The Satellite DNAs Populating the Genome of Trigona hyalinata and the Sharing of a Highly Abundant satDNA in Trigona Genus
Abstract
1. Introduction
2. Materials and Methods
2.1. Genomic DNA Isolation and Digestion by Restriction Enzymes
2.2. Cloning, Sequencing, and Sequence Analysis
2.3. Genome Sequencing and Satellite DNA Sequence Analysis
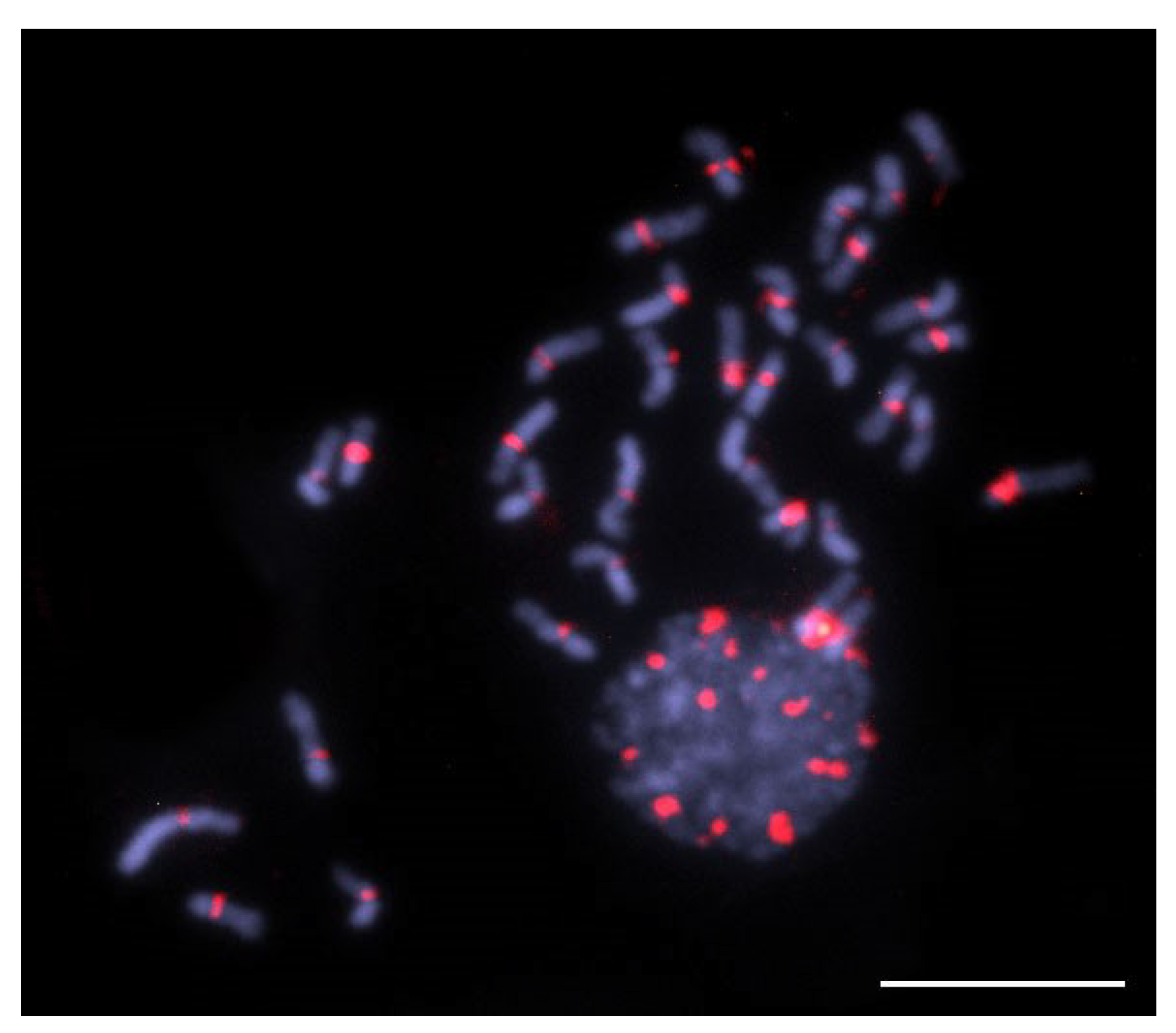
2.4. Fluorescence In Situ Hybridization (FISH)
3. Results
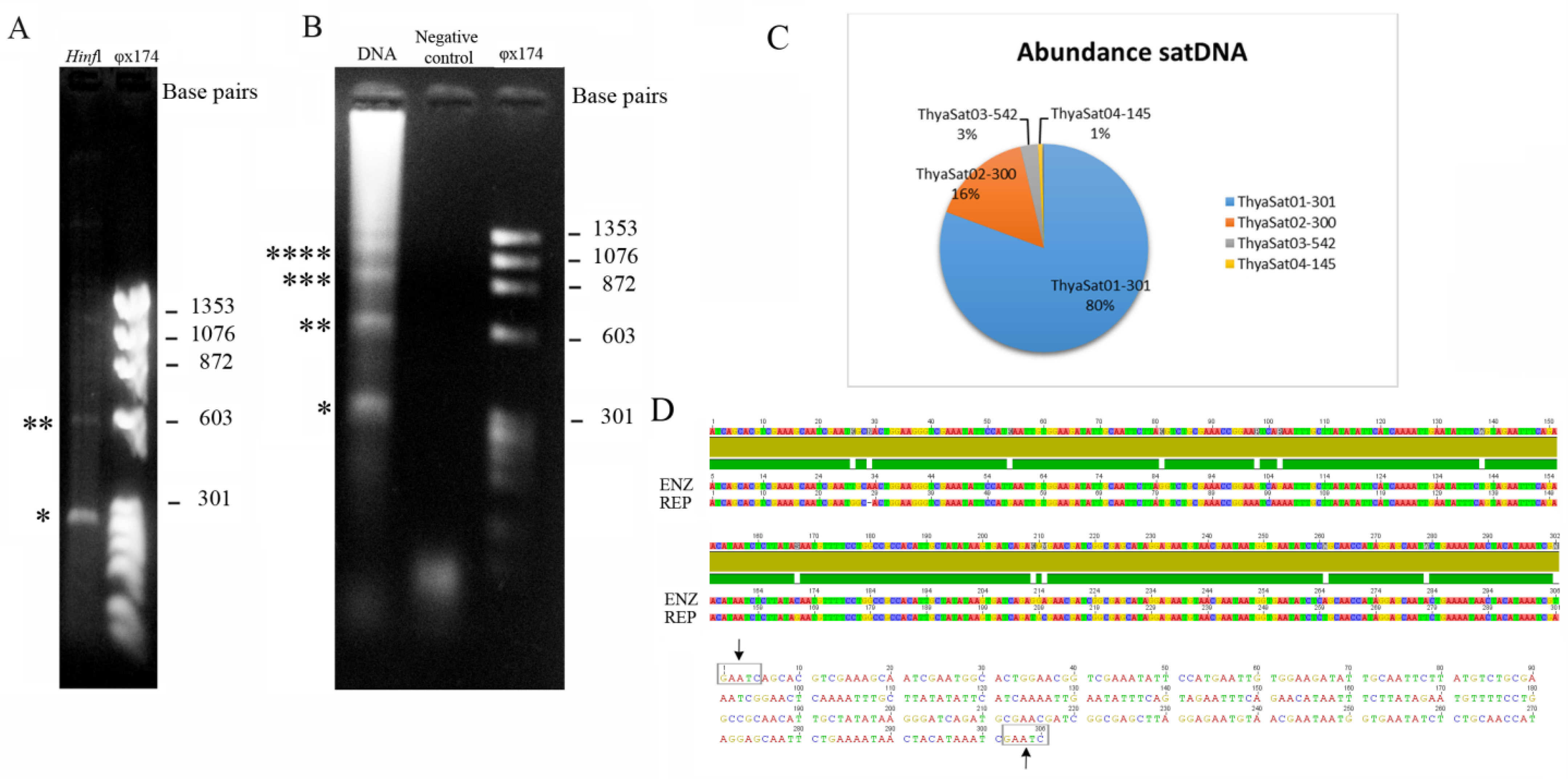
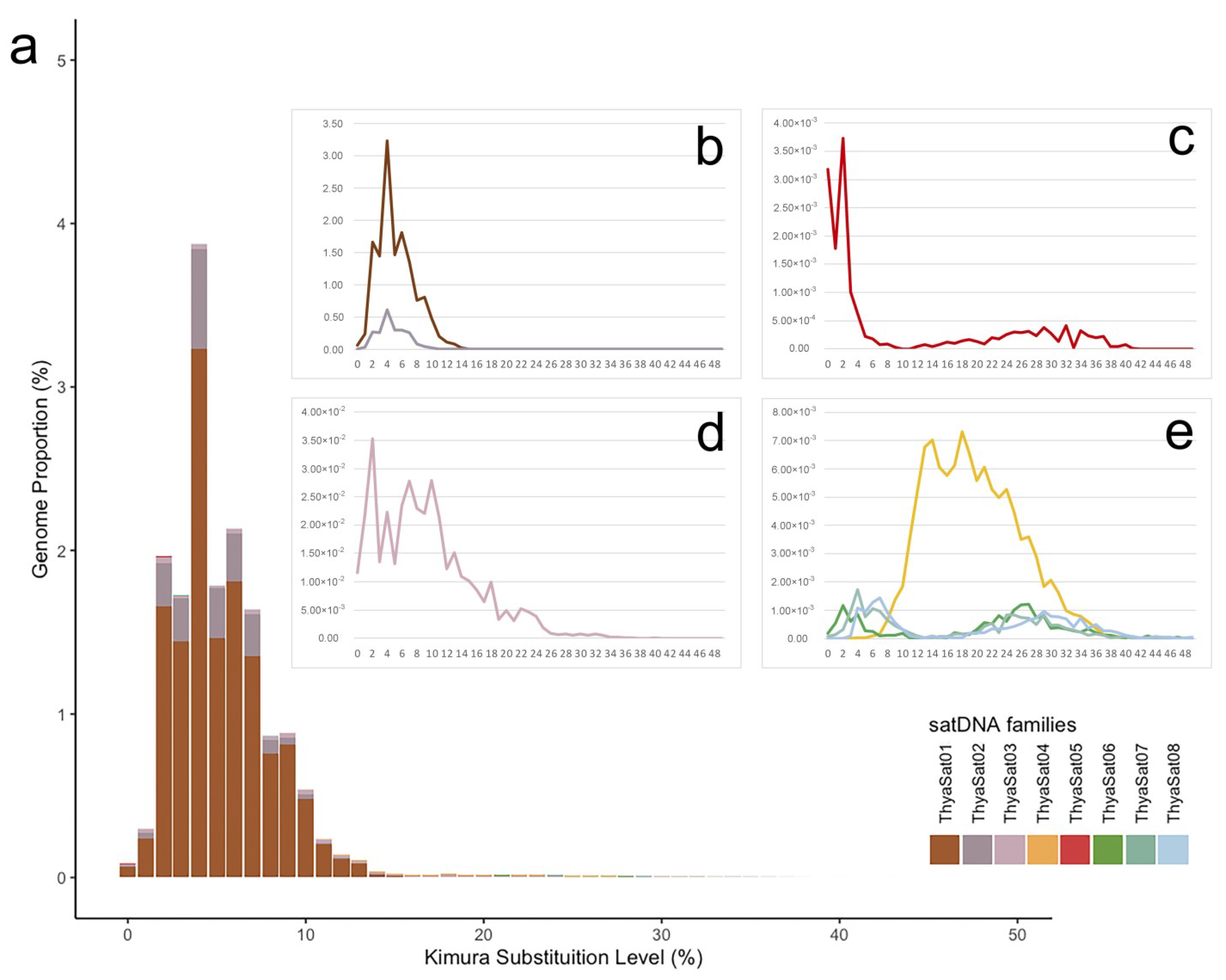
3.1. Extensive Amplification of a satDNA Superfamily on the Genome of T. hyalinata
3.2. A Phylogenetically Conserved satDNA on Heterochromatin of Trigona Species
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlesworth, B.; Sniegowski, P.; Stephan, W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 1994, 371, 215–220. [Google Scholar] [CrossRef] [PubMed]
- López-Flores, I.; Garrido-Ramos, M.A. The Repetitive DNA Content of Eukaryotic Genomes. Genome Dyn. 2012, 7, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramos, M. Satellite DNA: An Evolving Topic. Genes 2017, 8, 230. [Google Scholar] [CrossRef]
- Lower, S.S.; McGurk, M.P.; Clark, A.G.; Barbash, D.A. Satellite DNA evolution: Old ideas, new approaches. Curr. Opin. Genet. Dev. 2018, 49, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Plohl, M.; Meštrović, N.; Mravinac, B. Satellite DNA evolution. Repetitive DNA 2012, 7, 126–152. [Google Scholar]
- Fry, K.; Salser, W. Nucleotide Sequences of HS-α Satellite DNA from Kangaroo Rat Dipodomys Ordii and Characterization of Similar Sequences in Other Rodents. Cell 1977, 12, 1069–1084. [Google Scholar] [CrossRef]
