Abstract
Treatment-resistant schizophrenia (TRS) is often associated with severe burden of disease, poor quality of life and functional impairment. Clozapine is the gold standard for the treatment of TRS, although it is also known to cause significant side effects in some patients. In view of the burgeoning interest in the role of genetic factors in precision psychiatry, we conducted a scoping review to narratively summarize the current genetic factors associated with TRS, clozapine resistance and side effects to clozapine treatment. We searched PubMed from inception to December 2022 and included 104 relevant studies in this review. Extant evidence comprised associations between TRS and clozapine resistance with genetic factors related to mainly dopaminergic and serotoninergic neurotransmitter systems, specifically, TRS and rs4680, rs4818 within COMT, and rs1799978 within DRD2; clozapine resistance and DRD3 polymorphisms, CYP1A2 polymorphisms; weight gain with LEP and SNAP-25 genes; and agranulocytosis risk with HLA-related polymorphisms. Future studies, including replication in larger multi-site samples, are still needed to elucidate putative risk genes and the interactions between different genes and their correlations with relevant clinical factors such as psychopathology, psychosocial functioning, cognition and progressive changes with treatment over time in TRS and clozapine resistance.
1. Introduction
Schizophrenia is a debilitating major mental illness, and it affects about 1% of the population [1]. Prevalent cases of schizophrenia globally have been reported to increase from 13.1 million in 1990 to 20.9 million in 2016, contributing almost 13.4 million years of life lived with disability to the global disease burden [2]. The economic burden of schizophrenia in the United States was estimated to be more than USD 60 billion annually [3]. Unfortunately, not all patients with schizophrenia respond adequately to treatment. Although the mainstay of treatment for schizophrenia is antipsychotic medication, approximately one third of patients have a limited response to antipsychotic medication treatment and are assessed to have treatment-resistant schizophrenia (TRS) [4]. Patients with TRS, compared with schizophrenia in remission, are reported to have an increased rate of suicidal ideation [5], impaired cognitive functioning [6], greater medical comorbidities [7], lower quality of life and higher cost of treatment [5]. Several hypotheses have been proposed for the underlying neurobiology of TRS [8]. One hypothesis suggests that TRS is a result of dopamine supersensitivity as a result of a continuous blockade of dopamine receptors by antipsychotic medications [9,10]. Another hypothesis states that TRS is a result of glutamate dysregulation, which in turn stimulates the activity of dopaminergic projections from the midbrain to the striatum, giving rise to positive symptoms of schizophrenia [11,12].
The criteria to assess for TRS have been continuously updated since 1966 [13] and have been mentioned in several practice guidelines, including those from the American Psychiatric Association [14], The Royal Australian and New Zealand College of Psychiatrists [15], and the British Association for Psychopharmacology [16]. In order to establish consensus criteria to standardize the definition of TRS, the Treatment Response and Resistance in Psychosis (TRRIP) working group was formed, and it proposed that the TRS criteria should include the following: (1) current symptoms of at least moderate severity; (2) treatment with at least two different antipsychotic medications; (3) treatment duration of at least 6 weeks with a total daily dose equivalent of at least 600 mg of chlorpromazine; and (4) medication adherence of at least 80% of the prescribed doses taken [17].
Of note, clozapine is considered as a gold standard for the treatment of TRS and is also often considered as a proxy indicator of treatment resistance [18]. Historically, in 1988, Kane and colleagues first demonstrated the efficacy of clozapine in treating TRS [19]. Subsequently, more studies have reported that clozapine exhibits superiority over other antipsychotics for TRS [20,21]. A meta-analysis included 2530 randomly assigned participants in 30 clinical trials and found that clozapine was more effective than conventional neuroleptics in reducing symptoms in patients with TRS [20]. Another meta-analysis involved 1916 independent patients in 12 controlled studies and confirmed that clozapine was superior in controlling psychotic symptoms in TRS [21]. However, clozapine has significant side effects, some of which are severe and life-threatening. Common side effects of clozapine include tachycardia, metabolic syndrome, hyper-salivation and constipation, while severe side effects (with relative lethality (RL) calculated as fatal outcomes out of reported cases) include pneumonia (RL 30%), myocarditis (RL 12%), arrhythmia (RL 5%), seizure (RL 5%) and agranulocytosis (RL 2%) [22,23,24]. There are several factors that can modulate the clozapine effect, including genetic factors and drug–drug interactions. To reduce the risk of clozapine-related side effects, a recent international guideline suggests considering three parameters: (1) a DNA ancestry group, (2) a sex-smoking subgroup, and (3) the presence or absence of clozapine poor metabolizer status [24].
To better understand the underlying biology of TRS and optimize its treatment, there is increasing interest in the genetic factors associated with TRS and clozapine treatment. For example, a recent genetic association study found that an interaction between the dopaminergic and γ-aminobutyric acid (GABA) signal intensities could differentiate patients with and without TRS [25]. Another recent study reported that the Glutamate Decarboxylase 1 (GAD1) gene and the GABA Type B Receptor 2 (GABBR2) gene were associated with TRS [26]. Other than genes related to TRS, genes associated with clozapine treatment are explored in other studies as well. One study focused on the variability in cytochrome P450 (CYP) enzyme genes and found that CYP2C19 variants were associated with clozapine response [27]. Another study assessed the genes related to clozapine side effects and demonstrated that polymorphism in the α 2A adrenergic receptor gene was associated with clozapine-induced sialorrhea [28].
The aim of the current scoping review is to provide a comprehensive and up-to-date overview of the genes associated with three main areas, namely (1) TRS, (2) clozapine resistance, and (3) clozapine-related side effects. In the interest of exploring the clinical effects of clozapine, this review will concentrate on the pharmacodynamic genes that are pertinent to clozapine treatment. A better understanding of the underlying genetic underpinnings can potentially help to uncover the complex biology of TRS, including clozapine resistance and side effects, with the hope of better optimized treatments of TRS.
2. Materials and Methods
The literature search was performed in the PubMed database by including the keyword “schizophrenia” in the title of the article, linked with a combination of the following keywords: “resistant”, “refractory”, “response”, “treatment”, “clozapine” and “gene” in the title or abstract. We included papers published from database inception to December 2022, removing any duplicates prior to screening. In accordance with the inclusion criteria and exclusion criteria, two authors (JY and QHC) independently screened the retrieved literature. In the case of a disagreement, both authors would discuss with the rest of the team until a consensus was reached. Selection criteria were as follows: (1) original papers published in English, (2) definition of TRS was provided by the study, and/or it was explicitly stated that participants recruited were treatment-resistant or on clozapine, (3) genes related to TRS or clozapine were analyzed, (4) most, if not all, of the participants in the primary group of interest had TRS or were on clozapine, (5) studies on human participants. Exclusion criteria were (1) uncertainty about whether participants were treatment-resistant or on clozapine, (2) studies on animals, (3) there was no main gene(s) of interest investigated in relation to TRS or clozapine resistance, (4) the paper was a meta-analysis or systematic review, (5) sample size smaller than five. For each included study, we extracted variables that included the characteristics of subjects, the definition of TRS, and the significant genetic associations.
3. Results
Overall, 104 studies were included in this review (see Figure 1). The studies were conducted in Asia (n= 38, 36.5%), the United States (n = 25, 24.0%), Europe (n = 23, 22.1%), South America (n = 9, 8.65%), both Europe and Asia (n = 5, 4.81%), Australia (n = 3, 2.88%), and Africa (n = 1, 0.96%). The sample size varied from less than 10 to a few thousand, with an average age of 38.8 ± 5.21 years for patients with TRS examined. The proportion of males with TRS across all studies was 66.1%, and the average duration of illness was 16.3 ± 4.54 years.
