Expanding the Chromosomal Evolution Understanding of Lygaeioid True Bugs (Lygaeoidea, Pentatomomorpha, Heteroptera) by Classical and Molecular Cytogenetic Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Family Rhyparochromidae
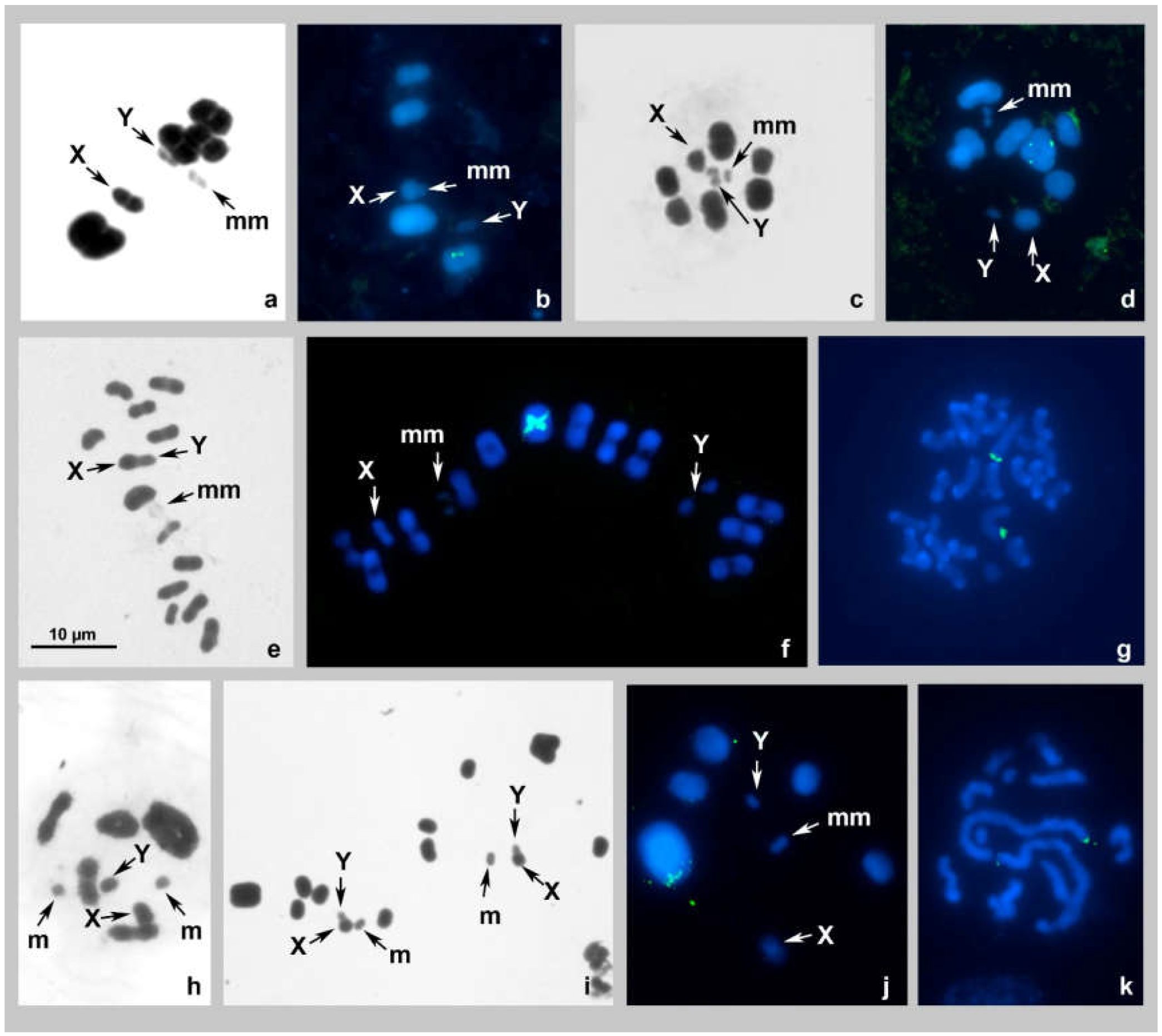
Elasmolomus squalidus, 2n = 12(8A + 2m + XY) (Figure 1a,b)
3.2. Family Heterogastridae
3.2.1. Nerthus sp. 1, 2n = 16(12A + 2m + XY) (Figure 1c)
3.2.2. Nerthus sp. 2, 2n = 18(14A + 2m + XY) (Figure 1d)
3.3. Family Cymidae
Cymus claviculus, 2n = 28(24A + 2m + XY) (Figure 1e–g)
3.4. Family Blissidae
Dimorphopterus spinolae 2n = 14(10A + 2m + XY) (Figure 1h–k)
3.5. Family Lygaeidae
3.5.1. Subfamily Ischnorhynchinae
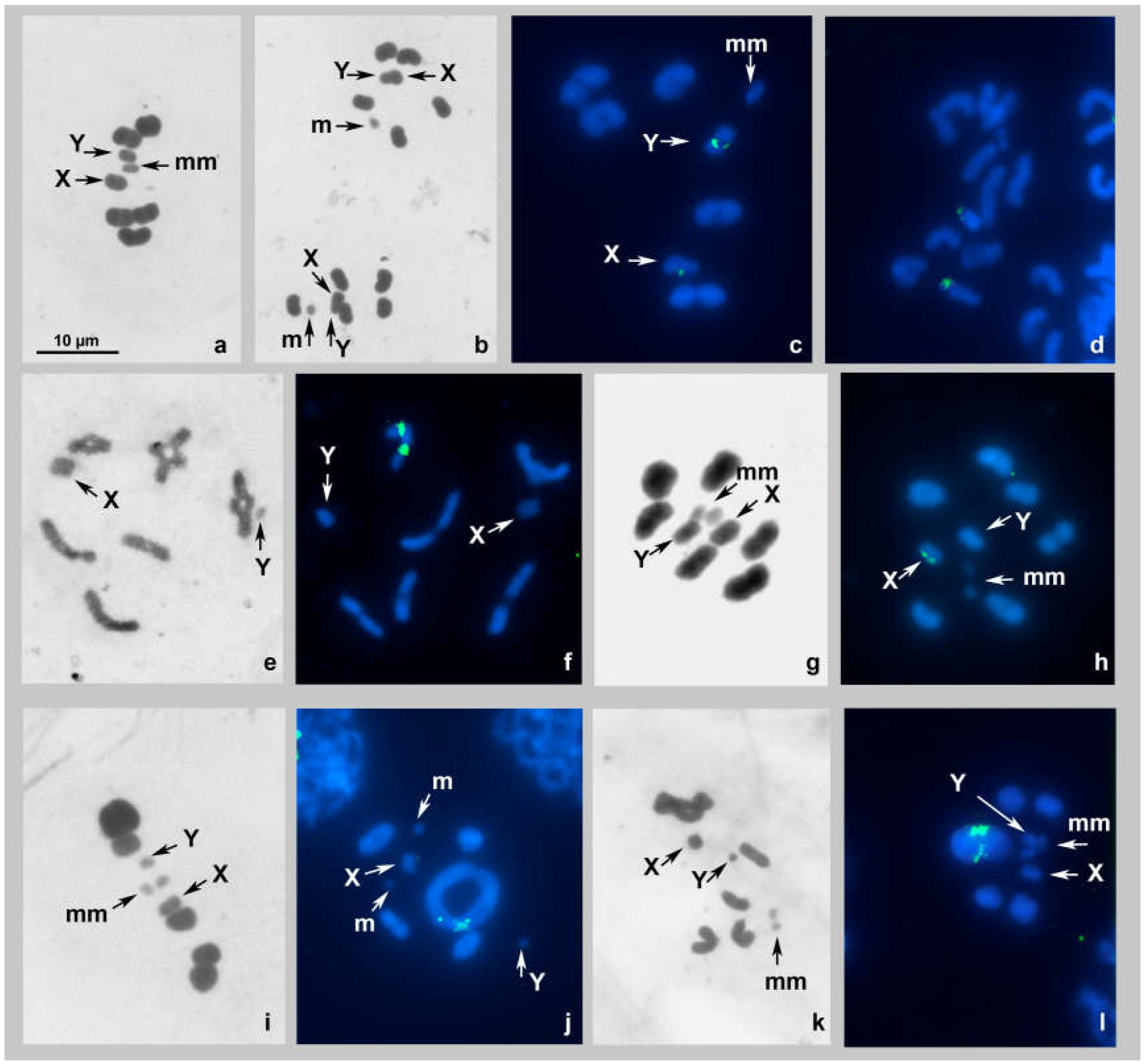
Kleidocerys resedae, 2n = 14(10A + 2m + XY) (Figure 2a–d)
3.5.2. Subfamily Lygaeinae
Spilostethus saxatilis 2n = 14(12A + XY) (Figure 2e,f)
Thunbergia floridulus 2n = 16(12A + 2m + XY) (Figure 2g,h)
3.5.3. Subfamily Orsillinae
Nysius cymoides, 2n = 14(10A + 2m + XY) (Figure 2i,j)
Nysius helveticus, 2n = 14(10A + 2m + XY) (Figure 2k,l)
4. Discussion
4.1. Standard Karyotypes
4.1.1. Spermatocyte Meiosis
4.1.2. 45S rDNA-FISH
4.1.3. (TTAGG)n-FISH
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, T.J.; Dellapé, P.M.; de Paula, A.S. The big-eyed bugs, chinch bugs, and seed bugs (Lygaeoidea). In True Bugs (Heteroptera) of the Neotropics, Entomology in Focus 2; Panizzi, A.R., Grazia, J., Eds.; Springer: Dordrecht, The Netherlands; Springer: Heidelberg, Germany; Springer: New York, NY, USA; Springer: London, UK, 2015; pp. 459–514. [Google Scholar] [CrossRef]
- Dellapé, P.M.; Henry, T.J. Lygaeoidea Species File. Version 5.0/5.0. Available online: http://Lygaeoidea.SpeciesFile.org/ (accessed on 10 November 2022).
- Ueshima, N. Animal Cytogenetics. Insecta 6. Hemiptera II: Heteroptera; Gebrüder Borntraeger: Berlin/Stuttgart, Germany, 1979; Volume 117, 528p. [Google Scholar]
- Ueshima, N.; Ashlock, P.D. Cytotaxonomy of the Lygaeidae (Hemiptera-Heteroptera). Univ. Kans. Sci. Bull. 1980, 51, 717–801. [Google Scholar] [CrossRef]
- Grozeva, S.; Kuznetsova, V.G. Notes on the karyotypes of some lygaeid bugs (Heteroptera, Pentatomomorpha, Lygaeidae). Folia Biol. 1993, 41, 65–75. [Google Scholar]
- Papeschi, A.G.; Bressa, M.J. Evolutionary cytogenetics in Heteroptera. J. Biol. Res. 2006, 5, 3–21. [Google Scholar]