- Piccoli, M.C.A.; Bardella, V.B.; Cabral-de-Mello, D.C. Repetitive DNAs in Melipona scutellaris (Hymenoptera: Apidae: Meliponidae): Chromosomal distribution and test of multiple heterochromatin amplification in the genus. Apidologie 2018, 49, 497–504. [Google Scholar] [CrossRef]
- Cunha, M.S.; Campos, L.A.O.; Lopes, D.M. Insights into the heterochromatin evolution in the genus Melipona (Apidae: Meliponini). Insectes Soc. 2020, 67, 391–398. [Google Scholar] [CrossRef]
- Pereira, J.A.; Salomão, T.M.F.; Lopes, D.M. Different repetitive DNA sequences make up heterochromatin in Meliponini. Apidologie 2020, 51, 855–860. [Google Scholar] [CrossRef]
- Pereira, J.A.; Travenzoli, N.M.; de Oliveira, M.P.; de Azevedo Werneck, H.; Salomão, T.M.F.; Lopes, D.M. Molecular cytogenetics in the study of repetitive sequences helping to understand the evolution of heterochromatin in Melipona (Hymenoptera, Meliponini). Genetica 2021, 149, 55–62. [Google Scholar] [CrossRef]
- Pereira, J.A.; Milani, D.; Ferretti, A.B.S.M.; Bardella, V.B.; Cabral-de-Mello, D.C.; Lopes, D.M. The extensive amplification of heterochromatin in Melipona bees revealed by high throughput genomic and chromosomal analysis. Chromosoma 2021, 130, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kerr, W.E. Estudos sobre o gênero Melipona. An. Esc. Super. Agric. Luiz Queiroz 1948, 5, 181–276. [Google Scholar] [CrossRef]
- Brito, R.M.; Caixeiro, A.P.d.A.; Pompolo, S.d.G.; Azevedo, G.G. Cytogenetic data of Partamona peckolti (Hymenoptera, Apidae, Meliponini) by C banding and fluorochrome staining with DA/CMA3 and DA/DAPI. Genet. Mol. Biol. 2003, 26, 53–57. [Google Scholar] [CrossRef]
