Maternal Immune Activation and Enriched Environments Impact B2 SINE Expression in Stress Sensitive Brain Regions of Rodent Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. Brain Region Extraction
2.3. RNA Isolation
2.4. Reverse Transcription
2.5. Polymerase Chain Reaction
2.6. Statistics
3. Results
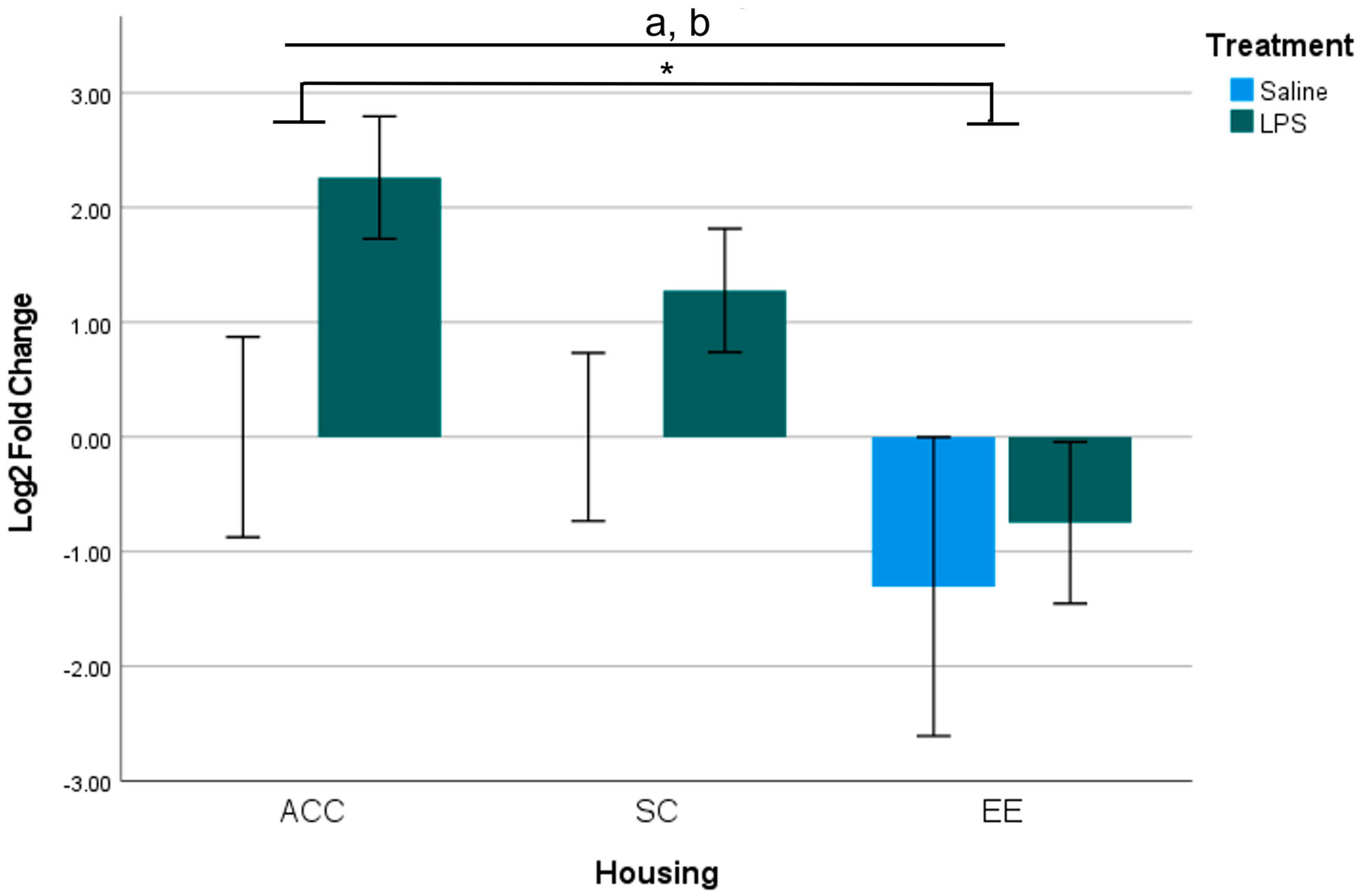
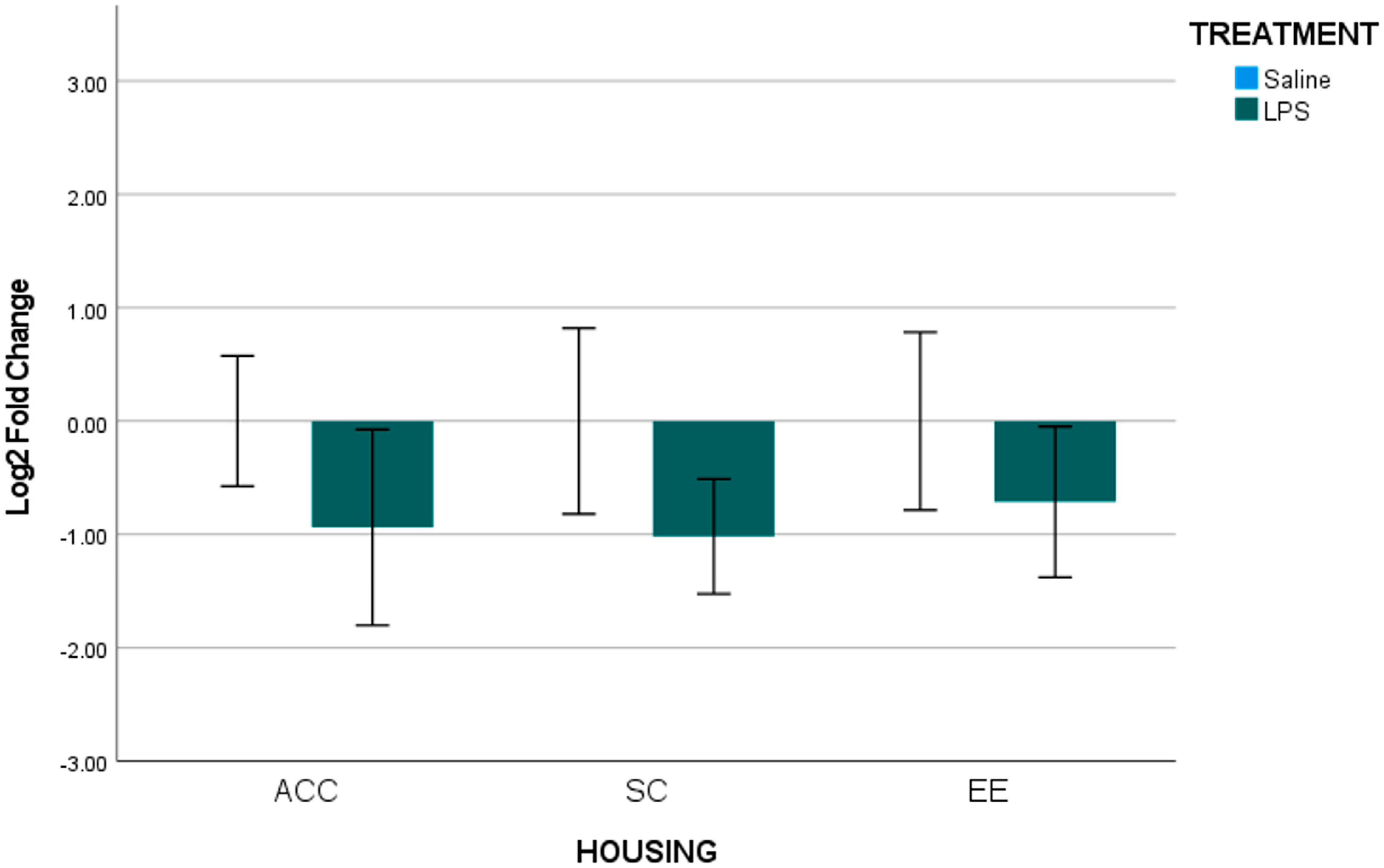
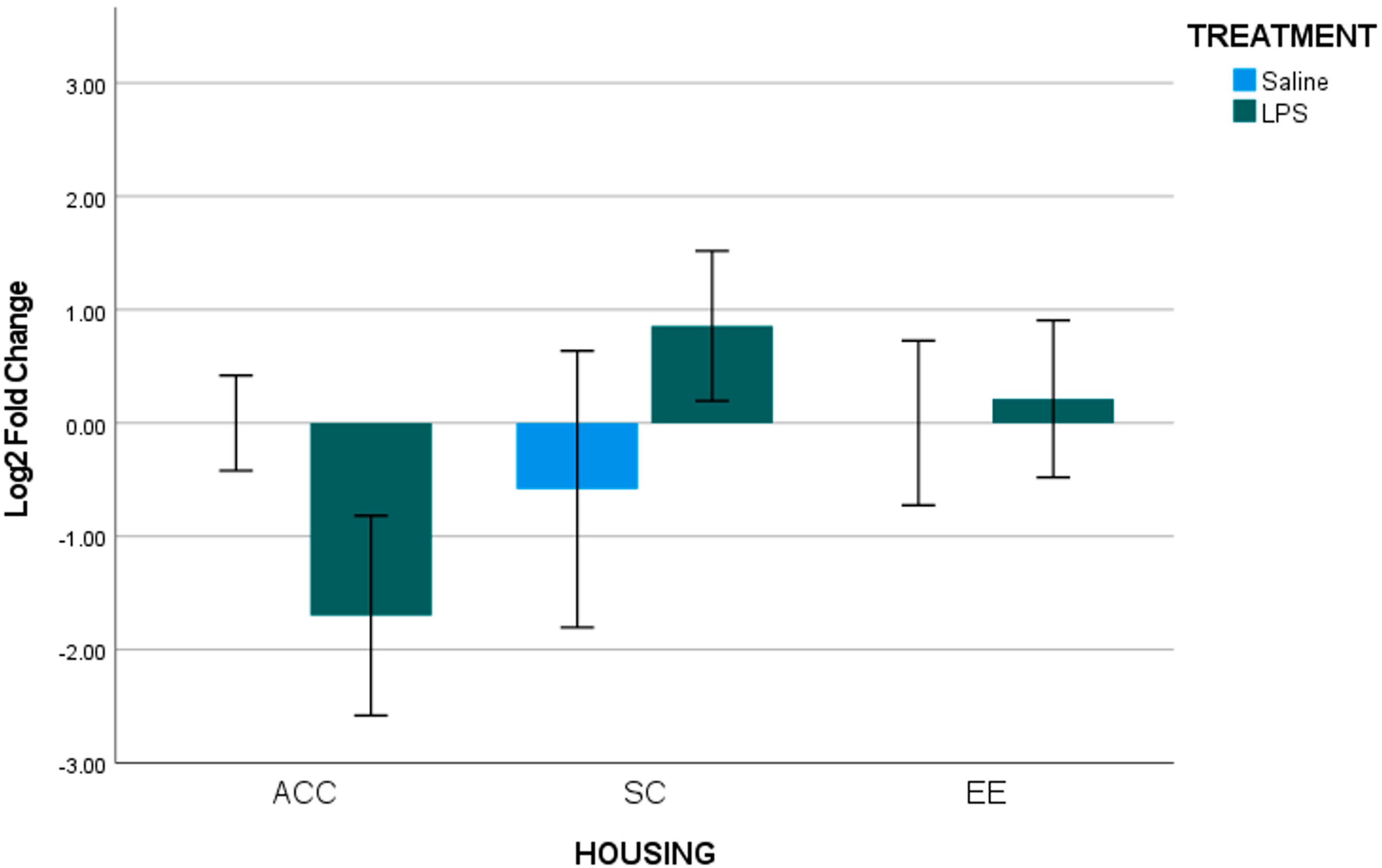
3.1. Analysis for Each Brain Region of Interest
3.2. Summary of Gestational LPS Impacts Offspring B2 Expression
3.3. Summary of Housing Environment on B2 Expression of Offspring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, R.G. Stress, Adaptation, and the Deep Genome: Why Transposons Matter. Integr. Comp. Biol. 2020, 60, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S. So Much “Junk” DNA in Our Genome. Brookhaven Symp. Biol. 1972, 23, 366–370. [Google Scholar] [PubMed]
- Orgel, L.E.; Crick, F.H.C. Selfish DNA: The Ultimate Parasite. Nature 1980, 284, 604–607. [Google Scholar] [CrossRef] [PubMed]
- McClintock, B. Controlling Elements and the Gene. Cold Spring Harb. Symp. Quant. Biol. 1956, 21, 197–216. [Google Scholar] [CrossRef]
- McClintock, B. The Significance of Responses of the Genome to Challenge. Science 1984, 226, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Bundo, M.; Toyoshima, M.; Okada, Y.; Akamatsu, W.; Ueda, J.; Nemoto-Miyauchi, T.; Sunaga, F.; Toritsuka, M.; Ikawa, D.; Kakita, A.; et al. Increased L1 Retrotransposition in the Neuronal Genome in Schizophrenia. Neuron 2014, 81, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Daskalakis, N.P.; Provost, A.C.; Hunter, R.G.; Guffanti, G. Noncoding RNAs: Stress, Glucocorticoids, and Posttraumatic Stress Disorder. Biol. Psychiatry 2018, 83, 849–865. [Google Scholar] [CrossRef] [Green Version]
- DeRosa, H.; Richter, T.; Wilkinson, C.; Hunter, R.G. Bridging the Gap Between Environmental Adversity and Neuropsychiatric Disorders: The Role of Transposable Elements. Front. Genet. 2022, 13, 813510. [Google Scholar] [CrossRef]
- Hunter, R.G.; McEwen, B.S.; Pfaff, D.W. Environmental Stress and Transposon Transcription in the Mammalian Brain. Mob. Genet. Elem. 2013, 3, e24555. [Google Scholar] [CrossRef] [Green Version]
- Lapp, H.E.; Hunter, R.G. Early Life Exposures, Neurodevelopmental Disorders, and Transposable Elements. Neurobiol. Stress 2019, 11, 100174. [Google Scholar] [CrossRef]
- Ponomarev, I.; Rau, V.; Eger, E.I.; Harris, R.A.; Fanselow, M.S. Amygdala Transcriptome and Cellular Mechanisms Underlying Stress-Enhanced Fear Learning in a Rat Model of Posttraumatic Stress Disorder. Neuropsychopharmacol 2010, 35, 1402–1411. [Google Scholar] [CrossRef] [Green Version]
- Ponomarev, I.; Wang, S.; Zhang, L.; Harris, R.A.; Mayfield, R.D. Gene Coexpression Networks in Human Brain Identify Epigenetic Modifications in Alcohol Dependence. J. Neurosci. 2012, 32, 1884–1897. [Google Scholar] [CrossRef] [Green Version]
- Reilly, M.T.; Faulkner, G.J.; Dubnau, J.; Ponomarev, I.; Gage, F.H. The Role of Transposable Elements in Health and Diseases of the Central Nervous System. J. Neurosci. 2013, 33, 17577–17586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, T.A.; Hunter, R.G. Chapter 19—Epigenetics in Posttraumatic Stress Disorder. In Epigenetics in Psychiatry, 2nd ed.; Peedicayil, J., Grayson, D.R., Avramopoulos, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 429–450. ISBN 978-0-12-823577-5. [Google Scholar]