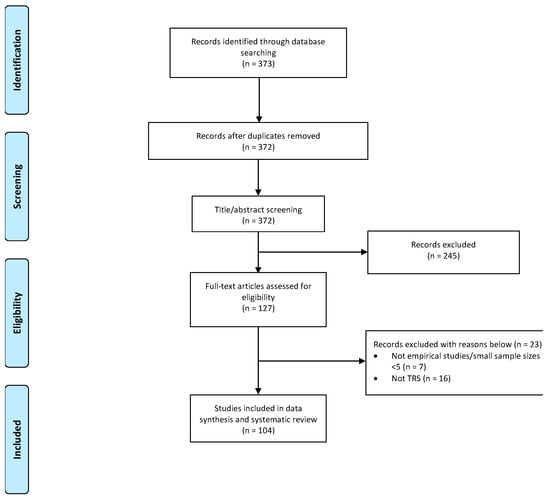
Figure 1.
PRISMA chart of studies included in this review.
3.1. Risk Genes/Polymorphisms for Treatment-Resistant Schizophrenia
The most common definition of TRS reported in the included studies consisted of three major elements. First, there is no sufficient improvement in symptoms, or a persistence of moderate-to-severe symptoms, as assessed with the Positive and Negative Syndrome Scale (PANSS), Brief Psychotic Rating Scale (BPRS), and Global Assessment of Functioning (GAF). Second, sufficient doses of two or more trials of antipsychotics (chlorpromazine-equivalent dose of 600 mg or higher) had been used prior to the diagnosis of TRS. Some studies specified that the antipsychotics should be from at least two different biochemical classes. Third, the antipsychotics should have been trialed for a sufficient duration, often defined as a period of at least 4 to 6 weeks. The genes and polymorphisms that are associated with an increased risk of TRS are listed in Table 1.

Table 1.
Risk genes/polymorphisms for treatment-resistant schizophrenia.
3.1.1. Dopaminergic System—Genes Related to TRS
- Catechol-O-methyltransferase (COMT)
COMT plays a key role in the regulation of dopamine activity in the prefrontal cortex and could indirectly affect dopamine levels in the striatum, leading to treatment resistance [55]. Two main single-nucleotide polymorphisms (SNPs) in COMT (rs4680 and rs4818) were associated with an increased risk of TRS (See Table 1). COMT rs4680 met allele carriers who were male [29], as well as females with the A/A genotype [30], were at increased risk. Females with the COMT rs4818 C/C genotype were similarly vulnerable [30]. A haplotype of rs4818-rs4680 non G-G/G-G was associated with TRS in females only [30].
- Dopamine Receptor D2 (DRD2), Dopa Decarboxylase (DDC)
The DRD2 gene codes for the D2 dopamine receptor, which is the primary site of the therapeutic action of antipsychotics [56] and has been implicated in the pathophysiology of schizophrenia [57]. DRD2 rs1799978 is associated with an increased risk of TRS [32] in G allele carriers [33]. DDC is an enzyme required for dopamine synthesis, and aberrant dopamine synthesis is hypothesized to underlie symptoms related to schizophrenia [58]. DDC rs10499696 A/A genotype carriers were also more susceptible to TRS [32]. GRB10, a neighboring gene of DDC, was also associated with TRS, with a higher proportion of rs2237457 T/T genotype carriers found in the TRS group [42].
- Combination of dopaminergic genes
A combination of the COMT rs4680 met/met genotype and the DRD3 rs6280 Ser/Gly genotype was also predictive of TRS [33].
3.1.2. Serotonergic System—Genes Related to TRS
- 5-Hydroxytryptamine Receptor 2A (HTR2A), 5-Hydroxytryptamine Receptor 2C (HTR2C)
Serotonin or 5-hydroxytryptamine (5-HT) also plays an important role in schizophrenia. HTR2C receptors are able to inhibit dopamine release in the limbic brain areas, and this has been hypothesized to cause psychotic symptoms and affect the efficacy of antipsychotics [59]. Similarly, HTR2A receptors showed a reduction in binding in schizophrenia patients and has been implicated in the pathophysiology of schizophrenia [60]. Studies included in our review only reported significant findings for these two genes in male patients. The HTR2A 2/2 genotype [34], as well as the HTR2C rs6318 Ser non-carriers and HTR2C rs3813929-rs6318 non C-Ser haplotype carriers [35] were at increased risk for TRS.
- Serotonin Transporter Gene-linked Polymorphic Region (SERT-PR)
Given serotonin’s importance in the pathophysiology of schizophrenia, there has been interest in investigating regional changes in 5-HT sites, such as the SERT promoter region. The SS genotype of SERT-PR was predictive of TRS in a study by Bilic et al. [31].
- Combination of dopamine and serotonergic genes
In patients with Dopamine Transporter Gene (SLC6A3) 10/10 or 10/12 genotype, those who had the SERT-in2 ls or ss genotype were more susceptible to TRS [31]. In patients with the SERT-in2 ll genotype, the SLC6A3 9/10, 9/11, 9/9, or 6/6 genotype was also associated with TRS [31].
3.1.3. GABA/Glutamatergic System—Genes Related to TRS
- Glutamate decarboxylase 1 (GAD1), γ-Aminobutyric Acid Type B Receptor Subunit 2 (GABBR2)
The compound γ aminobutyric acid (GABA) is implicated in the pathophysiology of schizophrenia, affecting three main areas relating to learning, memory, and executive functions [61,62]. GAD1, the rate-limiting enzyme of GABA, is posited to contribute to GABA dysfunction in the brain of schizophrenia patients [63]. One study has found showing that GAD1 rs3749034 and GABBR2 rs10985765 are related to TRS as well [26].
- Glutamate Metabotropic Receptor 3 (GRM3)
GRM3 codes for the mGluR3 protein, which is needed for optimal glutamate signaling in the brain [64], and it has been associated with response to antipsychotic treatment [65]. The GRM3 rs1989796 TT genotype, as well as the rs1476455 CC genotype, were associated with TRS [36].
- Combination of GABA/Glutamatergic system and dopaminergic system
Kogure and colleagues [25] reported that COMT rs4680/GAD1 rs3749034 met(+)/T(𢄡) carriers were more likely to be found in the TRS group.
3.1.4. Other Gene Variants—Genes Related to TRS
- Endocannabinoid
The endocannabinoid system is hypothesized to contribute to psychotic symptoms, and the Cannabinoid Receptor 1 (CNR1) gene, which codes for endocannabinoid receptors, has been investigated for its possible links to schizophrenia [66]. There was an increase in the expression of CNR1 found amongst TRS patients, and the rs806368 T/T and T/T genotype carriers as well as rs1049353 G/G genotype carriers [32] had an increased risk of being diagnosed with TRS.
- Oxytocin (OXT)
Abnormalities in the dopaminergic and oxytocinergic reward system signaling in the amygdala could underlie the social deficits often witnessed in schizophrenia [67]. This ranges from the negative symptoms of withdrawal and isolation to the positive symptoms of suspicion and paranoia [67]. The OXT rs2740210 C allele and the OXTR rs2228485 A allele as well as the A/A genotype were associated with TRS [32].
- Brain Derived Neurotrophic Factor (BDNF)
Neurodevelopmental models have proposed that synaptic function is negatively affected when BDNF concentrations are reduced, altering neurotransmission and giving rise to symptoms seen in schizophrenia [68,69]. Badrlou and colleagues [38] found higher expression levels of BDNF as well as the BDNF-associated lncRNA PKNY in TRS patients. Zhang and colleagues [39] reported an increased incidence of TRS in minor allele carriers of multiple BDNF SNPs.