- Lopes, D.M.; Pompolo, S.d.G.; Campos, L.A.d.O.; Tavares, M.G. Cytogenetic characterization of Melipona rufiventris Lepeletier 1836 and Melipona mondury Smith 1863 (Hymenoptera, Apidae) by C banding and fluorochromes staining. Genet. Mol. Biol. 2008, 31, 49–52. [Google Scholar] [CrossRef]
- Barth, A.; Fernandes, A.; das Pompolo, S.G.; Costa, M.A. Occurrence of b chromosomes in Tetragonisca Latreille, 1811 (Hymenoptera, Apidae, Meliponini): A new contribution to the cytotaxonomy of the genus. Genet. Mol. Biol. 2011, 34, 77–79. [Google Scholar] [CrossRef]
- Rocha, M.P.; Pompolo, S.d.G. Karyotypes and heterochromatin variation (C-bands) in Melipona species (Hymenoptera, Apidae, Meliponinae). Genet. Mol. Biol. 1998, 21, 41–45. [Google Scholar] [CrossRef]
- Costa, K.F.; Brito, R.M.; Miyazawa, C.S. Karyotypic description of four species of Trigona (Jurine, 1807) (Hymenoptera, Apidae, Meliponini) from the State of Mato Grosso, Brazil. Genet. Mol. Biol. 2004, 27, 187–190. [Google Scholar] [CrossRef]
- Godoy, D.C.; Ferreira, R.P.; Lopes, D.M. Chromosomal Variation and Cytogenetics of Plebeia lucii and P. phrynostoma (Hymenoptera: Apidae). Fla. Entomol. 2013, 96, 1559–1566. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Diniz, D.; Rodrigues, T.A.S.; Cioffi, M.D.B.; Waldschmidt, A.M. Heterochromatin Distribution and Chromosomal Mapping of Microsatellite Repeats in the Genome of Frieseomelitta Stingless Bees (Hymenoptera: Apidae: Meliponini). Fla. Entomol. 2018, 101, 33–39. [Google Scholar] [CrossRef]
- Rasmussen, C.; Camargo, J.M.F. A molecular phylogeny and the evolution of nest architecture and behavior in Trigona s.s. (Hymenoptera: Apidae: Meliponini). Apidologie 2008, 39, 102–118. [Google Scholar] [CrossRef]