- Rusiecki, J.A.; Chen, L.; Srikantan, V.; Zhang, L.; Yan, L.; Polin, M.L.; Baccarelli, A. DNA Methylation in Repetitive Elements and Post-Traumatic Stress Disorder: A Case–Control Study of US Military Service Members. Epigenomics 2012, 4, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estecio, M.R.H.; Gallegos, J.; Dekmezian, M.; Lu, Y.; Liang, S.; Issa, J.-P.J. SINE Retrotransposons Cause Epigenetic Reprogramming of Adjacent Gene Promoters. Mol. Cancer Res. 2012, 10, 1332–1342. [Google Scholar] [CrossRef] [Green Version]
- Ichiyanagi, K. Epigenetic Regulation of Transcription and Possible Functions of Mammalian Short Interspersed Elements, SINEs. Genes Genet. Syst. 2013, 88, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulkner, G.J.; Kimura, Y.; Daub, C.O.; Wani, S.; Plessy, C.; Irvine, K.M.; Schroder, K.; Cloonan, N.; Steptoe, A.L.; Lassmann, T.; et al. The Regulated Retrotransposon Transcriptome of Mammalian Cells. Nat. Genet. 2009, 41, 563–571. [Google Scholar] [CrossRef]
- Lunyak, V.V.; Prefontaine, G.G.; Nunez, E.; Cramer, T.; Ju, B.-G.; Ohgi, K.A.; Hutt, K.; Roy, R.; Garcia-Diaz, A.; Zhu, X.; et al. Developmentally Regulated Activation of a SINE B2 Repeat as a Domain Boundary in Organogenesis. Science 2007, 317, 248–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, T.; Nishihara, H.; Hirakawa, M.; Fujimura, K.; Tanaka, M.; Kokubo, N.; Kimura-Yoshida, C.; Matsuo, I.; Sumiyama, K.; Saitou, N.; et al. Possible Involvement of SINEs in Mammalian-Specific Brain Formation. Proc. Natl. Acad. Sci. USA 2008, 105, 4220–4225. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.A.; Von Kaenel, S.; Goodrich, J.A.; Kugel, J.F. The SINE-Encoded Mouse B2 RNA Represses MRNA Transcription in Response to Heat Shock. Nat. Struct. Mol. Biol. 2004, 11, 816–821. [Google Scholar] [CrossRef]
- Mariner, P.D.; Walters, R.D.; Espinoza, C.A.; Drullinger, L.F.; Wagner, S.D.; Kugel, J.F.; Goodrich, J.A. Human Alu RNA Is a Modular Transacting Repressor of MRNA Transcription during Heat Shock. Mol. Cell 2008, 29, 499–509. [Google Scholar] [CrossRef]
- Hunter, R.G.; Murakami, G.; Dewell, S.; Seligsohn, M.; Baker, M.E.R.; Datson, N.A.; McEwen, B.S.; Pfaff, D.W. Acute Stress and Hippocampal Histone H3 Lysine 9 Trimethylation, a Retrotransposon Silencing Response. Proc. Natl. Acad. Sci. USA 2012, 109, 17657–17662. [Google Scholar] [CrossRef] [Green Version]
- Dyrvig, M.; Gøtzsche, C.R.; Woldbye, D.P.D.; Lichota, J. Epigenetic Regulation of Dnmt3a and Arc Gene Expression after Electroconvulsive Stimulation in the Rat. Mol. Cell. Neurosci. 2015, 67, 137–143. [Google Scholar] [CrossRef]
- Monteggia, L.M.; Zarate, C. Antidepressant Actions of Ketamine: From Molecular Mechanisms to Clinical Practice. Curr. Opin. Neurobiol. 2015, 30, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, A.A.; Lapp, H.E.; Hunter, R.G. Epigenetic Mechanisms of the Glucocorticoid Receptor. Trends Endocrinol. Metab. 2019, 30, 807–818. [Google Scholar] [CrossRef]
- Bartlett, A.A.; Guffanti, G.; Hunter, R.G. B2 SINE RNA as a Novel Regulator of Glucocorticoid Receptor Transcriptional Activity. Neurobiol. Stress 2023, 23, 100522. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.A.; DeRosa, H.; Clark, M.; Lapp, H.E.; Guffanti, G.; Hunter, R.G. Corticosterone Dynamically Regulates Retrotransposable Element Expression in the Rat Hippocampus and C6 Cells. Neurobiol. Stress 2021, 15, 100397. [Google Scholar] [CrossRef]