- Kaur, H.; Suman, V. Chromosomes and their meiotic behavior in two species of Dieuches Dohrn, 1860 (Heteroptera: Lygaeidae: Rhyparochrominae). Comp. Cytogenet. 2009, 3, 43–50. [Google Scholar] [CrossRef]
- Suman, V.; Kaur, H. Meiotic studies in seven Heteropteran species. Cytologia 2012, 77, 311–322. [Google Scholar] [CrossRef]
- Souza, H.V.; Bicudo, H.E.M.C.; Itoyama, M.M. Study of chromosomal and nucleolar aspects in testes of Nysius californicus (Heteroptera-Lygaediae). Genet. Mol. Res. 2007, 6, 33–40. [Google Scholar]
- Souza, H.V.; Castanhole, M.M.U.; Gomes, M.O.; Murakami, A.S.; Souza-Firmino, T.S.; Saran, P.S.; Banho, C.A.; Monteiro, L.D.; Silva, J.C.; Itoyama, M.M. Meiotic behavior of 18 species from eight families of terrestrial Heteroptera. Insect Sci. 2014, 14, 149. [Google Scholar] [CrossRef]
- Bardella, V.B.; Sampaio, T.R.; Venturelli, N.B.; Dias, A.; Giuliano-Caetano, L.; Fernandes, J.A.M.; da Rosa, R. Physical mapping of 18S rDNA and heterochromatin in species of family Lygaeidae (Hemiptera: Heteroptera). Genet. Mol. Res. 2014, 13, 2186–2199. [Google Scholar] [CrossRef]
- Toscani, M.A.; Pigozzi, M.I.; Papeschi, A.G.; Bressa, M.J. Histone H3 methylation and autosomal vs. sex chromosome segregation during male meiosis in Heteroptera. Front. Ecol. Evol. 2022, 10, 836786. [Google Scholar] [CrossRef]
- Grozeva, S.; Kuznetsova, V.G. Karyotypes and some structural properties of the reproductive system of bugs of the subfamily Artheneinae (Heteroptera, Pentatomomorpha, Lygaeidae). Entomol. Rev. 1990, 69, 14–26. [Google Scholar]
- Grozeva, S. Karyotypes, male reproductive system, and abdominal trichobothria of the Berytidae (Heteroptera) with phylogenetic considerations. Syst. Entomol. 1995, 20, 207–216. [Google Scholar] [CrossRef]
- Grozeva, S. Karyotype and structure of the reproductive system in Piesma (Heteroptera, Piesmatidae). Entomol. Rev. 1991, 70, 157–166. [Google Scholar]
- Kaur, H.; Suman, V.; Kaur, R. First report on C-banding and fluorescent banding in species of Dieuches (Rhyparochrominae: Lygaeidae: Heteroptera). Entomol. Res. 2010, 40, 1–7. [Google Scholar] [CrossRef]
- Grozeva, S.; Kuznetsova, V.G.; Anokhin, B.A. Karyotypes, male meiosis and comparative FISH mapping of 18S ribosomal DNA and telomeric (TTAGG)n repeat in eight species of true bugs (Hemiptera, Heteroptera). Comp. Cytogenet. 2011, 5, 355–374. [Google Scholar] [CrossRef]
- Suman, V.; Kaur, H. First report on C-banding, fluorochrome staining and NOR location in holocentric chromosomes of Elasmolomus (Aphanus) sordidus Fabricius, 1787 (Heteroptera, Rhyparochromidae). Adv. Hemipterology 2013, 319, 283–291. [Google Scholar] [CrossRef]