- Rasmussen, C.; Cameron, S.A. Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biol. J. Linn. Soc. 2010, 99, 206–232. [Google Scholar] [CrossRef]
- Domingues, A.M.T.; Waldschmidt, A.M.; Andrade, S.E.; Andrade-Souza, V.; de Oliveira Alves, R.M.; da Silva, J.C.; Costa, M.A. Karyotype characterization of Trigona fulviventris Guérin, 1835 (Hymenoptera, Meliponini) by C banding and fluorochrome staining: Report of a new chromosome number in the genus. Genet. Mol. Biol. 2005, 28, 390–393. [Google Scholar] [CrossRef]
- Rocha, M.; Pompolo, S.; Campos, L. Citogenética da Tribo Meliponini (Hymenoptera, Apidae). In Apoidea Neotropica. Homenagem aos 90 anos de Jesus Santiago Moure; Melo, G.A.R., Santos, I.A., Eds.; UNESC: Criciúma, Brazil, 2003; pp. 311–320. [Google Scholar]
- Fernandes, A.; Barth, A.; Sampaio, W.S. Caracterização citogenética da espécie Trigona chanchamayoensis (Hymenoptera, Apidae, Meliponini) encontrada no cerrado brasileiro. Evolução E Conserv. Biodivers. 2013, 4, 63. [Google Scholar] [CrossRef]
- Barboza, V.P.; Costa, M.A. Cytogenetic Analysis in Trigona spinipes Fabricius (Hymenoptera, Meliponina) Reveals Intraspecific Variation. Neotrop. Entomol. 2021, 50, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.G.; Ferreira, R.d.P.; Travenzoli, N.M.; Lopes, D.M. Karyotypic variation in the stingless bee Trigona spinipes (Hymenoptera: Apidae: Meliponini) from different geographical regions of Brazil. Apidologie 2021, 52, 1358–1367. [Google Scholar] [CrossRef]
- Waldschmidt, A.M.; Fernandes Salomão, T.M.; De Barros, E.G.; De Antônio Oliveira Campos, L. Extraction of genomic DNA from Melipona quadrifasciata (Hymenoptera: Apidae, Meliponinae). Brazilian J. Genet. 1997, 20, S0100–S84551997000300011. [Google Scholar] [CrossRef]