- Cytochrome P450s (CYP)
CYP-related genes code for drug-metabolizing enzymes, such as those involved in metabolizing antipsychotics. TRS was associated with higher mRNA transcript levels of CYP2A6 and a reduced mRNA expression of CYP2D6 and CYP3A4 [40]. Martínez-Magaña and colleagues [41] also reported a loss-of-function variant carrier amongst the TRS group.
- MicroRNAs (miRNA) and associated biogenesis machinery
DICER1 is a main component of the miRNA biogenesis machinery [70] and was upregulated in TRS patients [37]. Expression levels of multiple miRNAs were also elevated in TRS patients, including miR-181b-5p, miR-195-5p, miR-301a-3p [46], hsa-miR-218-5p, hsa-miR-1262 [47], and hsa-miR-675-3p [48].
- Inflammation and oxidative stress
Oxidative stress has been associated with the pathophysiology of schizophrenia [71], and Glutathione S-transferase (GST) is involved in detoxification, thereby protecting cells and tissues from oxidative stress damage. Among the GSTs, double-null genotype carriers of GSTT1 and GSTM1 were found to have increased risk of TRS [43]. Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine produced by both neurons and glial cells and may be related to psychiatric symptoms [72]. Aytac and colleagues [53] reported a significant relationship between TRS and the TNF-α-238 GG genotype.
- Transcripts within the NRG–ErbB signaling pathway
Neuregulin-1 (NRG1) is involved in various neurodevelopmental processes, including cell migration, synaptic formation, plasticity, and myelination [73]. NRG1 regulates both excitatory and inhibitory synaptic transmission in the adult brain, and abnormal neurotransmission and/or synaptic plasticity have been reported in both glutamatergic and GABAergic pathways in the brain of schizophrenia patients (e.g., [74]). In our included studies, NRG1 rs7834206 was found to be associated with TRS [49]. AKT serine/threonine kinase 1 (AKT1) acts downstream of the dopamine D2 receptor and has also been associated with schizophrenia in several studies [75,76,77]. It was upregulated in TRS patients in a study by Moretti and colleagues [37]. P70S6K, a protein kinase linked to protein synthesis, cell growth, and cell proliferation, was elevated in TRS patients [50].
- Genes involved in synaptic functioning
One of the lines of evidence supporting the neurodevelopmental hypothesis of schizophrenia emerges from the finding of abnormal expression of genes involved in neuronal and glial migration, cell proliferation, and synaptogenesis (e.g., [78]). Altered reelin expression may result in the impairment of neuronal connectivity and synaptic plasticity, leading to the cognitive deficits observed in schizophrenia [79]. The study by Goldberger and colleagues [51] provides further support for the involvement of reelin in schizophrenia, with (CGG)10 alleles and genotypes in particular being associated with TRS. Another gene involved in synaptic functioning, SNAP25 synaptosome-associated protein 25 (SNAP-25), was found to be related to TRS. The MnlI T/G or G/G genotype, as well as the TaiI T/T or T/C genotypes, were associated with an increased risk of TRS [52]. Neurexin 1 (NRXN1), which has a fundamental role in synaptogenesis and synaptic maintenance, had a lower methylation rate in TRS patients, as compared to HCs [44].
- Ubiquitin-related genes
Ubiquitin fusion degradation protein 1 (UFD1) is involved in protein degradation and was found to have increased expression amongst TRS patients [37]. Another ubiquitin conjugating enzyme (Ubiquitin Conjugating Enzyme E2 K; UBE2K) was also associated with schizophrenia, and this association is hypothesized to be driven by elevated levels of ubiquitinated proteins [80]. Higher mRNA levels of UBE2K have also been found in TRS patients, providing evidence for the involvement of this gene [54].
3.2. Risk Genes/Polymorphisms for Clozapine Resistance
Table 2 shows the genes and polymorphisms associated with increased risk for clozapine resistance. Clozapine is often prescribed to patients who have demonstrated inadequate response to at least two antipsychotics.

Table 2.
Risk genes/polymorphisms for Clozapine resistance.
The criterion for determining clozapine response/resistance was more varied among the included studies as compared to that of treatment resistance. Most studies employed change scores of rating scales (such as BPRS, PANSS, GAS) from pre-treatment baseline [52,84,101,107,108,109,110,111,112,113,114] or a reduction of more than or equal to 20% in scores, which is often used as an indication of clozapine response [35,81,85,88,96,102,103,106,115,116,117,118,119]. There were also studies that necessitated a greater reduction in scores before considering the patient to be clozapine responsive [83,87,92,104]. A minimum treatment period ranging from 6 to 8 weeks [101,107], 10 weeks [117], and 6 months [81,83,85,103,106] was part of the criteria used by most studies.
3.2.1. Dopaminergic System—Genes Related to Clozapine Resistance
- DRD2, DRD3, DRD4
Similar to that of treatment resistance, dopamine receptor genes (DRD2, DRD3, DRD4) were found to be related to clozapine resistance (See Table 2). Specifically, DRD2 rs1799978 T allele carriers [32], rs2514218 G/G genotype carriers [81], DRD3 Ser 9 allele carriers and Ser9/Ser9 genotype carriers [83], as well as 1-1 genotype carriers [84] had an increased risk of developing clozapine resistance. As for DRD4, most of the risk-gene polymorphisms found to be significantly related to clozapine resistance were specific to either African-Americans or Caucasian patients [85].
- Solute Carrier Family 6 Member 3 (SLC6A3)
Previous studies suggest that the human dopamine transporter (SLC6A3) plays an essential role in the accumulation of extracellular dopamine through which dopamine neurotransmission is controlled, and it is also the main action site of psychostimulants [120,121,122]. Psychotic disturbances have been linked to the aberrant release of dopamine and neurotransmission, which possibly implicates SLC6A3 in schizophrenia. The rs2975226-71A allele, as well as the rs2652511-844C × rs2975226-71A × rs2963238-1491C haplotype, was associated with an increased risk of poor response to clozapine [87].
COMT and combinations with other dopaminergic genes
COMT rs4680 met allele carriers were more likely to be clozapine resistant [33]. COMT val/val genotype carriers who also had DRD4 120/120 or 120/140 genotypes had an increased risk of developing clozapine resistance [86].
3.2.2. Serotonergic System—Genes Related to Clozapine Resistance
- HTR2A, HTR2C, HTR3A, HTR3B
HTR2A tyr452 allele carriers [88], HTR3A rs1062613 C allele or T/T genotype carriers [89,90], rs2276392 A allele carriers [89], as well as HTR3B-100_-102delAAG(del) minor allele carriers [91] were at higher risk of developing clozapine resistance. HTR2C rs6318 Ser non-carriers were also more likely to be clozapine resistant, although this effect was significant only among males [35].
Serotonin-Transporter-Linked Promoter Region (SLC6A4/HTTLPR)
The SLC6A4 rs25531 S′ allele and the S′/S′ or S′/L′ genotypes were associated with clozapine resistance [92].
3.2.3. GABA/Glutamatergic Systems—Genes Associated with Clozapine Resistance
GAD67 mRNA is an enzyme which has a key role in the production and release of brain GABA [123]. Higher mRNA levels of GAD67, GAD25, and GAD1 were detected in patients with clozapine resistance [93].
3.2.4. Other Genes Associated with Clozapine Resistance
- Endocannabinoid
The CNR1 rs8006379 C allele as well as the rs1043953 A allele carriers were at higher risk of developing clozapine resistance [32].
- BDNF
A higher expression of BDNF [95] and the Val/Val genotype [96] were associated with clozapine resistance in two of the included studies.
- CYP
CYP1A2 and CYP2C19 activity scores were elevated and reduced in patients with clozapine resistance, respectively [100]. In addition, the CYP1A2*1F AA and AC genotypes [98], as well as the CYP2C19 *1/*17 genotype and *2 allele carriers [27] were at increased risk of developing clozapine resistance. The CYP1A2-163A allele was associated with clozapine resistance in smokers only [99].
