The Effect of Fetal Bovine Acellular Dermal Matrix Seeded with Wharton’s Jelly Mesenchymal Stem Cells for Healing Full-Thickness Skin Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Acellular Dermal Matrices
2.2. DNA Quantification
2.3. Collagen Content Analysis
2.4. Glycosaminoglycan Evaluation
2.5. Isolation and Expansion of MSCs from Wharton’s Jelly
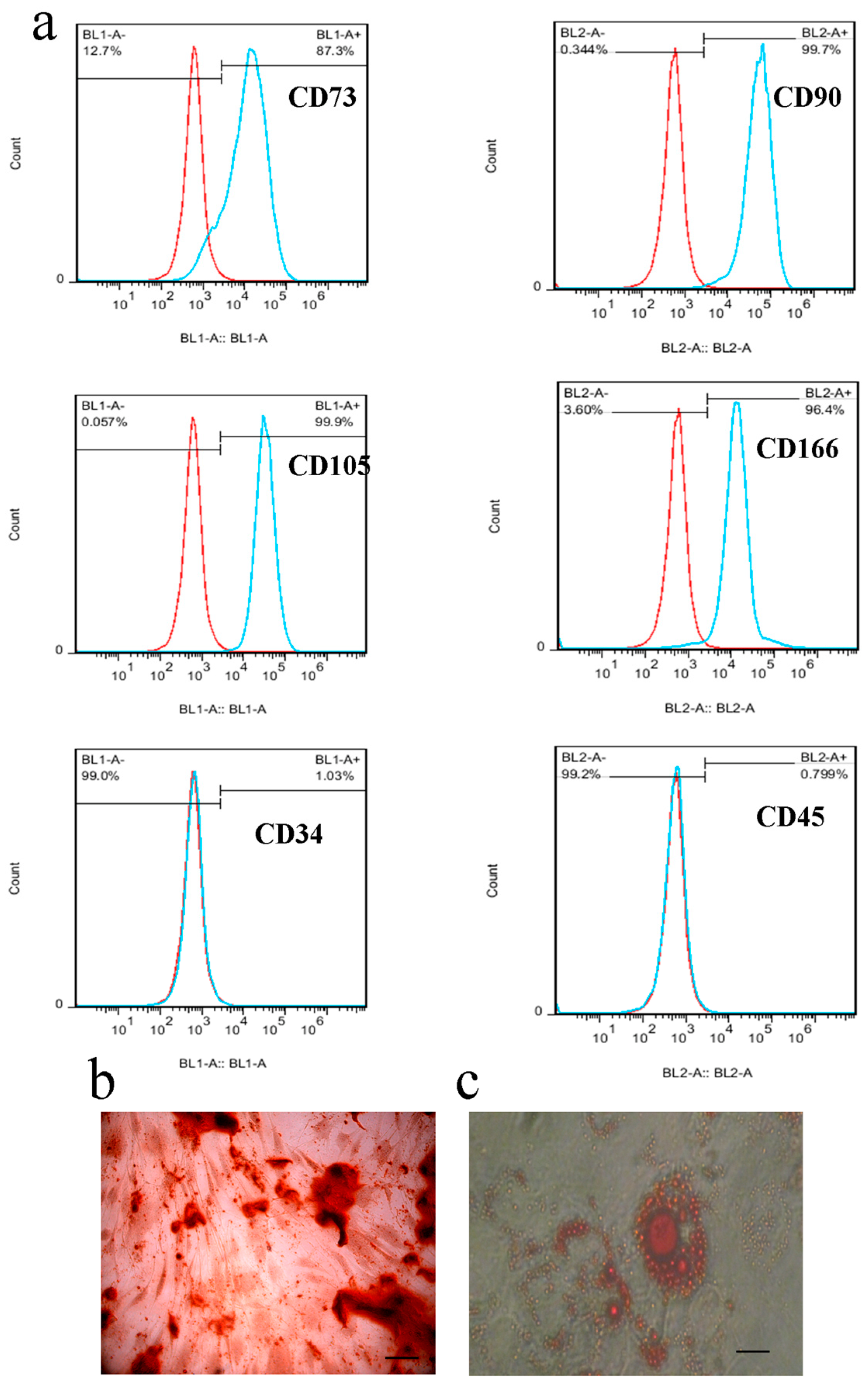
2.6. Stem Cell Characterization by Flow Cytometry
2.7. Multipotency of WJ-MSCs
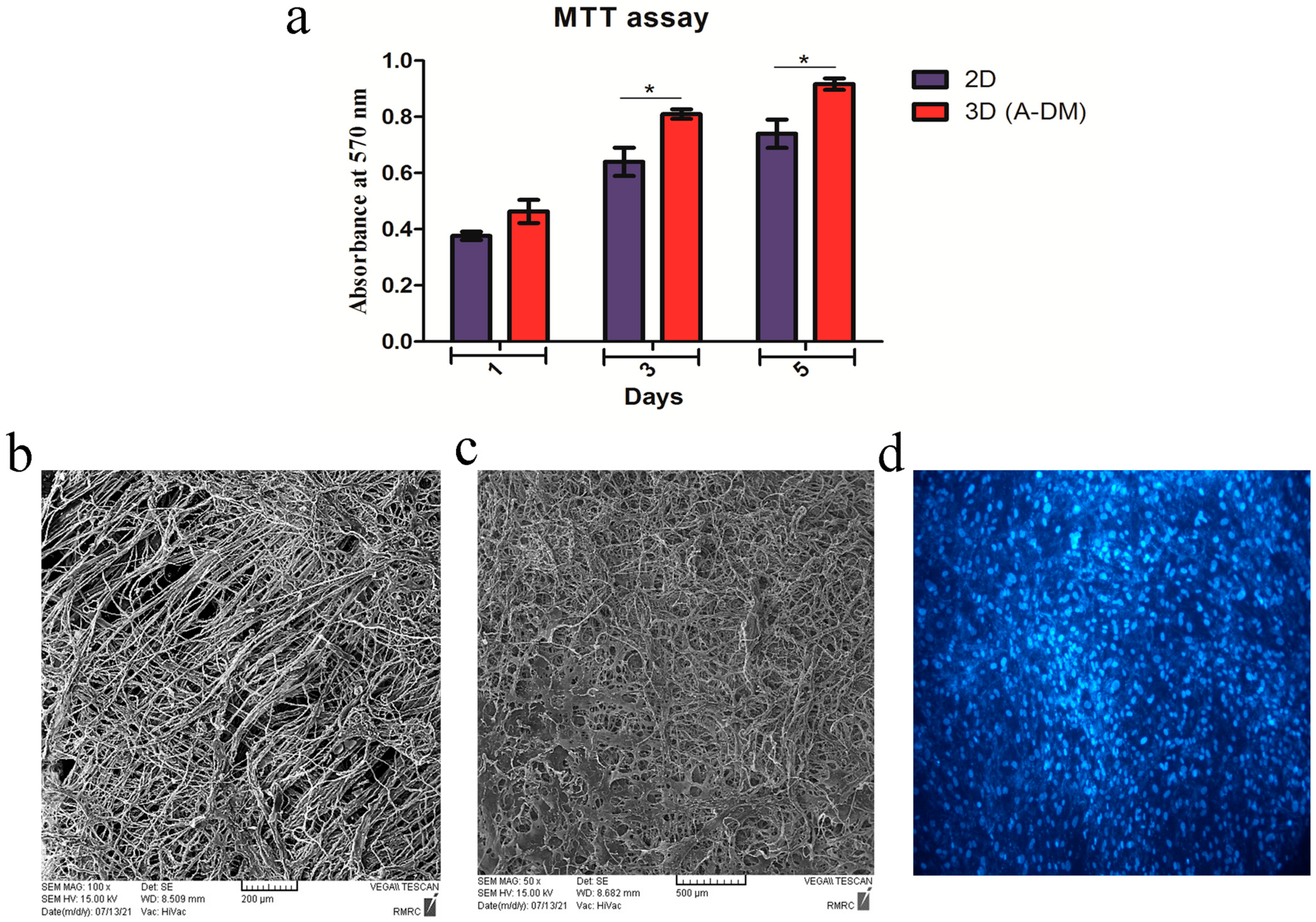
2.8. Cell Proliferation and Penetration Assay
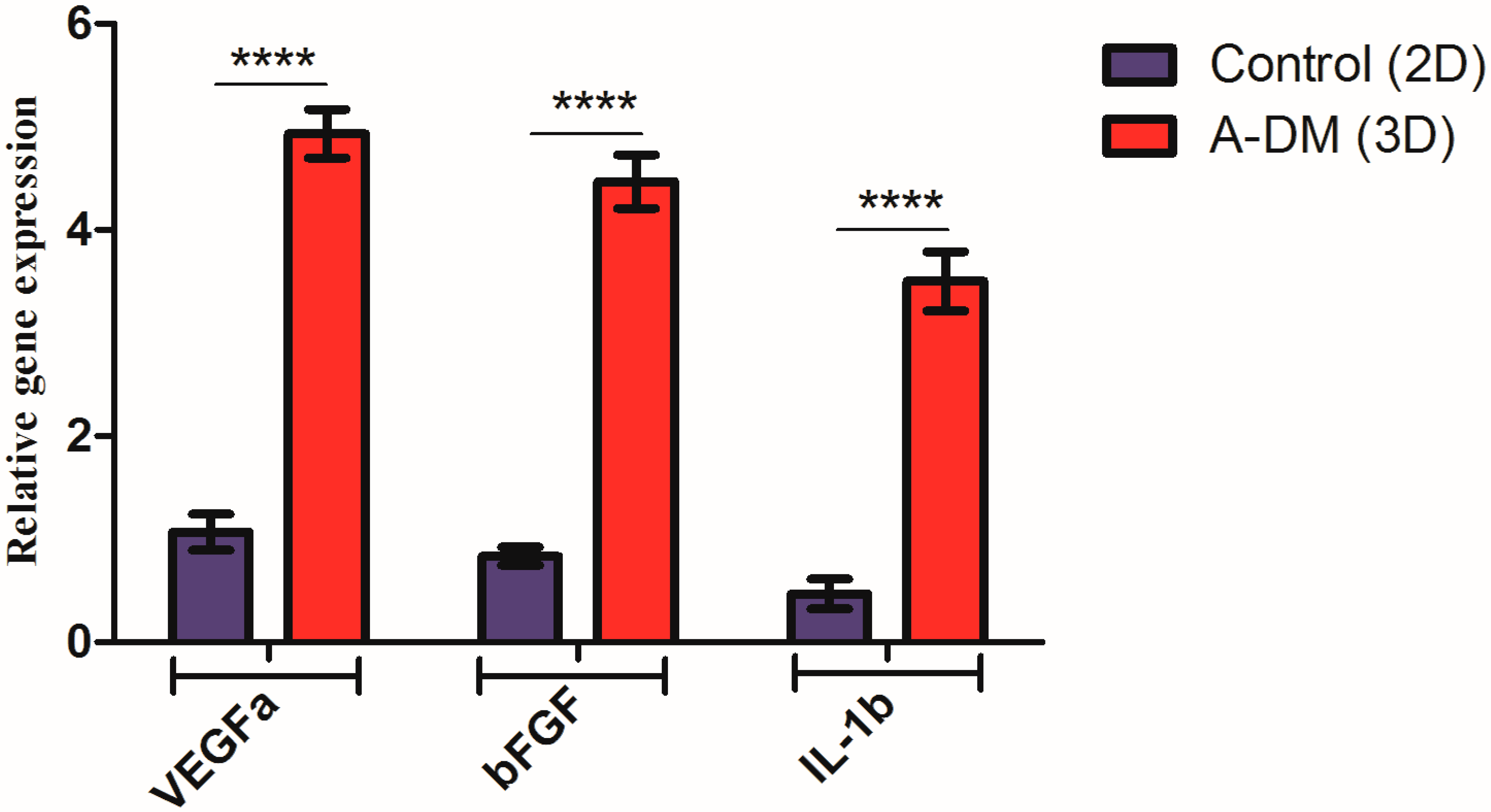
2.9. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
2.10. In Vivo Implantation of the ADMs
2.11. Histological Staining
2.12. Statistical Analysis
3. Results
3.1. Characterization of ADM
3.2. Characterization of the WJ-MSCs
3.3. Cell Proliferation and Penetration
3.4. Gene Expression Results
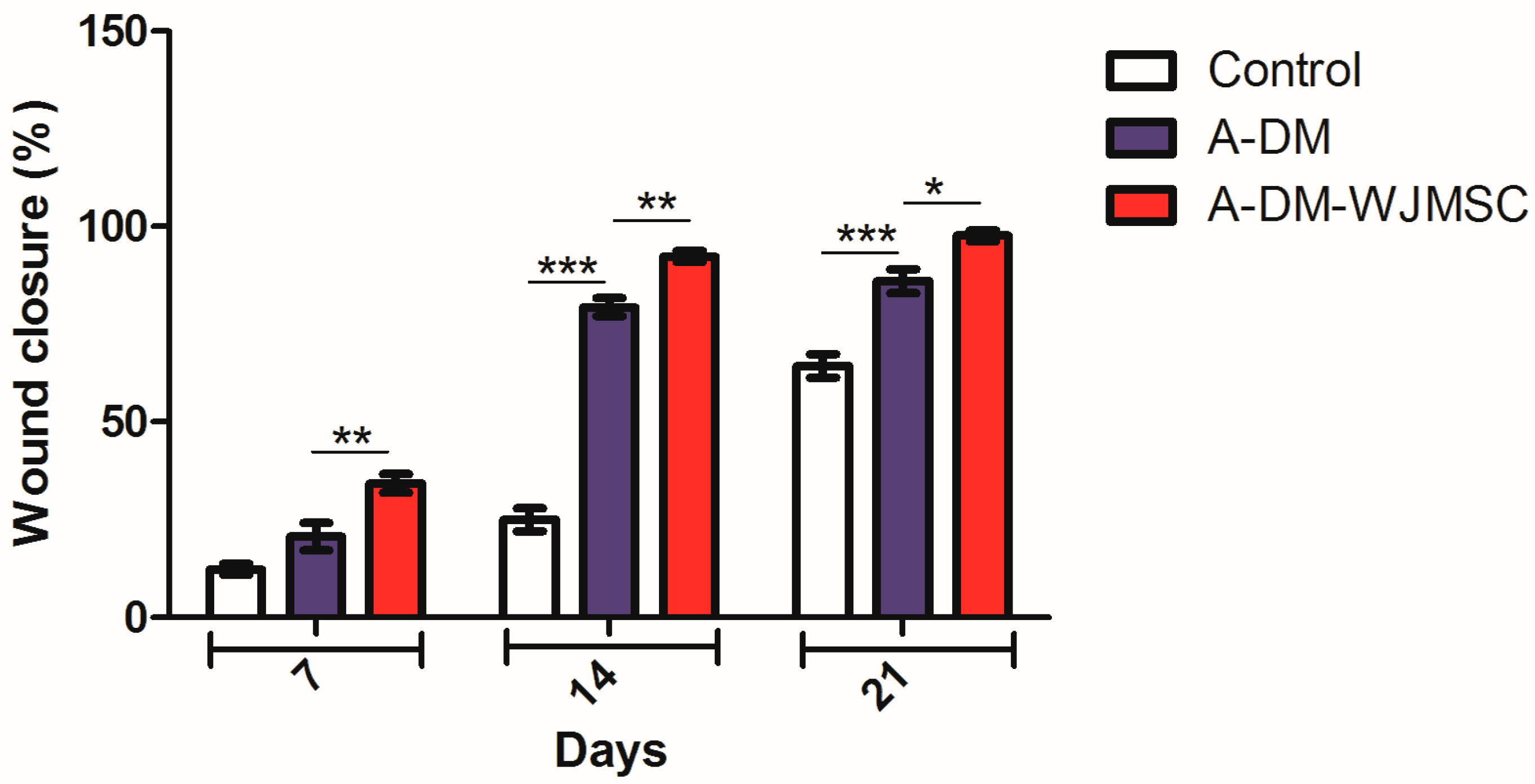
3.5. Wound Closure
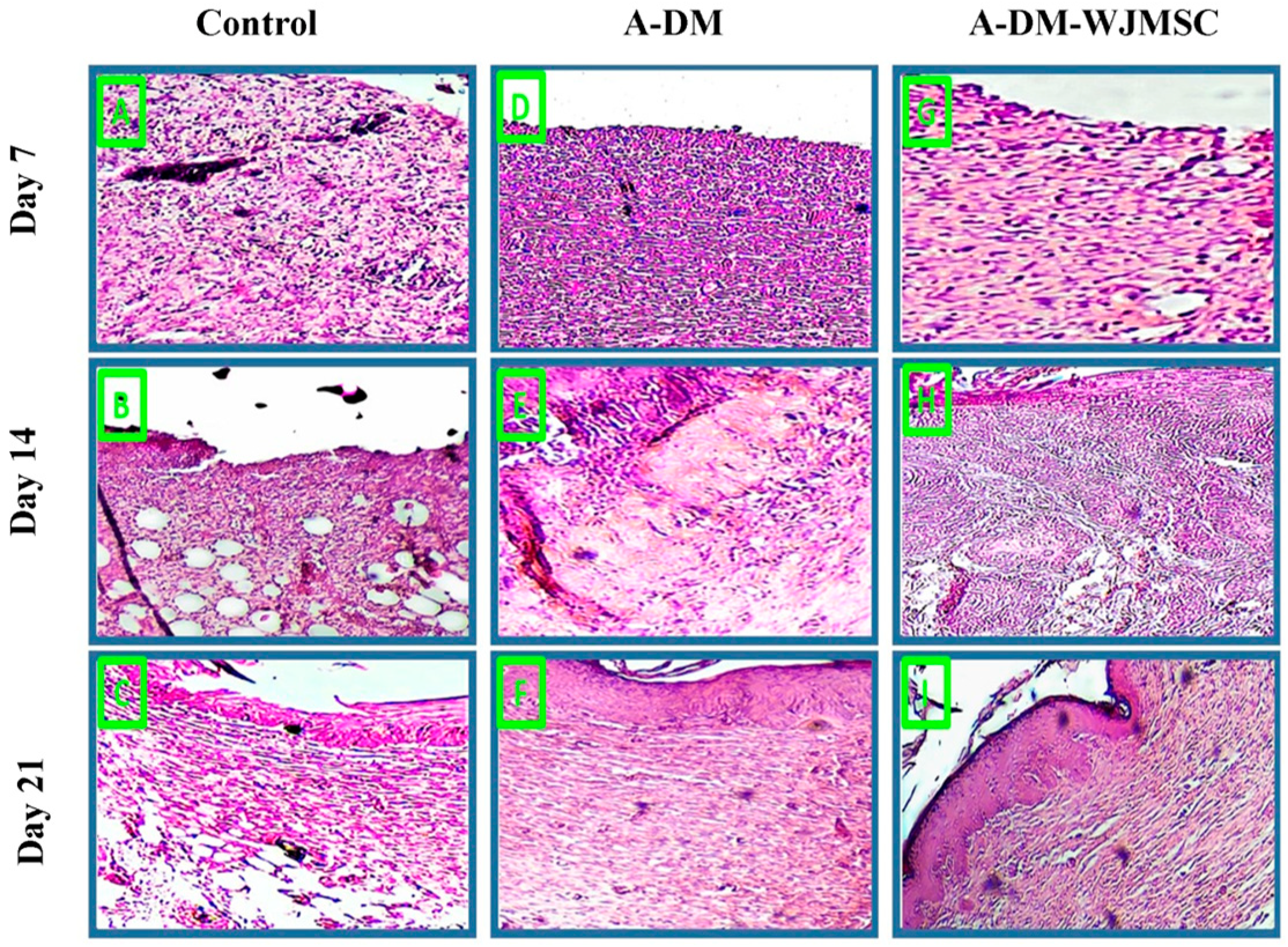
3.6. Histological Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robson, M.C. Wound healing; biologic features and approaches to maximize healing trajectories. Curr. Probl. Surg. 2001, 38, 61–140. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Williamson, D.; Stiltz, A.J.; Harding, K. Advances in Wound Care and Healing Technology. Am. J. Clin. Dermatol. 2000, 1, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Herndon, D.N.; Barrow, R.E.; Rutan, R.L.; Rutan, T.C.; Desai, M.H.; Abston, S. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann. Surg. 1989, 209, 547. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Chhabra, N.; Kaur, A.; Gupta, N. Wound Healing Concepts in Clinical Practice of OMFS. J. Maxillofac. Oral Surg. 2017, 16, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Gacto-Sanchez, P. Surgical treatment and management of the severely burn patient: Review and update. Med. Intensiv. Engl. Ed. 2017, 41, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef]