- Drummond, A.; Ashton, B.; Cheung, M.; Helid, J.; Kearse, M.; Moir, R.; Stones-Havas, S.; Thierer, T.; Wilson, S. Geneious v4.8. 2009. Available online: http://www.geneious.com (accessed on 1 August 2021).
- Bao, W.; Kojima, K.K.; Kohany, O. Repbase Update, a Database of Repetitive Elements in Eukaryotic Genomes. Mob. DNA 2015, 6, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Novák, P.; Neumann, P.; Macas, J. Global analysis of repetitive DNA from unassembled sequence reads using RepeatExplorer2. Nat. Protoc. 2020, 15, 3745–3776. [Google Scholar] [CrossRef]
- Novák, P.; Ávila Robledillo, L.; Koblížková, A.; Vrbová, I.; Neumann, P.; Macas, J. TAREAN: A computational tool for identification and characterization of satellite DNA from unassembled short reads. Nucleic Acids Res. 2017, 45, e111. [Google Scholar] [CrossRef]
- Smit, A.; Hubley, R.; Green, P. RepeatMasker Open-4.0. 2013–2015. 2017. Available online: http://www.repeatmasker.org/ (accessed on 1 August 2021).
- Ruiz-Ruano, F.J.; López-León, M.D.; Cabrero, J.; Camacho, J.P.M. High-throughput analysis of the satellitome illuminates satellite DNA evolution. Sci. Rep. 2016, 6, 28333. [Google Scholar] [CrossRef] [PubMed]
- Hirotami, T.I.; Robert, W.T.; Michael, W.J.C.; Ross, H.C. Modes of spontaneous chromosomal mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Jpn. J. Genet. 1988, 63, 159–185. [Google Scholar] [CrossRef]
- Pinkel, D.; Straume, T.; Gray, J.W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc. Natl. Acad. Sci. USA 1986, 83, 2934–2938. [Google Scholar] [CrossRef]
