e22172. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Nepusz, T.; Yu, H.; Paccanaro, A. Detecting overlapping protein complexes in protein-protein interaction networks. Nat. Methods 2012, 9, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A.; Kemp, J.P.; Youlten, S.E.; Laurent, L.; Logan, J.G.; Chai, R.C.; Vulpescu, N.A.; Forgetta, V.; Kleinman, A.; Mohanty, S.T.; et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2019, 51, 258–266. [Google Scholar] [CrossRef]
- Cawley, N.X.; Wetsel, W.C.; Murthy, S.R.; Park, J.J.; Pacak, K.; Loh, Y.P. New roles of carboxypeptidase E in endocrine and neural function and cancer. Endocr. Rev. 2012, 33, 216–253. [Google Scholar] [CrossRef]
- Kim, H.J.; Hong, J.; Yoon, H.J.; Yoon, Y.R.; Kim, S.Y. Carboxypeptidase E is a novel modulator of RANKL-induced osteoclast differentiation. Mol. Cells 2014, 37, 685–690. [Google Scholar] [CrossRef]
- Chang, E.J.; Ha, J.; Oerlemans, F.; Lee, Y.J.; Lee, S.W.; Ryu, J.; Kim, H.J.; Lee, Y.; Kim, H.M.; Choi, J.Y.; et al. Brain-type creatine kinase has a crucial role in osteoclast-mediated bone resorption. Nat. Med. 2008, 14, 966–972. [Google Scholar] [CrossRef]
- Mishra, Y.G.; Manavathi, B. Focal adhesion dynamics in cellular function and disease. Cell Signal 2021, 85, 110046. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Modzelewska, K.; Kwong, L.; Keely, P.J. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta 2004, 1692, 103–119. [Google Scholar] [CrossRef]
- Revach, O.Y.; Grosheva, I.; Geiger, B. Biomechanical regulation of focal adhesion and invadopodia formation. J. Cell Sci. 2020, 133, jcs244848. [Google Scholar] [CrossRef]
- Cao, H.; Yan, Q.; Wang, D.; Lai, Y.; Zhou, B.; Zhang, Q.; Jin, W.; Lin, S.; Lei, Y.; Ma, L.; et al. Focal adhesion protein Kindlin-2 regulates bone homeostasis in mice. Bone Res. 2020, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, X.; Lai, Y.; Chen, D.; Bai, X.; Liu, S.; Wu, Y.; Chen, M.; Lai, Y.; Cao, H.; et al. Kindlin-2 inhibits Nlrp3 inflammasome activation in nucleus pulposus to maintain homeostasis of the intervertebral disc. Bone Res. 2022, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Duong, L.T.; Lakkakorpi, P.; Nakamura, I.; Rodan, G.A. Integrins and signaling in osteoclast function. Matrix Biol. 2000, 19, 97–105. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.P.; Hodivala-Dilke, K.; Zheng, M.-H.; Namba, N.; Lam, J.; Novack, D.; Feng, X.; Ross, F.P.; Hynes, R.O.; Teitelbaum, S.L. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Investig. 2000, 105, 433–440. [Google Scholar] [CrossRef]
- Faccio, R.; Novack, D.V.; Zallone, A.; Ross, F.P.; Teitelbaum, S.L. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by beta3 integrin. J. Cell Biol. 2003, 162, 499–509. [Google Scholar] [CrossRef]
- Helfrich, M.H.; Nesbitt, S.A.; Dorey, E.L.; Horton, M.A. Rat osteoclasts adhere to a wide range of RGD (Arg-Gly-Asp) peptide-containing proteins, including the bone sialoproteins and fibronectin, via a beta 3 integrin. J. Bone Miner. Res. 1992, 7, 335–343. [Google Scholar] [CrossRef]
- Miyazaki, T.; Sanjay, A.; Neff, L.; Tanaka, S.; Horne, W.C.; Baron, R. Src kinase activity is essential for osteoclast function. J. Biol. Chem. 2004, 279, 17660–17666. [Google Scholar] [CrossRef]