- Zeng, R.; Lin, C.; Lin, Z.; Chen, H.; Lu, W.; Lin, C.; Li, H. Approaches to cutaneous wound healing: Basics and future directions. Cell Tissue Res. 2018, 374, 217–232. [Google Scholar] [CrossRef]
- Halim, A.S.; Khoo, T.L.; Yussof, S.J.M. Biologic and synthetic skin substitutes: An overview. Indian J. Plast. Surg. 2010, 43, S23–S28. [Google Scholar] [CrossRef]
- Gangwar, A.K.; Kumar, N.; Sharma, A.K.; Devi, S.; Negi, M.; Shrivastava, S.; Mathew, D.D.; Remya, V.; Sonal, S.; Arundeep, P. Bioengineered acellular dermal matrix for the repair of full thickness skin wounds in rats. Trends Biomater. Artif. Organs 2013, 27, 67–80. [Google Scholar]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.-L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef]
- Lamme, E.N.; Van Leeuwen, R.T.; Brandsma, K.; Van Marle, J.; Middelkoop, E. Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2000, 190, 595–603. [Google Scholar] [CrossRef]
- Bo, Q.; Yan, L.; Li, H.; Jia, Z.; Zhan, A.; Chen, J.; Yuan, Z.; Zhang, W.; Gao, B.; Chen, R. Decellularized dermal matrix-based photo-crosslinking hydrogels as a platform for delivery of adipose derived stem cells to accelerate cutaneous wound healing. Mater. Des. 2020, 196, 109152. [Google Scholar] [CrossRef]
- Chen, L.; Ma, J.; Chen, Y.; Huang, C.; Zheng, Z.; Gao, Y.; Jiang, Z.; Wei, X.; Peng, Y.; Yu, S.; et al. Polydopamine modified acellular dermal matrix sponge scaffold loaded with a-FGF: Promoting wound healing of autologous skin grafts. Biomater. Adv. 2022, 136, 212790. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, W.Q.; Wang, T.; Gao, Y.; Wu, J.; Xie, Z.; Zhao, J.; He, C.; Zhu, M.; Zhang, S.; et al. Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Mater. Sci. Eng. C 2021, 127, 112202. [Google Scholar] [CrossRef]
- Gangwar, A.K.; Kumar, N.; Khangembam, S.D.; Kumar, V.; Singh, R. Primary chicken embryo fibroblasts seeded acellular dermal matrix (3-D ADM) improve regeneration of full thickness skin wounds in rats. Tissue Cell 2015, 47, 311–322. [Google Scholar] [CrossRef]