- Levsky, J.F.; Singer, R.H. Fluorescence in situ hybridization: Past, present and future. J. Cell Sci. 2003, 116, 2833–2838. [Google Scholar] [CrossRef]
- Frydrychová, R.; Grossmann, P.; Trubac, P.; Vitková, M.; Marec, F.E. Phylogenetic distribution of TTAGG telomeric repeats in insects. Genome 2004, 47, 16–178. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Grozeva, S.; Gokhman, V. Telomere structure in insects: A review. J. Zool. Syst. Evol. Res. 2020, 58, 127–158. [Google Scholar] [CrossRef]
- Golub, N.V.; Golub, V.B.; Anokhin, B.A.; Kuznetsova, V.G. Comparative Cytogenetics of Lace Bugs (Tingidae, Heteroptera): New Data and a Brief Overview. Insects 2022, 13, 608. [Google Scholar] [CrossRef]
- Grozeva, S.; Nokkala, S. Chromosomes and their meiotic behavior in two families of the primitive infraorder Dipsocoromorpha (Heteroptera). Hereditas 1996, 125, 189–192. [Google Scholar] [CrossRef]
- Grozeva, S.; Anokhin, B.; Kuznetsova, V.G. Bed bugs (Hemiptera). In Protocols for Cytogenetic Mapping of Arthropod Genomes; Sharachov, I., Ed.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2015; pp. 285–326. [Google Scholar] [CrossRef]
- Golub, N.; Anokhin, B.; Kuznetsova, V. Comparative FISH mapping of ribosomal DNA clusters and TTAGG telomeric sequences to holokinetic chromosomes of eight species of the insect order Psocoptera. Comp. Cytogenet. 2019, 13, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Pfaler-Collander, E. Vergleichend-Karyologische Untersuchungen an Lygaeiden. Acta Zool. Fenn. 1941, 30, 1–119. [Google Scholar]
- Muramoto, N. A chromosome study of five hemipterans (Hemiptera) and two hymenopterans (Hymenoptera). La Kromosomo 1993, 69, 2336–2341. [Google Scholar]
- Geitler, L. Heterochromatin der Geschlechtschromosomen bei Heteropteren. Chromosoma 1939, 1, 197–229. [Google Scholar] [CrossRef]
- Parshad, R. Cytological studies in Heteroptera IV. Chromosome complement and meiosis in twenty-six species of the Pentatomidae, Lygaeidae and Coreidae with the consideration of the cytological bearing on the status of these three superfamilies. Res. Bull. Punjab Univ. 1957, 133, 521–559. [Google Scholar]
- Satapathy, S.N.; Patnaik, S.C. Chromosome numbers in forty-one species of Indian Heteroptera. Chromosome Inform. Serv. 1989, 47, 3–5. [Google Scholar]