Drug transporters encoded by the human ATP-binding cassette (ABC) gene family are hypothesized to affect the pharmacokinetics and response to clozapine [124]. Breast Cancer Resistance Protein (BCRP) encoded by the ABCG2 gene may be inhibited by clozapine and affect its plasma concentrations [125]. The ABCG2 421 C/C genotype was associated with clozapine resistance in a study by Akamine and colleagues [94].
- cAMP-Response Element Binding Protein (CREB)
The CREB binding protein is a co-activator of the CREB1 gene, which has been associated with response to treatment (in terms of cognitive improvement) in schizophrenia patients [126]. The CpG site cg05151055 of CREBBP showed decreased methylation in patients with clozapine resistance [97].
Mitochondrial uncoupling protein 4 (SLC25A27/UCP4) regulates the production of reactive oxygen species [127] as well as cellular calcium homeostasis [128] and has a neuroprotective function. Oxidative stress and aberrant calcium signaling affect mitochondrial function and consequently neuronal plasticity and neurotransmission, thereby increasing the risk of schizophrenia [129,130]. Non-carriers of the CCAC haplotype of rs3757241 × rs10807344 × rs9395206 × rs2270450 were more likely to demonstrate poor response to clozapine [107].
Genes That Are Involved in Cellular Interactions and Responses
Contactin-associated protein-like 2 (CNTNAP2) encodes for a group of transmembrane proteins that control cell–cell interactions in the nervous system [131]. The internal and overlapping deletions in the CNTNAP2 gene were associated with an increased risk of schizophrenia [132]. Higher mRNA levels of CNTNAP2 were detected in patients with a poor clozapine response [93].
Guanine nucleotide binding proteins (G-proteins) regulate cellular responses. The abnormal expression and function of these proteins [133] and their subunits have been associated with various mental health conditions [134] as well as response to treatment [135]. In our included studies, the G-protein β subunit 3 (GNB3) 825 T/T genotype was also associated with a poorer response to clozapine [104].
Genes That Affect Neuronal Development
Disrupted in Schizophrenia 1 (DISC1) is posited to be involved in synaptic pruning [136], and an over-pruning of cortical synapses during critical neurodevelopmental periods is hypothesized to contribute to the development of schizophrenia [137,138]. The DISC1 rs3738401 minor allele A carriers, A/A or A/G genotype carriers, as well as rs6675281 T allele non-carriers had a poorer response to clozapine [101].
There is evidence to suggest that Dystrobrevin-binding Protein 1 (DTNBP1) may be involved in regulating neuronal growth and neurotransmission, contributing to the pathogenesis of schizophrenia as a result [139]. The association between DTNBP1 and clozapine response was only found in the African-American population for our included studies, with the rs742105 C allele carriers and the genotype C/C carriers demonstrating poorer responses [102].
Evidence indicates that Glial cell line-derived Neurotrophic Factor (GDNF) plays a role in mammalian neuronal development [140], and GDNF family receptor α-1 (GFRA) proteins act as co-receptors to allow GDNF proteins to bind to the receptors [103], suggesting that GFRA may be involved in the pathophysiology of schizophrenia. The GFRA2 1-1-2 SNP27-SNP34-SNP37 haplotype non-carriers showed a poorer response to clozapine in the study by Souza and colleagues [103].
Inositol Monophosphatase 2 (IMPA2) is hypothesized to be involved in the phospholipase C signalling pathway, which mediates the action of several neurotransmitters and hormones [141] and which may underlie schizophrenia. Patients with clozapine resistance had higher mRNA levels of IMPA2 in the study by Sershen and colleagues [93].
A lack of Potassium Inwardly Rectifying Channel Subfamily J Member 10 (KCNJ10) in mice has previously been associated with myelin compaction failure and axonal degeneration [142]. In line with this finding, a lower expression of KCNJ10 was also found in patients with a poorer clozapine response [95].
Ligand Of Numb-Protein X 1 (LNX1) is responsible for the proteasomal degradation of NUMB protein, which is an important regulator of neurogenesis and neuronal differentiation [143]. LNX1 expression was higher in patients with a poorer response to clozapine [95].
Nuclear factor 1 B-type (NFIB) is necessary for normal cortical formation and development [144,145]. This gene was implicated in clozapine response as well, with the rs28379954 C allele associated with a poorer response [105].
NRXN1 encodes the neurexin-1α protein, the lack of which results in a loss of synaptic strength in the excitatory synapses of the hippocampus [146,147]. The rs1045881 T allele was associated with a poorer clozapine response [106].
Serpin Family A Member 5 (SERPINA5) encodes a protein that plays a role in synaptic plasticity and memory formation [148]. Lower expression levels of SERPINA5 were associated with a poorer response to clozapine [95].
Tet Methylcytosine Dioxygenase 1 (TET1) appears to be involved in catalyzing 5hmC formation in oligodendrocytes [149] and is crucial for myelin formation and repair [63,150]. Lower mRNA levels of TET1 were associated with poorer clozapine response in patients [93].
3.3. Genes/Polymorphisms Associated with Clozapine Side Effects
Despite the efficacy of clozapine for treatment-resistant schizophrenia, there are significant side effects associated with its use, some of which are severe and life-threatening [22,23,24]. Table 3 shows the common genes and polymorphisms associated with clozapine side effects in patients.

Table 3.
Genes/polymorphisms associated with Clozapine side effects.
There were several common side effects reported by the included studies, with the two major categories being metabolic side effects and blood disorders. Low high-density lipoprotein was found in patients with the DRD2 141 Ins C allele homozygous genotype [151]. Weight gain was associated with the Leptin (LEP)-2548A/G polymorphism in two studies, with the A/A genotype in Kang et al. [157], and the G/A or G/G genotypes in Zhang et al. [122]. The SNAP-25 MnlI T/T genotype as well as the TaiI C/C genotype were associated with weight gain in another separate study [52]. Higher blood pressure, which is also part of metabolic syndrome, was found in SH2B1 minor allele carriers [158]. Metabolic syndrome as a whole was also linked to the presence of the Sterol Regulatory Element Binding Transcription Factor 2 (SREBF2) A allele in patients [159]. Of note, there were genes linked to the response to metformin, which had an effect on symptoms associated with metabolic syndrome. Transmembrane protein 18 (TMEM18) and Glucosamine-6-Phosphate Deaminase 2 (GNPDA2) minor allele carriers on metformin had a greater reduction in insulin levels and were more likely to lose more than 7% of their body weight after metformin treatment [158].
Blood disorders mainly presented in the form of agranulocytosis or granulocytopenia, which is a known possible severe side effect of clozapine. Elevated expression levels of the proapoptotic genes TP53, Bax α and Bik were found in patients experiencing agranulocytosis [152]. The Human Leukocyte Antigen (HLA) system encodes molecules necessary for pro-inflammatory responses, and several HLA polymorphisms were also linked to an increased risk of granulocytopenia/agranulocytosis in four separate studies [153,154,155,156]. Two other side effects were reported by the included studies, with Adrenoceptor α 2A (ADRA2A) rs1800544 C/C genotype being linked to sialorrhea [28]. ADRA2A receptors are hypothesized to play a role in the regulation of neurotransmitter release [160]. GNB3 T825, on the other hand, was associated with convulsive episodes [104].
4. Discussion
This review provided an updated summary of the evidence of specific genetic variants related to TRS, clozapine resistance and clozapine side effects. Extant evidence comprised of associations between TRS and clozapine resistance with genetic factors related to mainly dopaminergic and serotoninergic neurotransmitter systems. For example, TRS was associated with rs4680 and rs4818 within COMT and rs1799978 within DRD2, and clozapine resistance was associated with DRD3 polymorphisms and CYP1A2 polymorphisms. In addition, clozapine-related weight gain was associated with LEP, SNAP-25 genes, and agranulocytosis risk with HLA-related polymorphisms.