- Zou, W.; Kitaura, H.; Reeve, J.; Long, F.; Tybulewicz, V.L.; Shattil, S.J.; Ginsberg, M.H.; Ross, F.P.; Teitelbaum, S.L. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J. Cell Biol. 2007, 176, 877–888. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, L.; Huang, Y.; Shi, K.; Zhang, L.; Huang, C.; Liang, J.; Zeng, Q.; Wang, J.; He, X.; et al. Puerarin specifically disrupts osteoclast activation via blocking integrin-beta3 Pyk2/Src/Cbl signaling pathway. J. Orthop. Translat. 2022, 33, 55–69. [Google Scholar] [CrossRef]
- Faccio, R.; Teitelbaum, S.L.; Fujikawa, K.; Chappel, J.; Zallone, A.; Tybulewicz, V.L.; Ross, F.P.; Swat, W. Vav3 regulates osteoclast function and bone mass. Nat. Med. 2005, 11, 284–290. [Google Scholar] [CrossRef]
- Park-Min, K.H. Metabolic reprogramming in osteoclasts. Semin. Immunopathol. 2019, 41, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.A.; Fumoto, T.; Iwai, K.; Takeshita, S.; Ito, M.; Shimohata, N.; Aburatani, H.; Taketani, S.; Lelliott, C.J.; Vidal-Puig, A.; et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat. Med. 2009, 15, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Faccio, R.; Novack, D.V. Alternative NF-kappaB Regulates RANKL-Induced Osteoclast Differentiation and Mitochondrial Biogenesis via Independent Mechanisms. J. Bone Miner. Res. 2015, 30, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Indo, Y.; Takeshita, S.; Ishii, K.A.; Hoshii, T.; Aburatani, H.; Hirao, A.; Ikeda, K. Metabolic regulation of osteoclast differentiation and function. J. Bone Miner. Res. 2013, 28, 2392–2399. [Google Scholar] [CrossRef] [PubMed]
- Karner, C.M.; Long, F. Glucose metabolism in bone. Bone 2018, 115, 2–7. [Google Scholar] [CrossRef]
- Suzuki, A.; Minamide, M.; Iwaya, C.; Ogata, K.; Iwata, J. Role of Metabolism in Bone Development and Homeostasis. Int. J. Mol. Sci. 2020, 21, 8992. [Google Scholar] [CrossRef]
- Wilson, D.F. Oxidative phosphorylation: Regulation and role in cellular and tissue metabolism. J. Physiol. 2017, 595, 7023–7038. [Google Scholar] [CrossRef]
- Jin, Z.; Wei, W.; Yang, M.; Du, Y.; Wan, Y. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 2014, 20, 483–498. [Google Scholar] [CrossRef]
- Bae, S.; Lee, M.J.; Mun, S.H.; Giannopoulou, E.G.; Yong-Gonzalez, V.; Cross, J.R.; Murata, K.; Giguere, V.; van der Meulen, M.; Park-Min, K.H. MYC-dependent oxidative metabolism regulates osteoclastogenesis via nuclear receptor ERRalpha. J. Clin. Investig. 2017, 127, 2555–2568. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Blair, H.C.; McDonald, J.M.; McKenna, M.A.; Jordan, S.E.; Williford, J.; Hardy, R.W. Regulation of osteoclastic bone resorption by glucose. Biochem. Biophys. Res. Commun. 1997, 235, 646–651. [Google Scholar] [CrossRef]