- Grozeva, S.; Nokkala, S.; Simov, N. First evidence of sex chromosomes pre-reduction in male meiosis in the Miridae bugs (Heteroptera). Folia Biol. 2006, 54, 9–12. [Google Scholar] [CrossRef]
- Grozeva, S.; Kuznetsova, V.G.; Simov, N.; Langourov, M.; Dalakchieva, S. Sex chromosome pre-reduction in male meiosis of Lethocerus patruelis (Stal, 1854) (Heteroptera, Belostomatidae) with some notes on the distribution of the species. ZooKeys 2013, 319, 119–135. [Google Scholar] [CrossRef]
- Ferretti, A.; Ruiz-Ruano, F.J.; Milani, D.; Loreto, V.; Marti, D.A.; Ramos, E.; Martins, C.; Cabral-de-Mello, D.C. How dynamic could be the 45S rDNA cistron? An intriguing variability in a grasshopper species revealed by integration of chromosomal and genomic data. Chromosoma 2019, 128, 165–175. [Google Scholar] [CrossRef]
- Cabral-de-Mello, D.C.; Oliveira, S.G.; de Moura, R.C.; Martins, C. Chromosomal organization of the 18S and 5S rRNAs and histone H3 genes in Scarabaeinae coleopterans: Insights into the evolutionary dynamics of multigene families and heterochromatin. BMC Genet. 2011, 12, 88. [Google Scholar] [CrossRef]
- Panzera, Y.; Pita, S.; Ferreiro, M.J.; Ferrandis, I.; Lages, C.; Pérez, R.; Silva, A.E.; Guerra, M.; Panzera, F. High dynamics of rDNA cluster location in kissing bug holocentric chromosomes (Triatominae, Heteroptera). Cytogenet. Genome Res. 2012, 38, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Bardella, V.B.; Fernandes, J.A.M.; Cabral-de-Mello, D.C. Chromosomal evolutionary dynamics of four multigene families in Coreidae and Pentatomidae (Heteroptera) true bugs. Mol. Genet. Genomics 2016, 291, 1919–1925. [Google Scholar] [CrossRef]
- Souza-Firmino, T.S.; Alevi, K.C.C.; Itoyama, M.M. Chromosomal divergence and volutionary inferences in Pentatomomorpha infraorder (Hemiptera, Heteroptera) based on the chromosomal location of ribosomal genes. PLoS ONE 2020, 15, e0228631. [Google Scholar] [CrossRef]
- Panzera, F.; Pita, S.; Lorite, P. Chromosome structure and evolution of Triatominae: A review. In Triatominae—The Biology of Chagas Disease Vectors. Entomology in Focus; Guarneri, A., Lorenzo, M., Eds.; Springer: New York, NY, USA, 2021; Volume 5, pp. 65–99. [Google Scholar] [CrossRef]
- Gapon, D.A.; Kuznetsova, V.G.; Maryańska-Nadachowska, A. A new species of the genus Rhaphidosoma Amyot et Serville, 1843 (Heteroptera, Reduviidae), with data on its chromosome complement. Comp. Cytogenet. 2021, 15, 467–505. [Google Scholar] [CrossRef] [PubMed]