This study found that most genes related to TRS were mainly associated with dopaminergic systems and serotoninergic systems. This is understandable, as the proposed hypotheses for the neurobiological mechanisms underlying TRS include dopaminergic and serotoninergic dysregulation with inter-relationships with glutamatergic abnormality [8]. The dopamine supersensitivity hypothesis has been proposed as a potential etiology for TRS [161]. It is also acknowledged that not all patients with TRS display hyperdopaminergic activity, and some TRS cases can have normal dopamine regulation or hypodopaminergic activity [162]. Other neurotransmitters, such as glutamate and serotonin, also play a role in the etiology of TRS. For example, it has been reported that elevated glutamate levels may contribute to TRS [11]. There is also evidence that serotonin affects the clinical efficacy of antipsychotics [163]. The interactions between SLC6A3 and the variable number tandem repeat polymorphism inside SERT-in2 may also contribute to the development of TRS [31].
Genes related to other systems, such as the endocannabinoid, oxytocin and miRNA, were also found to be related to TRS in the current review, highlighting the complex genetic etiology of TRS. The endocannabinoid system has been reported to affect neurodevelopment [164], and changes in cannabinoid receptors in certain brain regions were found in patients with schizophrenia [165]. Oxytocin may be involved in negative symptoms of schizophrenia, such as social withdrawal and flattened affect, and certain genotypes related to oxytocin were associated with a response to antipsychotic drugs [32]. The expression changes of miRNA also played a significant role in the pathogenesis of schizophrenia [166]. Recently the expression levels of miRNA have been found to be different between TRS and non-TRS [48].
For clozapine resistance, COMT activity had been reported to be related to the clozapine-induced release of dopamine in the prefrontal area [167]. The gene–gene interaction between DRD4 and COMT may also modulate the clinical response to clozapine in TRS [86]. Polymorphisms in serotonin receptors were also involved in clozapine response [168], and serotonin modulators may be used to augment the effects of antipsychotics in schizophrenia [169]. Of note, in a neuroimaging study, glutamate and glutamine levels were lower in clozapine-resistant TRS compared to clozapine-responsive TRS [12], suggesting a possible role of glutamate in clozapine treatment response.
Notably, the genetic variants related to CYP enzymes were associated with clozapine resistance. Clozapine is primarily metabolized in the liver by the CYP450 enzymes. The main CYP enzyme involved in clozapine metabolism is CYP1A2, which is a potential determinant of clozapine dose requirement [170]. There are other CYP enzymes involved in clozapine metabolism, including CYP2C19, CYP2D6 and CYP3A4 [171]. CYP1A2 and CYP3A4 are mainly responsible for the demethylation of clozapine, while CYP3A4 is involved in the N-oxidation of clozapine [172]. A few studies have shed light on the association between CYP-genetic polymorphisms and clozapine response. For example, a recent study has shown that CYP2C19 polymorphisms influenced clozapine responses during the treatment of schizophrenia [27].
Several genetic variants, such as LEP and HLA polymorphisms, were reported to be associated with clozapine side effects, such as weight gain and agranulocytosis in the current review. Leptin, a hormone-regulating adipose tissue, is increased in the circulatory levels by clozapine, and this could lead to weight gain during clozapine treatment [173]. The LEP AA genotype has been associated with higher weight gain, and the A allele group may be related to a slow adaption response to environmental factors that are predisposed to weight gain [157]. Other than weight gain, neutropenia or agranulocytosis is another significant side effect of clozapine. One of the genetic risk factors for agranulocytosis are the HLA polymorphisms, which were associated with a range of immunogenetic phenomena, and specific HLA alleles may contribute to other adverse drug reactions, such as Stevens-Johnson Syndrome [174].
The emergence of genetic findings in major psychiatric disorders has inspired the development of Precision Psychiatry, which aims to provide personalized treatment approaches and acknowledges that a singular medication, dose, and treatment plan may not be effective for all patients with similar diagnoses [175]. The current diagnostic process for TRS requires the patient to try at least two different antipsychotics before the confirmation of TRS [17]. With the development of Precision Psychiatry, it is possible that TRS, clozapine resistance or potentially serious adverse effects for clozapine can be identified earlier, with suitable biological markers and managed accordingly. For example, to prevent life-threatening Stevens-Johnson syndrome among some Asians, HLA-B*1502 allele genotyping prior to the initiation of carbamazepine therapy in new patients of Asian ancestry is now considered the standard of care [176]. In addition, traditionally, the process of selecting an appropriate antipsychotic often involves a “trial and error” approach, where clinicians prescribe different antipsychotic at different dosages until an acceptable efficacy and tolerable side effects are achieved clinically. With the help of genetic technology, Precision Psychiatry may potentially guide more accurate and effective treatment. For example, a patient with risk genes suggesting clozapine resistance may be offered an alternative treatment, such as other pharmacotherapies or electroconvulsive therapy, to control the persistent psychotic symptoms. Though they are still developing, genetic markers in psychiatry offer a promising opportunity to complement other germane biological markers to improve precision in the clinical management of patients with schizophrenia.
There are several limitations in this review. First, the sample size varies widely across the studies, and the smaller sample sizes may limit the power to detect differences. Second, fewer studies compared the differences between TRS and clozapine resistance. For this review, a total of forty-two studies looked at TRS only (40.4%), fifty-three studies looked at resistance to or side effects from clozapine (51.0%), and only nine studies looked at both TRS and clozapine resistance (8.6%). Third, there is less correlation with clinical factors such as symptomatology, psychotropic drug treatment, and cognitive functioning. Fourth, there is a dearth of longitudinal studies and inter-relationships with clinical course. Fifth, despite the fact that genetic factors account for some variability in TRS, clozapine resistance and tolerability, other biological factors such as inflammation, oxidative stress, and neuronal and synaptic functioning may be contributory, as suggested by the genetic signals, thus making up the larger biosignature overall.
What are the implications for the future research on TRS? The historical candidate association studies have several limitations, including inadequate statistical power, inconsistent results, and false associations [177,178]. With the advancements of gene detection technology, there is promise of better elucidation of the underlying genetic architecture of TRS and clozapine resistance. Following the cost reduction in new genotyping technology, it has been pointed out that psychiatric genetics is shifting away from candidate association studies based on historical choices [179]. Genome-wide association studies (GWAS) adopt an atheoretical approach, have improved our understanding of the genetic basis of schizophrenia and other psychiatric disorders [57,180], and have the potential to examine common genetic variants related to TRS [181]. A combination of GWAS with fine mapping and functional genomic data has been conducted on schizophrenia [182] and can potentially unravel insights into the neurobiology of TRS and prioritize the genetic factors for further evaluation. More recent investigations have also applied novel techniques, such as whole exome sequencing (WES) and polygenic risk score (PRS) analysis, to further explore the multiple genetic variants contributing to disease risk. WES can capture rare types of genetic variants, but there is only a small number of such studies on schizophrenia [183]. PRS provides a weighted sum of the number of risk alleles in an individual, with higher scores indicating a higher genetic risk burden [184], and there is some evidence that a higher genetic risk burden indicates a higher likelihood of developing TRS [185]. The development of a PRS for TRS may eventually lead to a high predictive accuracy of TRS [186]. Future work may also want to focus on greater correlation between the genetic factors and clinical phenotype as well as studying the inter-relationships with other measures such as neuroimaging and cognitive and longitudinal outcome variables.