- Gitau, R.; Fisk, N.M.; Glover, V. Maternal Stress in Pregnancy and Its Effect on the Human Fetus: An Overview of Research Findings. Stress 2001, 4, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Binder, E.B. Current Research Trends in Early Life Stress and Depression: Review of Human Studies on Sensitive Periods, Gene–Environment Interactions, and Epigenetics. Exp. Neurol. 2012, 233, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Nemeroff, C.B. The Role of Childhood Trauma in the Neurobiology of Mood and Anxiety Disorders: Preclinical and Clincal Studies. Biol. Psychiatry 2001, 49, 1023–1039. [Google Scholar] [CrossRef] [Green Version]
- Meijer, A. Child Psychiatric Sequelae of Maternal War Stress. Acata Psychiatr. Scand. 1985, 72, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, F.; Molteni, R.; Racagni, G.; Rivarola, M.A. Stress during Development: Impact on Neuroplasticity and Relevance to Psychopathology. Prog. Neurobiol. 2007, 81, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, M.K.; Hamelmann, E.; Arck, P.C. Mothers in Stress: Consequences for the Offspring. Am. J. Reprod. Immunol. 2005, 54, 63–69. [Google Scholar] [CrossRef]
- Kofman, O. The Role of Prenatal Stress in the Etiology of Developmental Behavioural Disorders. Neurosci. Biobehav. Rev. 2002, 26, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M. The Long-Term Behavioural Consequences of Prenatal Stress. Neurosci. Behav. Rev. 2008, 32, 1073–1086. [Google Scholar] [CrossRef]
- Bowman, R.E.; Maclusky, N.J.; Sarmiento, Y.; Frankfurt, M.; Gordon, M.; Luiene, V.N. Sexually Dimorphic Effects of Prenatal Stress on Cognition. Hormonal Responses, and Central Neurotransmitters. Endocrinology 2004, 145, 3778–3787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, H.N.; Zorilla, E.P.; Mandyam, C.; River, C.L. Exposure to Repetitive versus Varied Stress during Prenatal Development Generates Two Distinct Anxiogenic and Neuroendocrine Profiles in Adulthood. Endocrinology 2006, 147, 2506–2517. [Google Scholar] [CrossRef] [Green Version]
- Weinstock, M. The Potential Influence of Maternal Stress Hormones on Development and Mental Health of the Offspring. Brain Behav. Immun. 2005, 19, 296–308. [Google Scholar] [CrossRef]
- Berger, M.A.; Barros, V.G.; Sarchi, M.I.; Tarazi, F.I.; Antonelli, M.C. Long-Term Effects of Prenatal Stress on Dopamine and Glutamate Receptors in Adult Rat Brain. Neurochem. Res. 2002, 27, 1525–1533. [Google Scholar] [CrossRef]
- Koeniga, J.I.; Elmera, G.I.; Sheparda, P.D.; Leeb, P.R.; Mayoa, C.; Joya, B.; Herchera, E.; Bradya, D.L. Prenatal Exposure to a Repeated Variable Stress Paradigm Elicits Behavioral and Neuroendocrinological Changes in the Adult Offspring: Potential Relevance to Schizophrenia. Behav. Brain Res. 2005, 156, 251–561. [Google Scholar] [CrossRef]
- Newport, D.J.; Stowe, Z.S.; Nemeroff, C. Parental Depression: Animal Models of an Adverse Life Event. Am. J. Psychiatry 2002, 159, 1265–1283. [Google Scholar] [CrossRef]