- Huang, S.-P.; Hsu, C.-C.; Chang, S.-C.; Wang, C.-H.; Deng, S.-C.; Dai, N.-T.; Chen, T.-M.; Chan, J.Y.-H.; Chen, S.-G.; Huang, S.-M. Adipose-derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full-thickness defect. Ann. Plast. Surg. 2012, 69, 656–662. [Google Scholar] [CrossRef]
- Kamolz, L.-P.; Keck, M.; Kasper, C. Wharton’s jelly mesenchymal stem cells promote wound healing and tissue regeneration. Stem Cell Res. Ther. 2014, 5, 62. [Google Scholar] [CrossRef]
- Mehrabani, D.; Nazempour, M.; Mehdinavaz-Aghdam, R.; Hashemi, S.-S.; Jalli, R.; Moghadam, M.S.; Zare, S.; Jamhiri, I.; Moayedi, J.; Karimi-Busheri, F. MRI tracking of human Wharton’s jelly stem cells seeded onto acellular dermal matrix labeled with superparamagnetic iron oxide nanoparticles in burn wounds. Burn. Trauma 2022, 10, tkac018. [Google Scholar] [CrossRef]
- Pourfath, M.R.; Behzad-Behbahani, A.; Hashemi, S.S.; Derakhsahnfar, A.; Taheri, M.N.; Salehi, S. Monitoring wound healing of burn in rat model using human Wharton’s jelly mesenchymal stem cells containing cGFP integrated by lentiviral vectors. Iran. J. Basic Med. Sci. 2018, 21, 70–76. [Google Scholar] [CrossRef]
- Huang, Z.; Shen, Y.; Sun, A.; Huang, G.; Zhu, H.; Huang, B.; Xu, J.; Song, Y.; Pei, N.; Ma, J.; et al. Magnetic targeting enhances retrograde cell retention in a rat model of myocardial infarction. Stem Cell Res. Ther. 2013, 4, 149. [Google Scholar] [CrossRef]
- Jehangir, S.; Ramesh, S.; Thomas, M.; Madhuri, V. Wharton’s Jelly Mesenchymal Stem Cells on a Novel Aloe Vera-Polycaprolactone (A-PCL) Composite Scaffold in Burns. Regen. Eng. Transl. Med. 2022, 8, 437–445. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Pourfath, M.R.; Derakhshanfar, A.; Behzad-Behbahani, A.; Moayedi, J. The role of labeled cell therapy with and without scaffold in early excision burn wounds in a rat animal model. Iran. J. Basic Med. Sci. 2020, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, S.; Jarraya, M. Technical note: Comparison of the PrestoBlue and LDH release assays with the MTT assay for skin viability assessment. Cell Tissue Bank. 2015, 16, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Garcés, V.; Molina Aguilar, P.; Bea Serrano, C.; García Bustos, V.; Benavent Seguí, J.; Ferrández Izquierdo, A.; Ruiz-Saurí, A. Age-related dermal collagen changes during development, maturation and ageing—A morphometric and comparative study. J. Anat. 2014, 225, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.N.; Karimizade, A.; Enderami, S.E.; Abasi, M.; Talebpour Amiri, F.; Jafarirad, A.; Mellati, A. The effect of source animal age, decellularization protocol, and sterilization method on bovine acellular dermal matrix as a scaffold for wound healing and skin regeneration. Artif. Organs 2023, 47, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, S.H.; El-Shal, A.S.; Shalaby, S.M.; Abd-Elbary, E.; Mazen, N.F.; Abdel Kader, R.R. The role of placenta-derived mesenchymal stem cells in healing of induced full-thickness skin wound in a mouse model. IUBMB Life 2015, 67, 701–709. [Google Scholar] [CrossRef]
- Hamra, N.F.; Putra, A.; Tjipta, A.; Amalina, N.D.; Nasihun, T. Hypoxia mesenchymal stem cells accelerate wound closure improvement by controlling α-smooth muscle actin expression in the full-thickness animal model. Open Access Maced. J. Med. Sci. 2021, 9, 35–41. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Vander Beken, S.; Wlaschek, M.; et al. TSG-6 Released from Intradermally Injected Mesenchymal Stem Cells Accelerates Wound Healing and Reduces Tissue Fibrosis in Murine Full-Thickness Skin Wounds. J. Investig. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef]
- Jiang, D.; Qi, Y.; Walker, N.G.; Sindrilaru, A.; Hainzl, A.; Wlaschek, M.; MacNeil, S.; Scharffetter-Kochanek, K. The effect of adipose tissue derived MSCs delivered by a chemically defined carrier on full-thickness cutaneous wound healing. Biomaterials 2013, 34, 2501–2515. [Google Scholar] [CrossRef]