5. Conclusions
In conclusion, in view of the huge clinical and socio-economic burden associated with TRS, there is compelling reason to better understand the biological basis of TRS with the potential for identifying novel treatment targets to optimize clinical treatment. This review of the extant genetic studies in TRS provided insights into the complex genetic heterogeneity related to different neurotransmitter systems, neuronal development and cellular functioning. Conducting genome-wide investigations on substantial cohorts and performing replication studies with a well-selected panel of causal single nucleotide variants will advance our knowledge of TRS and clozapine pharmacogenomics. Further work, including replication in larger multi-site genetic studies, is needed to elucidate putative risk genes and the interactions between different genes and their correlations with relevant clinical factors such as psychopathology, psychosocial functioning, cognition and progressive changes with treatment over time in TRS and clozapine resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14030689/s1, Table S1: Summary of study and population characteristics, definition of treatment resistance, and correlates.
Author Contributions
Conceptualization, K.S.; methodology, J.Y., Q.H.C. and K.S.; formal analysis, J.Y. and Q.H.C.; data curation, J.Y., Q.H.C. and K.S.; writing—original draft preparation, J.Y. and Q.H.C.; writing—review and editing, K.S. and R.S.M.; supervision, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Details of included studies are found in Supplementary Table S1.
Conflicts of Interest
Dr. Roger S. McIntyre has received a research grant from the CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute, and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, Atai Life Sciences. Dr. Roger McIntyre is CEO of Braxia Scientific Corp. The other authors have no disclosures.
References
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef]
- Charlson, F.J.; Ferrari, A.J.; Santomauro, D.F.; Diminic, S.; Stockings, E.; Scott, J.G.; McGrath, J.J.; Whiteford, H.A. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef]
- Marcus, S.C.; Olfson, M. Outpatient antipsychotic treatment and inpatient costs of schizophrenia. Schizophr. Bull. 2008, 34, 173–180. [Google Scholar] [CrossRef]
- Elkis, H.; Buckley, P.F. Treatment-Resistant Schizophrenia. Psychiatr. Clin. North Am. 2016, 39, 239–265. [Google Scholar] [CrossRef]
- Kennedy, J.L.; Altar, C.A.; Taylor, D.L.; Degtiar, I.; Hornberger, J.C. The social and economic burden of treatment-resistant schizophrenia: A systematic literature review. Int. Clin. Psychopharmacol. 2014, 29, 63–76. [Google Scholar] [CrossRef]
- Iasevoli, F.; Giordano, S.; Balletta, R.; Latte, G.; Formato, M.V.; Prinzivalli, E.; De Berardis, D.; Tomasetti, C.; de Bartolomeis, A. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 34–48. [Google Scholar] [CrossRef]
- Firth, J.; Siddiqi, N.; Koyanagi, A.; Siskind, D.; Rosenbaum, S.; Galletly, C.; Allan, S.; Caneo, C.; Carney, R.; Carvalho, A.F.; et al. The Lancet Psychiatry Commission: A blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019, 6, 675–712. [Google Scholar] [CrossRef]
- Potkin, S.G.; Kane, J.M.; Correll, C.U.; Lindenmayer, J.P.; Agid, O.; Marder, S.R.; Olfson, M.; Howes, O.D. The neurobiology of treatment-resistant schizophrenia: Paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020, 6, 1. [Google Scholar] [CrossRef]
- Chouinard, G.; Chouinard, V.A. Atypical antipsychotics: CATIE study, drug-induced movement disorder and resulting iatrogenic psychiatric-like symptoms, supersensitivity rebound psychosis and withdrawal discontinuation syndromes. Psychother. Psychosom. 2008, 77, 69–77. [Google Scholar] [CrossRef]
- Chouinard, G.; Jones, B.D.; Annable, L. Neuroleptic-induced supersensitivity psychosis. Am. J. Psychiatry 1978, 135, 1409–1410. [Google Scholar] [CrossRef]
- Demjaha, A.; Egerton, A.; Murray, R.M.; Kapur, S.; Howes, O.D.; Stone, J.M.; McGuire, P.K. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol. Psychiatry 2014, 75, e11–e13. [Google Scholar] [CrossRef]
- Goldstein, M.E.; Anderson, V.M.; Pillai, A.; Kydd, R.R.; Russell, B.R. Glutamatergic neurometabolites in clozapine-responsive and -resistant schizophrenia. Int. J. Neuropsychopharmacol. 2015, 18, pyu117. [Google Scholar] [CrossRef]
- Itil, T.M.; Keskiner, A.; Fink, M. Therapeutic studies in "therapy resistant" schizophrenic patients. Compr. Psychiatry 1966, 7, 488–493. [Google Scholar] [CrossRef]
- Lehman, A.F.; Lieberman, J.A.; Dixon, L.B.; McGlashan, T.H.; Miller, A.L.; Perkins, D.O.; Kreyenbuhl, J.; American Psychiatric, A.; Steering Committee on Practice, G. Practice guideline for the treatment of patients with schizophrenia, second edition. Am. J. Psychiatry 2004, 161, 1–56. [Google Scholar]
- Galletly, C.; Castle, D.; Dark, F.; Humberstone, V.; Jablensky, A.; Killackey, E.; Kulkarni, J.; McGorry, P.; Nielssen, O.; Tran, N. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust. N. Z. J. Psychiatry 2016, 50, 410–472. [Google Scholar] [CrossRef]
- Barnes, T.R.; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: Recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2011, 25, 567–620. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R.; Agid, O.; de Bartolomeis, A.; van Beveren, N.J.; Birnbaum, M.L.; Bloomfield, M.A.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T.; et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef]
- Khokhar, J.Y.; Henricks, A.M.; Sullivan, E.D.K.; Green, A.I. Unique Effects of Clozapine: A Pharmacological Perspective. Adv. Pharmacol. 2018, 82, 137–162. [Google Scholar] [CrossRef]
- Kane, J.; Honigfeld, G.; Singer, J.; Meltzer, H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry 1988, 45, 789–796. [Google Scholar] [CrossRef]
- Wahlbeck, K.; Cheine, M.; Essali, A.; Adams, C. Evidence of clozapine’s effectiveness in schizophrenia: A systematic review and meta-analysis of randomized trials. Am. J. Psychiatry 1999, 156, 990–999. [Google Scholar] [CrossRef]
- Chakos, M.; Lieberman, J.; Hoffman, E.; Bradford, D.; Sheitman, B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: A review and meta-analysis of randomized trials. Am. J. Psychiatry 2001, 158, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.L.; Freudenreich, O.; Sayer, M.A.; Love, R.C. Addressing Barriers to Clozapine Underutilization: A National Effort. Psychiatr. Serv. 2018, 69, 224–227. [Google Scholar] [CrossRef]