- Van den Hove, D.L.; Blanco, C.E.; Aendekerk, B.; Desbonnet, L.; Bruschettini, M.; Steinbusch, H.P.; Prickaerts, J.; Steinbusch, H.W. Prenatal Restraint Stress and Long-Term Affective Consequences. Dev. Neurosci. 2005, 27, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Maganga-Bakita, I.; Aiken, A.A.; Puracchio, M.J.; Kentner, A.C.; Hunter, R.G. Regulatory Effects of Maternal Immune Activation and Environmental Enrichment on Glucocorticoid Receptor and FKBP5 Expression in Stress-Sensitive Regions of the Offspring Brain. Neuroscience 2022, 505, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Connors, E.J.; Shaik, A.N.; Migliore, M.M.; Kentner, A.C. Environmental Enrichment Mitigates the Sex-Specific Effects of Gestational Inflammation on Social Engagement and the Hypothalamic Pituitary Adrenal Axis-Feedback System. Brain Behav. Immun. 2014, 42, 178–190. [Google Scholar] [CrossRef]
- Meyer, U.; Yee, B.K.; Feldon, J. The Developmental Impact of Prenatal Infections at Different Times of Pregnancy: The Earlier the Worse? Neuroscientist 2007, 13, 241–256. [Google Scholar] [CrossRef]
- Patterson, P.H. Immune Involvement in Schizophrenia and Autism: Etiology, Pathology, and Animal Models. Behav. Brain Res. 2009, 204, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.; Tran, N.; Fung, J.N.; Summers, B.L.; Rofe, A.M. Maternal Dietary Zinc Supplementation Prevents Aberrant Behaviour in an Object Recognition Task in Mice Offspring Exposed to LPS in Early Pregnancy. Behav. Brain Res. 2009, 197, 210–218. [Google Scholar] [CrossRef]
- Graciarena, M.; Depino, A.M.; Pitossi, F.J. Prenatal Inflammation Impairs Adult Neurogenesis and Memory Related Behavior through Persistent Hippocampal TGFb1 Downregulation. Brain Behav. Immun. 2010, 24, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Golan, H.M.; Lev, V.; Hallak, M.; Sorokin, Y.; Huleihel, M. Specific Neurodevelopmental Damage in Mice Offspring Following Maternal Inflammation during Pregnancy. Neuropharmacology 2005, 48, 903–917. [Google Scholar] [CrossRef]
- Howland, J.G.; Cazakoff, B.N.; Zhang, Y. Altered Object-in-Place Recognition Memory, Prepulse Inhibition, and Locomotor Activity in the Offspring of Rats Exposed to a Viral Mimetic during Pregnancy. Neuroscience 2012, 201, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Bitanihirwe, B.K.; Peleg-Raibstein, D.; Mouttet, F.; Feldon, J.; Meyer, U. Late Prenatal Immune Activation in Mice Leads to Behavioral and Neurochemical Abnormalities Relevant to the Negative Symptoms of Schizophrenia. Neuropsychopharmacology 2010, 35, 2462–2478. [Google Scholar] [CrossRef] [Green Version]
- Hava, G.; Vered, L.; Yael, M.; Mordechai, H.; Mahoud, H. Alterations in Behavior in Adult Offspring Mice Following Maternal Inflammation during Pregnancy. Devlopmental Psychobiol. 2006, 48, 162–168. [Google Scholar] [CrossRef]
- Malkova, N.V.; Yu, C.Z.; Hsiao, E.Y.; Moore, M.J.; Patterson, P.H. Maternal Immune Activation Yields Offspring Displaying Mouse Versions of the Three Core Symptoms of Autism. Brain Behav. Immun. 2012, 26, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Coyle, J.; Tsai, T.; Goff, D. Converging Evidence of NMDA Receptor Hypofunction in the Pathophysiology of Schizophrenia. Ann. N. Y. Acad. Sci. 2003, 1003, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Engler, A.; Weber, L.; Schedlowski, M.; Feldon, J. Preliminary Evidence for a Modulation of Fetal Dopaminergic Development by Maternal Immune Activation during Pregnancy. Neuroscience 2008, 154, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Laviola, G. On Mouse Pups and Their Lactating Dams: Behavioral Consequences of Prenatal Exposure to Oxazepam and Interacting Factors. Pharmacol. Biochem. Behav. 1996, 55, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Laviola, G.; Terranova, M.L. The Developmental Psychobiology of Behavioral Plasticity: The Role of Social Experiences in the Family Unit. Neurosci. Biobehav. Rev. 1998, 23, 197–213. [Google Scholar] [CrossRef]