- Arno, A.I.; Amini-Nik, S.; Blit, P.H.; Al-Shehab, M.; Belo, C.; Herer, E.; Tien, C.H.; Jeschke, M.G. Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res. Ther. 2014, 5, 28. [Google Scholar] [CrossRef]
- Nazempour, M.; Mehrabani, D.; Mehdinavaz-Aghdam, R.; Hashemi, S.-S.; Derakhshanfar, A.; Zare, S.; Zardosht, M.; Moayedi, J.; Vahedi, M. The effect of allogenic human Wharton’s jelly stem cells seeded onto acellular dermal matrix in healing of rat burn wounds. J. Cosmet. Dermatol. 2020, 19, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Deng, Z.; Han, S.; Liu, T.; Wen, N.; Lu, W.; Geng, X.; Huang, S.; Jin, Y. Tissue-Engineered Skin Containing Mesenchymal Stem Cells Improves Burn Wounds. Artif. Organs 2008, 32, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Rocco, P.R.M. Stem-cell extracellular vesicles and lung repair. Stem Cell Investig. 2017, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, Y.; Hou, Y.; Chai, J.; Duan, H.; Chu, W.; Zhang, H.; Hu, Q.; Du, J. Human Umbilical Cord Mesenchymal Stem Cells Transplantation Promotes Cutaneous Wound Healing of Severe Burned Rats. PLoS ONE 2014, 9, e88348. [Google Scholar] [CrossRef]
- Wang, H.; Yan, X.; Shen, L.; Li, S.; Lin, Y.; Wang, S.; Hou, X.L.; Shi, C.; Yang, Y.; Dai, J.; et al. Acceleration of wound healing in acute full-thickness skin wounds using a collagen-binding peptide with an affinity for MSCs. Burn. Trauma 2014, 2, 181–186. [Google Scholar] [CrossRef]
- Ma, K.; Liao, S.; He, L.; Lu, J.; Ramakrishna, S.; Chan, C.K. Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng. Part A 2011, 17, 1413–1424. [Google Scholar] [CrossRef]
- Khan, I.; Siddiqui, M.N.; Jameel, F.; Qazi, R.-e.-M.; Salim, A.; Aslam, S.; Zaidi, M.B. Potential of stem cell seeded three-dimensional scaffold for regeneration of full-thickness skin wounds. Interface Focus 2022, 12, 20220017. [Google Scholar] [CrossRef]