- Mijovic, A.; MacCabe, J.H. Clozapine-induced agranulocytosis. Ann. Hematol. 2020, 99, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- de Leon, J.; Schoretsanitis, G.; Smith, R.L.; Molden, E.; Solismaa, A.; Seppala, N.; Kopecek, M.; Svancer, P.; Olmos, I.; Ricciardi, C.; et al. An International Adult Guideline for Making Clozapine Titration Safer by Using Six Ancestry-Based Personalized Dosing Titrations, CRP, and Clozapine Levels. Pharmacopsychiatry 2022, 55, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Kogure, M.; Kanahara, N.; Miyazawa, A.; Oishi, K.; Nakata, Y.; Oda, Y.; Iyo, M. Interacting Roles of COMT and GAD1 Genes in Patients with Treatment-Resistant Schizophrenia: A Genetic Association Study of Schizophrenia Patients and Healthy Controls. J. Mol. Neurosci. 2021, 71, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, A.; Kanahara, N.; Kogure, M.; Otsuka, I.; Okazaki, S.; Watanabe, Y.; Yamasaki, F.; Nakata, Y.; Oda, Y.; Hishimoto, A.; et al. A preliminary genetic association study of GAD1 and GABAB receptor genes in patients with treatment-resistant schizophrenia. Mol. Biol. Rep. 2022, 49, 2015–2024. [Google Scholar] [CrossRef]
- Rodrigues-Silva, C.; Semedo, A.T.; Neri, H.; Vianello, R.P.; Galaviz-Hernandez, C.; Sosa-Macias, M.; de Brito, R.B.; Ghedini, P.C. The CYP2C19*2 and CYP2C19*17 Polymorphisms Influence Responses to Clozapine for the Treatment of Schizophrenia. Neuropsychiatr. Dis. Treat 2020, 16, 427–432. [Google Scholar] [CrossRef]
- Solismaa, A.; Kampman, O.; Seppälä, N.; Viikki, M.; Mäkelä, K.M.; Mononen, N.; Lehtimäki, T.; Leinonen, E. Polymorphism in alpha 2A adrenergic receptor gene is associated with sialorrhea in schizophrenia patients on clozapine treatment. Hum. Psychopharmacol. 2014, 29, 336–341. [Google Scholar] [CrossRef]
- Hajj, A.; Obeid, S.; Sahyoun, S.; Haddad, C.; Azar, J.; Rabbaa Khabbaz, L.; Hallit, S. Clinical and Genetic Factors Associated with Resistance to Treatment in Patients with Schizophrenia: A Case-Control Study. Int. J. Mol. Sci. 2019, 20, 4753. [Google Scholar] [CrossRef]
- Sagud, M.; Tudor, L.; Uzun, S.; Perkovic, M.N.; Zivkovic, M.; Konjevod, M.; Kozumplik, O.; Vuksan Cusa, B.; Svob Strac, D.; Rados, I.; et al. Haplotypic and Genotypic Association of Catechol-O-Methyltransferase rs4680 and rs4818 polymorphisms and treatment resistance in schizophrenia. Front. Pharmacol. 2018, 9, 705. [Google Scholar] [CrossRef]
- Bilic, P.; Jukic, V.; Vilibic, M.; Savic, A.; Bozina, N. Treatment-resistant schizophrenia and DAT and SERT polymorphisms. Gene 2014, 543, 125–132. [Google Scholar] [CrossRef]
- Zazueta, A.; Castillo, T.; Cavieres, A.; Gonzalez, R.; Abarca, M.; Nieto, R.R.; Deneken, J.; Araneda, C.; Moya, P.R.; Bustamante, M.L. Polymorphisms in Schizophrenia-Related Genes Are Potential Predictors of Antipsychotic Treatment Resistance and Refractoriness. Int. J. Neuropsychopharmacol. 2022, 25, 701–708. [Google Scholar] [CrossRef]
- Escamilla, R.; Camarena, B.; Saracco-Alvarez, R.; Fresán, A.; Hernández, S.; Aguilar-García, A. Association study between. Neuropsychiatr. Dis. Treat 2018, 14, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Joober, R.; Benkelfat, C.; Brisebois, K.; Toulouse, A.; Turecki, G.; Lal, S.; Bloom, D.; Labelle, A.; Lalonde, P.; Fortin, D.; et al. T102C polymorphism in the 5HT2A gene and schizophrenia: Relation to phenotype and drug response variability. J. Psychiatry Neurosci. 1999, 24, 141–146. [Google Scholar] [PubMed]
- Li, J.; Hashimoto, H.; Meltzer, H.Y. Association of Serotonin. Front. Psychiatry 2019, 10, 58. [Google Scholar] [CrossRef]
- Bishop, J.R.; Miller, d.D.; Ellingrod, V.L.; Holman, T. Association between type-three metabotropic glutamate receptor gene (GRM3) variants and symptom presentation in treatment refractory schizophrenia. Hum. Psychopharmacol. 2011, 26, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.N.; Ota, V.K.; Gouvea, E.S.; Pedrini, M.; Santoro, M.L.; Talarico, F.; Spindola, L.M.; Carvalho, C.M.; Noto, C.; Xavier, G.; et al. Accessing Gene Expression in Treatment-Resistant Schizophrenia. Mol. Neurobiol. 2018, 55, 7000–7008. [Google Scholar] [CrossRef] [PubMed]
- Badrlou, E.; Ghafouri-Fard, S.; Omrani, M.D.; Neishabouri, S.M.; Arsang-Jang, S.; Taheri, M.; Pouresmaeili, F. Expression of BDNF-Associated lncRNAs in Treatment-Resistant Schizophrenia Patients. J. Mol. Neurosci. 2021, 71, 2249–2259. [Google Scholar] [CrossRef]
- Zhang, J.P.; Lencz, T.; Geisler, S.; DeRosse, P.; Bromet, E.J.; Malhotra, A.K. Genetic variation in BDNF is associated with antipsychotic treatment resistance in patients with schizophrenia. Schizophr. Res. 2013, 146, 285–288. [Google Scholar] [CrossRef]
- Regen, F.; Cosma, N.C.; Otto, L.R.; Clemens, V.; Saksone, L.; Gellrich, J.; Uesekes, B.; Ta, T.M.T.; Hahn, E.; Dettling, M.; et al. Clozapine modulates retinoid homeostasis in human brain and normalizes serum retinoic acid deficit in patients with schizophrenia. Mol. Psychiatry 2021, 26, 5417–5428. [Google Scholar] [CrossRef]
- Martínez-Magaña, J.J.; Genís-Mendoza, A.D.; González-Covarrubias, V.; Jiménez-Guenchi, J.; Galindo-Chávez, A.G.; Roche-Bergua, A.; Castañeda-González, C.; Lanzagorta, N.; Soberón, X.; Nicolini, H. Exploratory analysis of rare and novel variants in mexican patients diagnosed with schizophrenia and dementia. Rev. Investig. Clin. 2019, 71, 246–254. [Google Scholar] [CrossRef]
- Li, J.; Meltzer, H.Y. A genetic locus in 7p12.2 associated with treatment resistant schizophrenia. Schizophr Res 2014, 159, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, D.S.; Santos, R.D.S.; de Brito, R.B.; Cruz, A.H.D.S.; Ghedini, P.C.; Reis, A.A.S. GSTM1/GSTT1 double-null genotype increases risk of treatment-resistant schizophrenia: A genetic association study in Brazilian patients. PLoS ONE 2017, 12, e0183812. [Google Scholar] [CrossRef] [PubMed]
- Jahn, K.; Heese, A.; Kebir, O.; Groh, A.; Bleich, S.; Krebs, M.O.; Frieling, H. Differential Methylation Pattern of Schizophrenia Candidate Genes in Tetrahydrocannabinol-Consuming Treatment-Resistant Schizophrenic Patients Compared to Non-Consumer Patients and Healthy Controls. Neuropsychobiology 2021, 80, 36–44. [Google Scholar] [CrossRef]
- Aytac, H.M.; Yazar, M.S.; Erol, A.; Pehlivan, S. Investigation of inflammation related gene polymorphism of the mannose-binding lectin 2 in schizophrenia and bipolar disorder. Neurosciences 2021, 26, 346–356. [Google Scholar] [CrossRef]
- Alacam, H.; Akgun, S.; Akca, H.; Ozturk, O.; Kabukcu, B.B.; Herken, H. miR-181b-5p, miR-195-5p and miR-301a-3p are related with treatment resistance in schizophrenia. Psychiatry Res. 2016, 245, 200–206. [Google Scholar] [CrossRef]