- Palomeque, T.; Lorite, P. Satellite DNA in insects: A review. Heredity 2008, 100, 564–573. [Google Scholar] [CrossRef]
- Pita, S.; Panzera, F.; Mora, P.; Vela, J.; Cuadrado, Á.; Sánchez, A.; Palomeque, T.; Lorite, P. Comparative repeatome analysis on Triatoma infestans Andean and Non-Andean lineages, main vector of Chagas disease. PLoS ONE 2017, 12, e0181635. [Google Scholar] [CrossRef] [PubMed]
- Mora, P.; Vela, J.; Ruiz-Ruano, F.J.; Ruiz-Mena, A.; Montiel, E.E.; Palomeque, T.; Lorite, P. Satellitome Analysis in the Ladybird Beetle Hippodamia variegata (Coleoptera, Coccinellidae). Genes 2020, 11, 783. [Google Scholar] [CrossRef]
- Bardella, V.B.; Milani, D.; Cabral-de-Mello, D.C. Analysis of Holhymenia histrio genome provides insight into the satDNA evolution in an insect with holocentric chromosomes. Chromosom. Res. 2020, 28, 369–380. [Google Scholar] [CrossRef]
- Feliciello, I.; Chinali, G.; Ugarković, Đ. Structure and population dynamics of the major satellite DNA in the red flour beetle Tribolium castaneum. Genetica 2011, 139, 999–1008. [Google Scholar] [CrossRef]
- Palacios-Gimenez, O.M.; Dias, G.B.; de Lima, L.G.; Kuhn, G.C.S.; Ramos, É.; Martins, C.; Cabral-de-Mello, D.C. High-throughput analysis of the satellitome revealed enormous diversity of satellite DNAs in the neo-Y chromosome of the cricket Eneoptera surinamensis. Sci. Rep. 2017, 7, 6422. [Google Scholar] [CrossRef]
- Palacios-Gimenez, O.M.; Bardella, V.B.; Lemos, B.; Cabral-de-Mello, D.C. Satellite DNAs are conserved and differentially transcribed among Gryllus cricket species. DNA Res. 2018, 25, 137–147. [Google Scholar] [CrossRef]