- Golchin, A.; Nourani, M.R. Effects of bilayer nanofibrillar scaffolds containing epidermal growth factor on full-thickness wound healing. Polym. Adv. Technol. 2020, 31, 2443–2452. [Google Scholar] [CrossRef]
- Biazar, E.; Keshel, S.H.; Sahebalzamani, A.; Hamidi, M.; Ebrahimi, M. The healing effect of unrestricted somatic stem cells loaded in nanofibrous poly hydroxybutyrate-co-hydroxyvalerate scaffold on full-thickness skin defects. J. Biomater. Tissue Eng. 2014, 4, 20–27. [Google Scholar] [CrossRef]
- Carvalho-Júnior, J.d.C.; Zanata, F.; Aloise, A.C.; Ferreira, L.M. Acellular dermal matrix in skin wound healing in rabbits-histological and histomorphometric analyses. Clinics 2021, 76, e2066. [Google Scholar] [CrossRef]
- Yan, W.; Liu, H.; Deng, X.; Jin, Y.; Wang, N.; Chu, J. Acellular dermal matrix scaffolds coated with connective tissue growth factor accelerate diabetic wound healing by increasing fibronectin through PKC signalling pathway. J. Tissue Eng. Regen. Med. 2018, 12, e1461–e1473. [Google Scholar] [CrossRef] [PubMed]
- Carlson, T.L.; Lee, K.W.; Pierce, L.M. Effect of Cross-Linked and Non–Cross-Linked Acellular Dermal Matrices on the Expression of Mediators Involved in Wound Healing and Matrix Remodeling. Plast. Reconstr. Surg. 2013, 131, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Truong, A.T.; Kowal-Vern, A.; Latenser, B.A.; Wiley, D.E.; Walter, R.J. Comparison of dermal substitutes in wound healing utilizing a nude mouse model. J. Burn. Wounds 2005, 4, e4. [Google Scholar]
- Hong, P.; Bezuhly, M.; Graham, M.E.; Gratzer, P.F. Efficient decellularization of rabbit trachea to generate a tissue engineering scaffold biomatrix. Int. J. Pediatr. Otorhinolaryngol. 