- Insel, T.R. Rethinking Schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.L.; Huang, K.P.; Wu, J.; Boucheron, C. Environmental Enrichment Enhances Neurogranin Expression and Hippocampal Learning and Memory but Fails to Rescue the Impairments of Neurogranin Null Mutant Mice. J. Neurosci. 2006, 26, 6230–6237. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Paizanis, E.; Natkunarajah, S.; Freret, T.; Boulouard, M.; Schumann-Bard, P. Environmental Enrichment Enhances Episodic-like Memory in Association with a Modified Neuronal Activation Profile in Adult Mice. PLoS ONE 2012, 7, e48043. [Google Scholar] [CrossRef]
- Morley-Fletcher, S.; Rea, M.; Maccari, S.; Laviola, G. Environmental Enrichment during Adolescence Reverses the Effects of Prenatal Stress on Play Behaviour and HPA Axis Reactivity in Rats. Eur. J. Neurosci. 2003, 18, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Turczak, J.; Przewlocki, R. Environmental Enrichment Reverses Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Issues for a Therapeutic Approach in Autism. Neuropsychopharmacology 2006, 31, 36–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kentner, A. Neuroprotection and Recovery from Early-Life Adversity: Considerations for Environmental Enrichment. Neural Regen. Res. 2015, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, M.; Ding, S.; Li, C.; Luo, X. Environmental Enrichment during Gestation Improves Behavioral Consequences and Synaptic Plasticity in Hippocampus of Prenatal-Stressed Offspring Rats. Acta Histochem. Cytochem. 2012, 45, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mychasiuk, R.; Muhammad, A.; Kolb, B. Environmental Enrichment Alters Structural Plasticity of the Adolescent Brain but Does Not Remediate the Effects of Prenatal Nicotine Exposure. Synapse 2014, 68, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Vivinetto, A.L.; Suarez, M.M.; Rivarola, M.A. Neurobiological Effects of Neonatal Maternal Separation and Post-Weaning Environmental Enrichment. Behav. Brain Res. 2013, 240, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.; Merali, Z.; Harrison, C. Therapeutic and Protective Effect of Environmental Enrichment against Psychogenic and Neurogenic Stress. Behav. Brain Res. 2006, 175, 1–8. [Google Scholar] [CrossRef]
- Hebb, D.O. The Effects of Early Experience on Problem-Solving at Maturity. Am. Psychol. 1947, 2, 306–307. [Google Scholar]
- Kramer, A.F.; Bherer, L.; Colcombe, S.J.; Dong, W.; Grennough, W.T. Environmental Influences on Cognitive and Brain Plasticity during Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59A, 940–957. [Google Scholar] [CrossRef] [Green Version]
- Rosenzweig, M.R.; Bennett, E.L. Psychobiology of Plasticity: Effects of Training and Experience on Brain and Behavior. Behav. Brain Res. 1996, 78, 57–65. [Google Scholar] [CrossRef]
- van Praag, H.; Kempermann, G.; Gage, F.H. Neural Consequences of Environmental Enrichment. Nat. Rev. Neurosci. 2000, 1, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Zocher, S.; Overall, R.W.; Lesche, M.; Dahl, A.; Kempermann, G. Epigenetic Rejuvenation of the Hippocampus by Environmental Enrichment. bioRxiv 2019, 776310. [Google Scholar] [CrossRef]