- Camacho, J.P.M.; Cabrero, J.; López-León, M.D.; Martín-Peciña, M.; Perfectti, F.; Garrido-Ramos, M.A.; Ruiz-Ruano, F.J. Satellitome comparison of two oedipodine grasshoppers highlights the contingent nature of satellite DNA evolution. BMC Biol. 2022, 20, 36. [Google Scholar] [CrossRef]
- Palacios-Gimenez, O.M.; Milani, D.; Song, H.; Marti, D.A.; López-León, M.D.; Ruiz-Ruano, F.J.; Camacho, J.P.M.; Cabral-De-Mello, D.C.; O’Neill, R. Eight Million Years of Satellite DNA Evolution in Grasshoppers of the Genus Schistocerca Illuminate the Ins and Outs of the Library Hypothesis. Genome Biol. Evol. 2020, 12, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Cabral-de-Mello, D.C.; Zrzavá, M.; Kubíčková, S.; Rendón, P.; Marec, F. The Role of Satellite DNAs in Genome Architecture and Sex Chromosome Evolution in Crambidae Moths. Front. Genet. 2021, 12, 661417. [Google Scholar] [CrossRef] [PubMed]
- de Lima, L.G.; Ruiz-Ruano, F.J. In-Depth Satellitome Analyses of 37 Drosophila Species Illuminate Repetitive DNA Evolution in the Drosophila Genus. Genome Biol. Evol. 2022, 14, evac064. [Google Scholar] [CrossRef]
- Teixeira, G.; Ferreira, R.; Lopes, D. Comparative Cytogenetic Analysis Reveals Chromosomal Variability in Five Stingless Bees of The Genus Trigona (Apidae, Apinae, Meliponini). 2023; (manuscript in preparation). [Google Scholar]
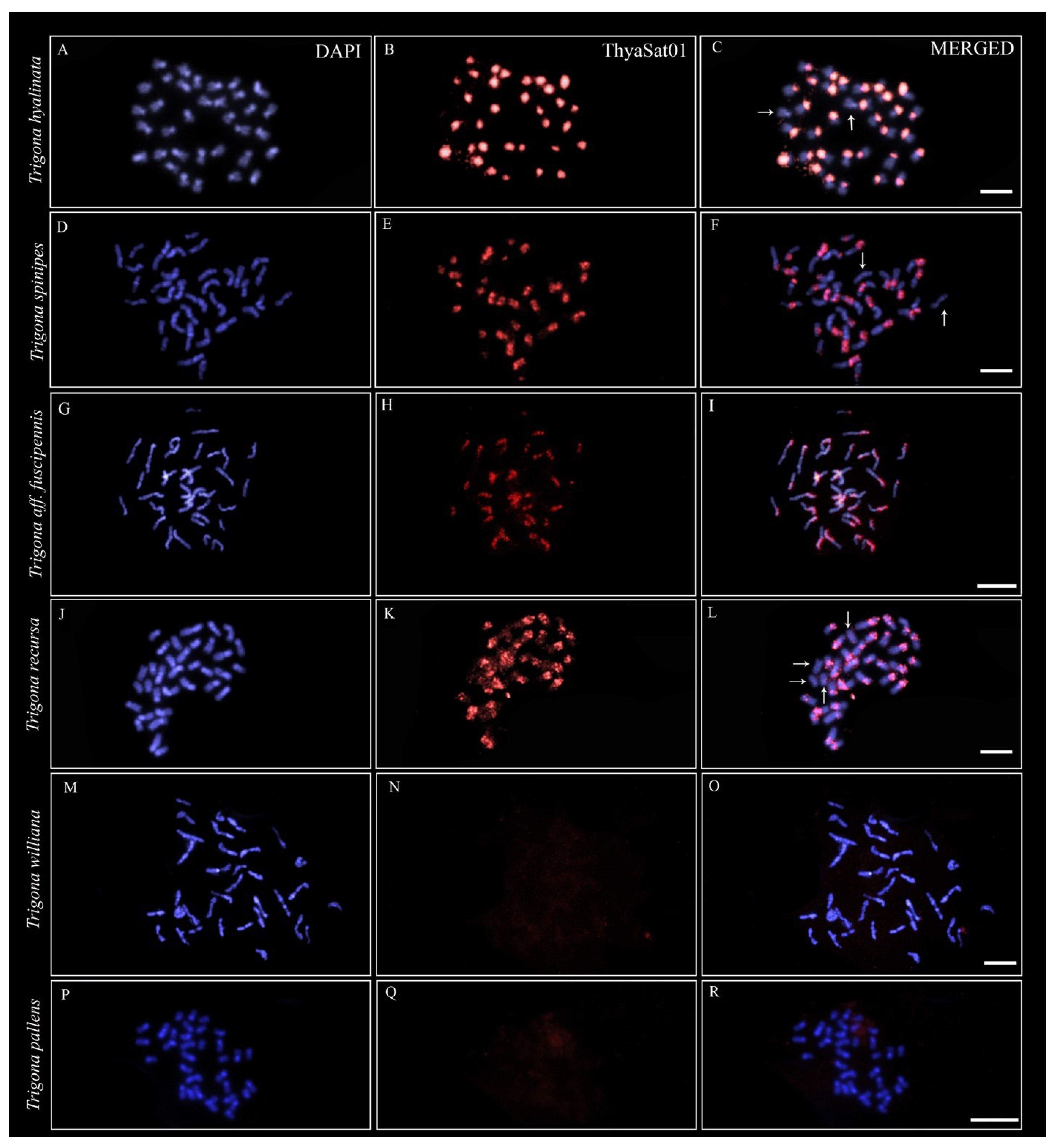
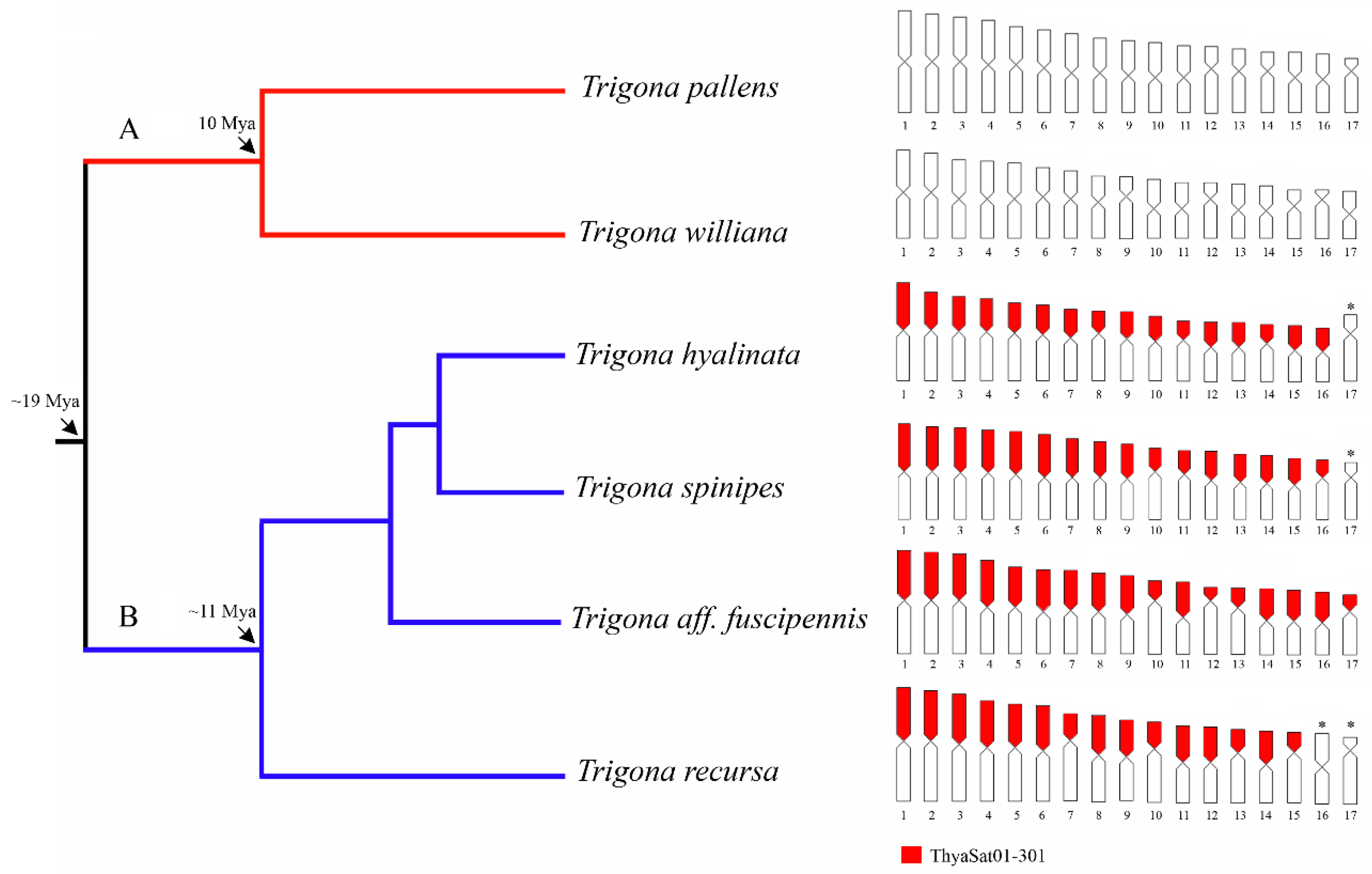
| Species | Species Group * | Clades * | Sample Locality |
|---|---|---|---|
| Trigona williana | “fulviventris” | A | Altamira, Pará |
| Trigona pallens | “pallens” | A | Altamira, Pará |
| T.hyalinata | “spinipes” | B | Viçosa, Minas Gerais |
| Trigona spinipes | “spinipes” | B | Ribeirão Preto, São Paulo |