2018, 112, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.F.; Sheena, Y.; Tarek Saleh, D.M.; Forouhi, P.; Benyon, S.L.; Irwin, M.S.; Malata, C.M. A direct comparison of porcine (Strattice™) and bovine (Surgimend™) acellular dermal matrices in implant-based immediate breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.R.; Lin, Y.N.; Wu, S.H.; Lin, S.D.; Srinivasan, P.; Hsieh, D.J.; Huang, S.H. Supercritical Carbon Dioxide-decellularized Porcine Acellular Dermal Matrix combined with Autologous Adipose-derived Stem Cells: Its Role in Accelerated Diabetic Wound Healing. Int. J. Med. Sci. 2020, 17, 354–367. [Google Scholar] [CrossRef]
- Magden, G.K.; Vural, C.; Bayrak, B.Y.; Ozdogan, C.Y.; Kenar, H. Composite sponges from sheep decellularized small intestinal submucosa for treatment of diabetic wounds. J. Biomater. Appl. 2021, 36, 113–127. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, Y.; Deng, X.; Liu, H.; Pang, H.; Shi, P.; Zhan, Z. Second-harmonic generation microscopy for assessment of mesenchymal stem cell-seeded acellular dermal matrix in wound-healing. Biomaterials 2015, 53, 659–668. [Google Scholar] [CrossRef]
- Yang, H.-y.; Fierro, F.; So, M.; Yoon, D.J.; Nguyen, A.V.; Gallegos, A.; Bagood, M.D.; Rojo-Castro, T.; Alex, A.; Stewart, H.; et al. Combination product of dermal matrix, human mesenchymal stem cells, and timolol promotes diabetic wound healing in mice. Stem Cells Transl. Med. 2020, 9, 1353–1364. [Google Scholar] [CrossRef]
- Orbay, H.; Takami, Y.; Hyakusoku, H.; Mizuno, H. Acellular Dermal Matrix Seeded with Adipose-Derived Stem Cells as a Subcutaneous Implant. Aesthetic Plast. Surg. 2011, 35, 756–763. [Google Scholar] [CrossRef]
- Motamed, S.; Taghiabadi, E.; Molaei, H.; Sodeifi, N.; Hassanpour, S.E.; Shafieyan, S.; Azargashb, E.; Farajzadeh-Vajari, F.; Aghdami, N.; Bajouri, A. Cell-based skin substitutes accelerate regeneration of extensive burn wounds in rats. Am. J. Surg. 2017, 214, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Foubert, P.; Barillas, S.; Gonzalez, A.D.; Alfonso, Z.; Zhao, S.; Hakim, I.; Meschter, C.; Tenenhaus, M.; Fraser, J.K. Uncultured adipose-derived regenerative cells (ADRCs) seeded in collagen scaffold improves dermal regeneration, enhancing early vascularization and structural organization following thermal burns. Burns 2015, 41, 1504–1516. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Accession Number | Sequence | Product Size (bp) |