- Khazipov, R.; Zaynutdinova, D.; Ogievetsky, E.; Valeeva, G.; Mitrukhina, O.; Manent, J.-B.; Represa, A. Atlas of the Postnatal Rat Brain in Stereotaxic Coordinates. Front. Neuroanat. 2015, 9, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, K.; Hunter, R.G.; Bartlett, A.A.; Lapp, H.E.; Kent, M. In Search of Optimal Resilience Ratios: Differential Influences of Neurobehavioral Factors Contributing to Stress-Resilience Spectra. Front. Neuroendocrinol. 2020, 56, 100802. [Google Scholar] [CrossRef]
- McKlveen, J.M.; Moloney, R.D.; Scheimann, J.R.; Myers, B.; Herman, J.P. “Braking” the Prefrontal Cortex: The Role of Glucocorticoids and Interneurons in Stress Adaptation and Pathology. Biol. Psychiatry 2019, 86, 669–681. [Google Scholar] [CrossRef]
- McKlveen, J.M.; Myers, B.; Flak, J.; Bundzikova, J.; Solomon, M.; Seroogy, K. Role of Prefrontal Cortex Glucocorticoid Receptors in Stress and Emotion. Biol. Psychiatry 2013, 74, 672–679. [Google Scholar] [CrossRef] [Green Version]
- McKlveen, J.M.; Myers, B.; Herman, J. The Medial Prefrontal Cortex: Coordinator of Autonomic, Neuroendocrine and Behavioural Responses to Stress. J. Neuroendocrinol. 2015, 27, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Schwabe, L. Stress and the Engagement of Multiple Memory Systems: Integration of Animal and Human Studies: Engagement of Multiple Memory Systems after Stress. Hippocampus 2013, 23, 1035–1043. [Google Scholar] [CrossRef]
- McClure, W.O.; Ishtoyana, A.; Lyon, M. Very Mild Stress of Pregnant Rats Reduces Volume and Cell Number in Nucleus Accumbens of Adult Offspring: Some Parallels to Schizophrenia. Dev. Brain Res. 2004, 149, 21–28. [Google Scholar] [CrossRef]
- Myers, B.; Mark-Dolgas, C.; Kasckow, J.; Cullinan, W.; Herman, J. Central Stress-Integrative Circuits: Forebrain Glutamatergic and GABAergic Projections to the Dorsomedial Hypothalamus, Medial Preoptic Area, and Bed Nucleus of the Stria Terminalis. Brain Struct. Funct. 2014, 219, 1287–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannerman, D.M.; Rawlins, J.N.; McHugh, S.B.; Deacon, R.M.; Yee, B.K.; Bast, T. Regional Dissociations within the Hippocampus—Memory and Anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.A. Barnes CA. Spatial Cognition and Functional Alterations of Aged Rat Hippocampus. Handb. Aged Brain 1998, 1998, 51–56. [Google Scholar]
- Eichenbaum, H. How Does the Hippocampus Contribute Tomemory? Trends Cogn. Sci. 2003, 7, 427–429. [Google Scholar] [CrossRef]
- Eichenbaum, H. Hippocampus: Cognitive Processes and Neural Representations That Underlie Declarative Memory. Neuron 2004, 44, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Herman, J.P.; McKlveen, J.M.; Solomon, M.B.; Carvalho-Netto, E.; Myers, B. Neural Regulation of the Stress Response: Glucocorticoid Feedback Mechanisms. Braz. J. Med. Biol. Res. 2012, 45, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesner, R.P. Role of the Hippocampus in Mediating Interference as Measured by Pattern Separation Processes. Behav. Process. 2013, 93, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Potvin, O.; Dore, F.Y.; Goulet, S. Contributions of the Dorsal Hippocampus and the Dorsal Subiculum to Processing of Idiothetic Information and Spatial Memory. Neurobiol. Learn. Mem. 2007, 87, 669–678. [Google Scholar] [CrossRef]
- Tracy, A.L.; Jarrard, L.E.; Davidson, T.L. The Hippocampus Andmotivation Revisited: Appetite and Activity. Behav. Brain Res. 2001, 127, 13–23. [Google Scholar] [CrossRef]
- Bodea, G.O.; McKelvey, E.G.Z.; Faulkner, G.J. Retrotransposon-Induced Mosaicism in the Neural Genome. Open Biol. 2018, 8, 180074. [Google Scholar] [CrossRef] [Green Version]