- Lemma, S.; Sboarina, M.; Porporato, P.E.; Zini, N.; Sonveaux, P.; Di Pompo, G.; Baldini, N.; Avnet, S. Energy metabolism in osteoclast formation and activity. Int. J. Biochem. Cell Biol. 2016, 79, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.I.; Falany, M.L.; Ponomareva, L.V.; Wang, W.; Williams, J.P. Glucose-dependent regulation of osteoclast H(+)-ATPase expression: Potential role of p38 MAP-kinase. J. Cell Biochem. 2002, 87, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Appelqvist, H.; Waster, P.; Kagedal, K.; Ollinger, K. The lysosome: From waste bag to potential therapeutic target. J. Mol. Cell Biol. 2013, 5, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Silvestre, T.; Neutzsky-Wulff, A.V.; Sorensen, M.G.; Christiansen, C.; Bollerslev, J.; Karsdal, M.A.; Henriksen, K. Advances in osteoclast biology resulting from the study of osteopetrotic mutations. Hum. Genet. 2009, 124, 561–577. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef]
- Zhao, H.; Patrick Ross, F. Mechanisms of osteoclastic secretion. Ann. N. Y. Acad. Sci. 2007, 1116, 238–244. [Google Scholar] [CrossRef]
- Duan, X.; Yang, S.; Zhang, L.; Yang, T. V-ATPases and osteoclasts: Ambiguous future of V-ATPases inhibitors in osteoporosis. Theranostics 2018, 8, 5379–5399. [Google Scholar] [CrossRef]
- Wu, H.; Xu, G.; Li, Y.P. Atp6v0d2 is an essential component of the osteoclast-specific proton pump that mediates extracellular acidification in bone resorption. J. Bone Miner. Res. 2009, 24, 871–885. [Google Scholar] [CrossRef]
- Matsumoto, N.; Daido, S.; Sun-Wada, G.H.; Wada, Y.; Futai, M.; Nakanishi-Matsui, M. Diversity of proton pumps in osteoclasts: V-ATPase with a3 and d2 isoforms is a major form in osteoclasts. Biochim. Biophys. Acta 2014, 1837, 744–749. [Google Scholar] [CrossRef]
- Feng, S.; Deng, L.; Chen, W.; Shao, J.; Xu, G.; Li, Y.P. Atp6v1c1 is an essential component of the osteoclast proton pump and in F-actin ring formation in osteoclasts. Biochem. J. 2009, 417, 195–203. [Google Scholar] [CrossRef]
- Zhao, H.; Ito, Y.; Chappel, J.; Andrews, N.W.; Teitelbaum, S.L.; Ross, F.P. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev. Cell 2008, 14, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.H.; Morse, L.R.; Battaglino, R.A. SNX10 is required for osteoclast formation and resorption activity. J. Cell Biochem. 2012, 113, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T. Do results obtained with RNA-sequencing require independent verification? Biofilm 2021, 3, 100043. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, S.; Wilson, S.G.; Zhu, K.; Walsh, J.P.; Xu, J.; Mullin, B.H. Identification of Differentially Expressed Genes and Molecular Pathways Involved in Osteoclastogenesis Using RNA-seq. Genes 2023, 14, 916. https://doi.org/10.3390/genes14040916
Rashid S, Wilson SG, Zhu K, Walsh JP, Xu J, Mullin BH. Identification of Differentially Expressed Genes and Molecular Pathways Involved in Osteoclastogenesis Using RNA-seq. Genes. 2023; 14(4):916. https://doi.org/10.3390/genes14040916
Chicago/Turabian StyleRashid, Sarah, Scott G. Wilson, Kun Zhu, John P. Walsh, Jiake Xu, and Benjamin H. Mullin. 2023. "Identification of Differentially Expressed Genes and Molecular Pathways Involved in Osteoclastogenesis Using RNA-seq" Genes 14, no. 4: 916. https://doi.org/10.3390/genes14040916
APA StyleRashid, S., Wilson, S. G., Zhu, K., Walsh, J. P., Xu, J., & Mullin, B. H. (2023). Identification of Differentially Expressed Genes and Molecular Pathways Involved in Osteoclastogenesis Using RNA-seq. Genes, 14(4), 916. https://doi.org/10.3390/genes14040916