| Trigona aff. fuscipennis | “fuscipennis” | B | Florestal, Minas Gerais |
| Trigona recursa | “recursa” | B | Januária, Minas Gerais |
| Satellite DNA | SF | ML | A + T% | Divergence | Abundance |
|---|---|---|---|---|---|
| ThyaSat01-301 | 1 | 301 | 63.5 | 5.74% | 13.774% |
| ThyaSat02-300 | 1 | 300 | 62.7 | 5.50% | 2.246% |
| ThyaSat03-542 | 542 | 66.1 | 8.99% | 0.370% | |
| ThyaSat04-145 | 2 | 145 | 55.9 | 20.05% | 0.110% |
| ThyaSat05-291 | 291 | 58.8 | 9.26% | 0.016% | |
| ThyaSat06-145 | 2 | 145 | 61.4 | 20.13% | 0.015% |
| ThyaSat07-145 | 2 | 145 | 58.6 | 16.87% | 0.017% |
| ThyaSat08-145 | 2 | 145 | 58.6 | 20.59% | 0.017% |
| Total | 16.565% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.A.; Cabral-de-Mello, D.C.; Lopes, D.M. The Satellite DNAs Populating the Genome of Trigona hyalinata and the Sharing of a Highly Abundant satDNA in Trigona Genus. Genes 2023, 14, 418. https://doi.org/10.3390/genes14020418
Pereira JA, Cabral-de-Mello DC, Lopes DM. The Satellite DNAs Populating the Genome of Trigona hyalinata and the Sharing of a Highly Abundant satDNA in Trigona Genus. Genes. 2023; 14(2):418. https://doi.org/10.3390/genes14020418
Chicago/Turabian StylePereira, Jaqueline A., Diogo C. Cabral-de-Mello, and Denilce M. Lopes. 2023. "The Satellite DNAs Populating the Genome of Trigona hyalinata and the Sharing of a Highly Abundant satDNA in Trigona Genus" Genes 14, no. 2: 418. https://doi.org/10.3390/genes14020418
APA StylePereira, J. A., Cabral-de-Mello, D. C., & Lopes, D. M. (2023). The Satellite DNAs Populating the Genome of Trigona hyalinata and the Sharing of a Highly Abundant satDNA in Trigona Genus. Genes, 14(2), 418. https://doi.org/10.3390/genes14020418