- Adzic, M.; Djordjevic, A.; Demonacos, C.; Krstic-Demonacos, M.; Radojcic, M.B. The Role of Phosphorylated Glucocorticoid Receptor in Mitochondrial Functions and Apoptotic Signalling in Brain Tissue of Stressed Wistar Rats. Int. J. Biochem. Cell Biol. 2009, 41, 2181–2188. [Google Scholar] [CrossRef]
- Aneichyk, T.; Hendriks, W.T.; Yadav, R.; Shin, D.; Gao, D.; Vaine, C.A.; Collins, R.L.; Domingo, A.; Currall, B.; Stortchevoi, A.; et al. Dissecting the Causal Mechanism of X-Linked Dystonia-Parkinsonism by Integrating Genome and Transcriptome Assembly. Cell 2018, 172, 897–909.e21. [Google Scholar] [CrossRef] [Green Version]
- Bragg, D.C.; Mangkalaphiban, K.; Vaine, C.A.; Kulkarni, N.J.; Shin, D.; Yadav, R.; Dhakal, J.; Ton, M.-L.; Cheng, A.; Russo, C.T.; et al. Disease Onset in X-Linked Dystonia-Parkinsonism Correlates with Expansion of a Hexameric Repeat within an SVA Retrotransposon in TAF1. Proc. Natl. Acad. Sci. USA 2017, 114, E11020–E11028. [Google Scholar] [CrossRef] [Green Version]
- Han, J.S.; Szak, S.T.; Boeke, J.D. Transcriptional Disruption by the L1 Retrotransposon and Implications for Mammalian Transcriptomes. Nature 2004, 429, 268–274. [Google Scholar] [CrossRef]
- Kazazian, H.H.; Wong, C.; Youssoufian, H.; Scott, A.F.; Phillips, D.G.; Antonarakis, S.E. Haemophilia A Resulting from de Novo Insertion of L1 Sequences Represents a Novel Mechanism for Mutation in Man. Nature 1988, 332, 164–166. [Google Scholar] [CrossRef]
- Sassaman, D.M.; Dombroski, B.A.; Moran, J.V.; Kimberland, M.L.; Naas, T.P.; DeBerardinis, R.J.; Gabriel, A.; Swergold, G.D.; Kazazian, H.H. Many Human L1 Elements Are Capable of Retrotransposition. Nat. Genet. 1997, 16, 37–43. [Google Scholar] [CrossRef]
- Shukla, R.; Upton, K.R.; Muñoz-Lopez, M.; Gerhardt, D.J.; Fisher, M.E.; Nguyen, T.; Brennan, P.M.; Baillie, J.K.; Collino, A.; Ghisletti, S.; et al. Endogenous Retrotransposition Activates Oncogenic Pathways in Hepatocellular Carcinoma. Cell 2013, 153, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of Stress in the Brain. Nat. Neurosci. 2015, 18, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.A.; Hunnicutt, K.E.; Larsen, R.J.; Yoder, A.D.; Saunders, A.M. Warning SINEs: Alu Elements, Evolution of the Human Brain, and the Spectrum of Neurological Disease. Chromosome Res. 2018, 26, 93–111. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Sharma, D.; Upadhyaya, K.C. L1 Retrotransposons Are Transcriptionally Active in Hippocampus of Rat Brain. Prague Med. Rep. 2016, 117, 42–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbarbary, R.A.; Lucas, B.A.; Maquat, L.E. Retrotransposons as Regulators of Gene Expression. Science 2016, 351, aac7247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrader, L.; Schmitz, J. The Impact of Transposable Elements in Adaptive Evolution. Mol. Ecol. 2019, 28, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- von Borell, C.J.; Weiss, A.; Penke, L. Developing Individual Differences in Primate Behavior: The Role of Genes, Environment, and Their Interplay. Behav. Ecol. Sociobiol. 2019, 73, 20. [Google Scholar] [CrossRef] [Green Version]
- André, V.; Gau, C.; Scheideler, A.; Aguilar-Pimentel, J.A.; Amarie, O.V.; Becker, L.; Garrett, L.; Hans, W.; Hölter, S.M.; Janik, D.; et al. Laboratory Mouse Housing Conditions Can Be Improved Using Common Environmental Enrichment without Compromising Data. PLoS Biol. 2018, 16, e2005019. [Google Scholar] [CrossRef] [Green Version]