- Bardella, V.B.; Fernandes, T.; Vanzela, A.L.L. The conservation of number and location of 18S sites indicates the relative stability of rDNA in species of Pentatomomorpha (Heteroptera). Genome 2013, 56, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Sochorová, J.; Garcia, S.; Gálvez, F.; Symonová, R.; Kovařík, A. Evolutionary trends in animal ribosomal DNA loci: Introduction to a new online database. Chromosoma 2018, 127, 141–150. [Google Scholar] [CrossRef]
- Teixeira, G.A.; de Aguiar, H.J.A.C.; Petitclerc, F.; Orivel, J.; Lopes, D.M.; Barros, L.A.C. Evolutionary insights into the genomic organization of major ribosomal DNA in ant chromosomes. Insect Mol. Biol. 2021, 30, 340–354. [Google Scholar] [CrossRef]
- Teixeira, G.A.; Barros, L.A.C.; de Aguiar, H.J.A.C.; Lopes, D.M. Distribution of GC-rich heterochromatin and ribosomal genes in three fungus-farming ants (Myrmicinae, Attini, Attina): Insights on chromosomal evolution. Comp. Cytogenet. 2021, 15, 413–428. [Google Scholar] [CrossRef]
- Sochorová, J.; Gálvez, F.; Matyášek, R.; Garcia, S.; Kovařík, A. Analyses of the updated “Animal rRDNA loci database” with an emphasis on its new features. Int. J. Mol. Sci. 2021, 22, 11403. [Google Scholar] [CrossRef]
- Buleu, O.G.; Jetybayev, I.Y.; Chobanov, D.P.; Bugrov, A.G. Comparative analysis of C-heterochromatin, ribosomal and telomeric DNA markers in chromosomes of Pamphagidae grasshoppers from Morocco. Comp. Cytogenet. 2019, 13, 61–74. [Google Scholar] [CrossRef]
- Nguyen, P.; Sahara, K.; Yoshido, A.; Marec, F. Evolutionary dynamics of rDNA clusters on chromosomes of moths and butterflies (Lepidoptera). Genetica 2010, 138, 343–354. [Google Scholar] [CrossRef]
- Anjos, A.; Paladini, A.; Evangelista, O.; Cabral-de-Mello, D.C. Insights into chromosomal evolution of Cicadomorpha using fluorochrome staining and mapping 18S rRNA and H3 histone genes. J. Zool. Syst. Evol. Res. 2018, 57, 314–322. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Maryańska-Nadachowska, A.; Anokhin, B.; Shapoval, N.; Shapoval, A. Chromosomal analysis of eight species of dragonflies (Anisoptera) and damselflies (Zygoptera) using conventional cytogenetics and fluorescence in situ hybridization: Insights into the karyotype evolution of the ancient insect order Odonata. J. Zool. Syst. Evol. Res. 2021, 59, 387–399. [Google Scholar] [CrossRef]