|---|---|---|---|
| Β-actin | NM_007393.5 | F:5′ CTTCTTGGGTATGGAATCCTG | 96 |
| R:5′ GTGTTGGCATAGAGGTCTTTAC | |||
| VEGFa | NM_001110268.1 | F:5′ TCGCTCCTCCACTTCTGAGG | 73 |
| R: 5′ GGCCATTACCAGGCCTCTTC | |||
| bFGF | NM_008006.2 | F:5′CCGGTCACGGAAATACTCCA | 89 |
| R:5′ CCTTCTGTCCAGGTCCCGTT | |||
| IL-1β | NM_000576 | F:5′ CCACAGACCTTCCAGGAGAATG | 84 |
| R:5′GTGCAGTTCAGTGATCGTACAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, R.N.; Hasanzadeh, E.; Abasi, M.; Gholipourmalekabadi, M.; Mellati, A.; Enderami, S.E. The Effect of Fetal Bovine Acellular Dermal Matrix Seeded with Wharton’s Jelly Mesenchymal Stem Cells for Healing Full-Thickness Skin Wounds. Genes 2023, 14, 909. https://doi.org/10.3390/genes14040909
Mansour RN, Hasanzadeh E, Abasi M, Gholipourmalekabadi M, Mellati A, Enderami SE. The Effect of Fetal Bovine Acellular Dermal Matrix Seeded with Wharton’s Jelly Mesenchymal Stem Cells for Healing Full-Thickness Skin Wounds. Genes. 2023; 14(4):909. https://doi.org/10.3390/genes14040909
Chicago/Turabian StyleMansour, Reyhaneh Nassiri, Elham Hasanzadeh, Mozhgan Abasi, Mazaher Gholipourmalekabadi, Amir Mellati, and Seyed Ehsan Enderami. 2023. "The Effect of Fetal Bovine Acellular Dermal Matrix Seeded with Wharton’s Jelly Mesenchymal Stem Cells for Healing Full-Thickness Skin Wounds" Genes 14, no. 4: 909. https://doi.org/10.3390/genes14040909
APA StyleMansour, R. N., Hasanzadeh, E., Abasi, M., Gholipourmalekabadi, M., Mellati, A., & Enderami, S. E. (2023). The Effect of Fetal Bovine Acellular Dermal Matrix Seeded with Wharton’s Jelly Mesenchymal Stem Cells for Healing Full-Thickness Skin Wounds. Genes, 14(4), 909. https://doi.org/10.3390/genes14040909