- Dirven, B.C.J.; Homberg, J.R.; Kozicz, T.; Henckens, M.J.A.G. Epigenetic Programming of the Neuroendocrine Stress Response by Adult Life Stress. J. Mol. Endocrinol. 2017, 59, R11–R31. [Google Scholar] [CrossRef] [Green Version]
- Elliott, B.; Grunberg, N. Effects of Social and Physical Enrichment on Open Field Activity Differ in Male and Female Sprague–Dawley Rats. Behav. Brain Res. 2005, 165, 187–196. [Google Scholar] [CrossRef]
- D’Andrea, I.; Gracci, F.; Alleva, E.; Branchi, I. Early Social Enrichment Provided by Communal Nest Increases Resilience to Depression-like Behavior More in Female than in Male Mice. Behav. Brain Res. 2010, 215, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, E.; Arpino, C.; Matteucci, C.; Sorrentino, R.; Pica, F.; Alessandrelli, R.; Coniglio, A.; Curatolo, P.; Rezza, G.; Macciardi, F.; et al. HERVs Expression in Autism Spectrum Disorders. PLoS ONE 2012, 7, e48831. [Google Scholar] [CrossRef] [Green Version]
- Doyle, G.A.; Doucet-O’Hare, T.T.; Hammond, M.J.; Crist, R.C.; Ewing, A.D.; Ferraro, T.N.; Mash, D.C.; Kazazian, H.H.; Berrettini, W.H. Reading LINEs within the Cocaine Addicted Brain. Brain Behav. 2017, 7, e00678. [Google Scholar] [CrossRef]
- Liu, S.; Du, T.; Liu, Z.; Shen, Y.; Xiu, J.; Xu, Q. Inverse Changes in L1 Retrotransposons between Blood and Brain in Major Depressive Disorder. Sci. Rep. 2016, 6, 37530. [Google Scholar] [CrossRef] [PubMed]
- Shpyleva, S.; Melnyk, S.; Pavliv, O.; Pogribny, I.; Jill James, S. Overexpression of LINE-1 Retrotransposons in Autism Brain. Mol. Neurobiol. 2018, 55, 1740–1749. [Google Scholar] [CrossRef]
- Weis, S.; Llenos, I.C.; Sabunciyan, S.; Dulay, J.R.; Isler, L.; Yolken, R.; Perron, H. Reduced Expression of Human Endogenous Retrovirus (HERV)-W GAG Protein in the Cingulate Gyrus and Hippocampus in Schizophrenia, Bipolar Disorder, and Depression. J. Neural Transm. 2007, 114, 645–655. [Google Scholar] [CrossRef] [PubMed]



| Housing Condition | N | Sex |
|---|---|---|
| Enriched Environment (EE) | 28 | 13 Females and 15 Males |
| Social Control (SC) | 27 | 12 Females and 15 Males |
| Animal Care Control (ACC) | 23 | 13 Females and 10 Males |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, T.A.; Aiken, A.A.; Puracchio, M.J.; Maganga-Bakita, I.; Hunter, R.G. Maternal Immune Activation and Enriched Environments Impact B2 SINE Expression in Stress Sensitive Brain Regions of Rodent Offspring. Genes 2023, 14, 858. https://doi.org/10.3390/genes14040858
Richter TA, Aiken AA, Puracchio MJ, Maganga-Bakita I, Hunter RG. Maternal Immune Activation and Enriched Environments Impact B2 SINE Expression in Stress Sensitive Brain Regions of Rodent Offspring. Genes. 2023; 14(4):858. https://doi.org/10.3390/genes14040858
Chicago/Turabian StyleRichter, Troy A., Ariel A. Aiken, Madeline J. Puracchio, Ismael Maganga-Bakita, and Richard G. Hunter. 2023. "Maternal Immune Activation and Enriched Environments Impact B2 SINE Expression in Stress Sensitive Brain Regions of Rodent Offspring" Genes 14, no. 4: 858. https://doi.org/10.3390/genes14040858
APA StyleRichter, T. A., Aiken, A. A., Puracchio, M. J., Maganga-Bakita, I., & Hunter, R. G. (2023). Maternal Immune Activation and Enriched Environments Impact B2 SINE Expression in Stress Sensitive Brain Regions of Rodent Offspring. Genes, 14(4), 858. https://doi.org/10.3390/genes14040858