- Pita, S.; Lorite, P.; Cuadrado, A.; Panzera, Y.; De Oliveira, J.; Alevi, K.C.C.; Rosa, J.A.; Freitas, S.P.C.; Gómez-Palacio, A.; Solari, A.; et al. High chromosomal mobility of rDNA clusters in holocentric chromosomes of Triatominae, vectors of Chagas disease (Hemiptera-Reduviidae). Med. Vet Entomol. 2022, 36, 66–80. [Google Scholar] [CrossRef]
- Vela, J.; Montiel, E.E.; Mora, P.; Lorite, P.; Palomeque, T. Aphids and ants, mutualistic species, share a mariner element with an unusual location on aphid chromosomes. Genes 2021, 12, 1966. [Google Scholar] [CrossRef]
- Okazaki, S.; Tsuchida, K.; Maekawa, H.; Ishikawa, H.; Fujiwara, H. Identification of a pentanucleotide telomeric sequence, (TTAGG)n, in the silk warm Bombyx mori and in other insects. J. Mol. Cell Biol. 1993, 15, 4545–4552. [Google Scholar] [CrossRef]
- Sahara, K.; Marec, F.; Traut, W. TTAGG telomeric repeats in chromosomes of some insects and other arthropods. Chromosome Res. 1999, 7, 449–460. [Google Scholar] [CrossRef]
- Mason, J.M.; Randall, T.A.; Frydrychova, R.C. Telomerase lost? Chromosoma 2016, 125, 65–73. [Google Scholar] [CrossRef]
- Lukhtanov, V.A. Diversity and evolution of telomere and subtelomere DNA sequences in insects. bioRxiv 2022. [Google Scholar] [CrossRef]
| Species | Number of Males Studied | Date and Place of Collection |
|---|---|---|
| Rhyparochromidae | ||
| Elasmolomus squalidus (Gmelin, 1790) | 3 | 2–4 November 2019, Popa mount, Myanmar (D. Gapon leg.) |
| Heterogastridae | ||
| Nerthus sp. 1 | 1 | 2 November 2019, Popa mount, Myanmar (D. Gapon leg.) |
| Nerthus sp. 2 | 1 | 2 November 2019, Popa mount, Myanmar (D. Gapon leg.) |
| Cymidae | ||
| Cymus claviculus (Fallen, 1807) | 2 | 16 August 2022, Voronezh region, Russia (V. Golub leg.) |
| Blissidae | ||
| Dimorphopterus spinolae (Signoret, 1857) | 3 | 14 August 2022, Voronezh region, Russia (V. Golub leg.) |
| Lygaeidae | ||
| Ischnorhynchinae | ||
| Kleidocerys resedae (Panzer, 1793) | 3 | 24 August 2022, Voronezh region, Russia (V. Golub leg.) |
| Lygaeinae | ||
| Spilostethus saxatilis (Scopoli, 1763) | 3 | 25 June 2021, Sevan vic., Armenia (D. Gapon leg.) |
| Thunbergia floridulus (Distant, 1918) | 2 | 13 November 2019, Pai, Thailand (D. Gapon leg.) |
| Orsillinae | ||
| Nysius cymoides (Spinola, 1837) | 2 | 1 August 2022, Goryachiy Kluch vic., Russia (V. Golub leg) |
| Nysius helveticus (Herrich-Schäffer, 1850) | 3 | 14–16 August 2022, Voronezh region, Russia (V. Golub leg.) |
| Species | 2n | 18S rDNA Location | TTAGG Repeat | Published Data |
|---|---|---|---|---|
| Rhyparochromidae | ||||
| Elasmolomus squalidus | 12(8A + 2m + XY) | AA2 | Not found | No |
| Heterogastridae | ||||
| Nerthus sp. 1 | 16(12A + 2m + XY) | Not studied | Not studied | No |
| Nerthus sp. 2 | 18 (14A + 2m + XY) | AA1 | Not found | No |
| Cymidae | ||||
| Cymus claviculus | 28(24A + 2m + XY) | AA1 (close to the ends) | Not found | [26]: n = 12 + m + X + Y |
| Blissidae | ||||
| Dimorphopterus spinolae | 14(10A + 2m+ XY) | AA1 (close to the ends) | Not found | [27]: “2n = 16 (and XY type)” |
| Lygaeidae | ||||
| Ischnorhynchinae | ||||
| Kleidocerys resedae | 14(10A + 2m + XY) | X and Y (close to the end) | Not found | [26] (as Ischnorhynchus lineatus): 2n = 14, n = 5 + m + X + Y |
| Lygaeinae | ||||
| Spilostethus saxatilis | 14(12A + XY) | AA (interstitially) | Not found | [28]: n = 6 + X(Y) |
| Thunbergia floridulus | 16(12A + 2m + XY) | X | Not found | No |
| Orsillinae | ||||
| Nysius cymoides | 14(10A + 2m + XY) | AA1 (close to the ends) | Not found | No |
| Nysius helveticus | 14(10A + 2m + XY) | AA1 (close to the ends) | Not found | [26] (as N. lineatus): 2n = 14, n = 5 + m + X + Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golub, N.V.; Maryańska-Nadachowska, A.; Anokhin, B.A.; Kuznetsova, V.G. Expanding the Chromosomal Evolution Understanding of Lygaeioid True Bugs (Lygaeoidea, Pentatomomorpha, Heteroptera) by Classical and Molecular Cytogenetic Analysis. Genes 2023, 14, 725. https://doi.org/10.3390/genes14030725
Golub NV, Maryańska-Nadachowska A, Anokhin BA, Kuznetsova VG. Expanding the Chromosomal Evolution Understanding of Lygaeioid True Bugs (Lygaeoidea, Pentatomomorpha, Heteroptera) by Classical and Molecular Cytogenetic Analysis. Genes. 2023; 14(3):725. https://doi.org/10.3390/genes14030725
Chicago/Turabian StyleGolub, Natalia V., Anna Maryańska-Nadachowska, Boris A. Anokhin, and Valentina G. Kuznetsova. 2023. "Expanding the Chromosomal Evolution Understanding of Lygaeioid True Bugs (Lygaeoidea, Pentatomomorpha, Heteroptera) by Classical and Molecular Cytogenetic Analysis" Genes 14, no. 3: 725. https://doi.org/10.3390/genes14030725
APA StyleGolub, N. V., Maryańska-Nadachowska, A., Anokhin, B. A., & Kuznetsova, V. G. (2023). Expanding the Chromosomal Evolution Understanding of Lygaeioid True Bugs (Lygaeoidea, Pentatomomorpha, Heteroptera) by Classical and Molecular Cytogenetic Analysis. Genes, 14(3), 725. https://doi.org/10.3390/genes14030725