- You, X.; Zhang, Y.; Long, Q.; Liu, Z.; Ma, X.; Lu, Z.; Yang, W.; Feng, Z.; Zhang, W.; Teng, Z.; et al. Investigating aberrantly expressed microRNAs in peripheral blood mononuclear cells from patients with treatment-resistant schizophrenia using miRNA sequencing and integrated bioinformatics. Mol. Med. Rep. 2020, 22, 4340–4350. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, Y.; Yoshino, Y.; Iga, J.I.; Ueno, S.I. Impact of clozapine on the expression of miR-675-3p in plasma exosomes derived from patients with schizophrenia. World J. Biol. Psychiatry 2022, 1–11. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Cong, Q.; Chen, D.; Yi, Z.; Huang, H.; Wang, C.; Li, M.; Zeng, R.; Liu, Y.; et al. miR143-3p-Mediated NRG-1-Dependent Mitochondrial Dysfunction Contributes to Olanzapine Resistance in Refractory Schizophrenia. Biol. Psychiatry 2022, 92, 419–433. [Google Scholar] [CrossRef]
- Mostaid, M.S.; Lee, T.T.; Chana, G.; Sundram, S.; Shannon Weickert, C.; Pantelis, C.; Everall, I.; Bousman, C. Peripheral Transcription of. Front. Psychiatry 2017, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, C.; Gourion, D.; Leroy, S.; Schürhoff, F.; Bourdel, M.C.; Leboyer, M.; Krebs, M.O. Population-based and family-based association study of 5’UTR polymorphism of the reelin gene and schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 137B, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.J.; Klempan, T.A.; De Luca, V.; Sicard, T.; Volavka, J.; Czobor, P.; Sheitman, B.B.; Lindenmayer, J.P.; Citrome, L.; McEvoy, J.P.; et al. The SNAP-25 gene may be associated with clinical response and weight gain in antipsychotic treatment of schizophrenia. Neurosci. Lett. 2005, 379, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Aytac, H.M.; Ozdilli, K.; Tuncel, F.C.; Pehlivan, M.; Pehlivan, S. Tumor Necrosis Factor-alpha (TNF-α) -238 G/A Polymorphism Is Associated with the Treatment Resistance and Attempted Suicide in Schizophrenia. Immunol. Investig. 2022, 51, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Meiklejohn, H.; Mostaid, M.S.; Luza, S.; Mancuso, S.G.; Kang, D.; Atherton, S.; Rothmond, D.A.; Weickert, C.S.; Opazo, C.M.; Pantelis, C.; et al. Blood and brain protein levels of ubiquitin-conjugating enzyme E2K (UBE2K) are elevated in individuals with schizophrenia. J. Psychiatr. Res. 2019, 113, 51–57. [Google Scholar] [CrossRef]
- Kim, E.; Howes, O.D.; Veronese, M.; Beck, K.; Seo, S.; Park, J.W.; Lee, J.S.; Lee, Y.-S.; Kwon, J.S. Presynaptic Dopamine Capacity in Patients with Treatment-Resistant Schizophrenia Taking Clozapine: An [18F]DOPA PET Study. Neuropsychopharmacology 2016, 42, 941–950. [Google Scholar] [CrossRef]
- Kaar, S.J.; Natesan, S.; McCutcheon, R.; Howes, O.D. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology 2020, 172, 107704. [Google Scholar] [CrossRef]
- Consortium, S.W.G.o.t.P.G. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef]
- Weinstein, J.J.; Chohan, M.O.; Slifstein, M.; Kegeles, L.S.; Moore, H.; Abi-Dargham, A. Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biol. Psychiatry 2017, 81, 31–42. [Google Scholar] [CrossRef]
- Giorgetti, M.; Tecott, L.H. Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. Eur. J. Pharmacol. 2004, 488, 1–9. [Google Scholar] [CrossRef]
- Selvaraj, S.; Arnone, D.; Cappai, A.; Howes, O. Alterations in the serotonin system in schizophrenia: A systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci. Biobehav. Rev. 2014, 45, 233–245. [Google Scholar] [CrossRef]
- Yoon, J.H.; Maddock, R.J.; Rokem, A.; Silver, M.A.; Minzenberg, M.J.; Ragland, J.D.; Carter, C.S. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J. Neurosci. 2010, 30, 3777–3781. [Google Scholar] [CrossRef]
- Schmidt, M.J.; Mirnics, K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology 2015, 40, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, J.; Zhang, K.; Lu, G.; Liu, Y.; Ren, K.; Wang, W.; Xin, D.; Xu, L.; Mao, H.; et al. Ten-eleven translocation 1 mediated-DNA hydroxymethylation is required for myelination and remyelination in the mouse brain. Nat. Commun. 2021, 12, 5091. [Google Scholar] [CrossRef]
- Cartmell, J.; Schoepp, D.D. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000, 75, 889–907. [Google Scholar] [CrossRef]
- Bishop, J.R.; Ellingrod, V.L.; Moline, J.; Miller, D. Association between the polymorphic GRM3 gene and negative symptom improvement during olanzapine treatment. Schizophr. Res. 2005, 77, 253–260. [Google Scholar] [CrossRef]
- Gouvêa, E.S.; Santos, A.F.; Ota, V.K.; Mrad, V.; Gadelha, A.; Bressan, R.A.; Cordeiro, Q.; Belangero, S.I. The role of the CNR1 gene in schizophrenia: A systematic review including unpublished data. Braz. J. Psychiatry 2017, 39, 160–171. [Google Scholar] [CrossRef]
- Goh, K.K.; Chen, C.H.; Lane, H.Y. Oxytocin in Schizophrenia: Pathophysiology and Implications for Future Treatment. Int. J. Mol. Sci. 2021, 22, 2143. [Google Scholar] [CrossRef] [PubMed]
- Favalli, G.; Li, J.; Belmonte-de-Abreu, P.; Wong, A.H.; Daskalakis, Z.J. The role of BDNF in the pathophysiology and treatment of schizophrenia. J. Psychiatr. Res. 2012, 46, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gören, J.L. Brain-derived neurotrophic factor and schizophrenia. Ment. Health Clin. 2016, 6, 285–288. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Upthegrove, R.; Khandaker, G.M. Cytokines, Oxidative Stress and Cellular Markers of Inflammation in Schizophrenia. Curr. Top Behav. Neurosci. 2020, 44, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Pae, C.U.; Serretti, A.; Artioli, P.; Kim, T.S.; Kim, J.J.; Lee, C.U.; Lee, S.J.; Paik, I.H.; Lee, C. Interaction analysis between 5-HTTLPR and TNFA -238/-308 polymorphisms in schizophrenia. J. Neural. Transm. 2006, 113, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Xiong, W.C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008, 9, 437–452. [Google Scholar] [CrossRef]
- Tsai, G.; Coyle, J.T. Glutamatergic mechanisms in schizophrenia. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Emamian, E.S.; Hall, D.; Birnbaum, M.J.; Karayiorgou, M.; Gogos, J.A. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat. Genet. 2004, 36, 131–137. [Google Scholar] [CrossRef]
- Schwab, S.G.; Hoefgen, B.; Hanses, C.; Hassenbach, M.B.; Albus, M.; Lerer, B.; Trixler, M.; Maier, W.; Wildenauer, D.B. Further evidence for association of variants in the AKT1 gene with schizophrenia in a sample of European sib-pair families. Biol. Psychiatry 2005, 58, 446–450. [Google Scholar] [CrossRef]
- Thiselton, D.L.; Vladimirov, V.I.; Kuo, P.H.; McClay, J.; Wormley, B.; Fanous, A.; O’Neill, F.A.; Walsh, D.; Van den Oord, E.J.; Kendler, K.S.; et al. AKT1 is associated with schizophrenia across multiple symptom dimensions in the Irish study of high density schizophrenia families. Biol. Psychiatry 2008, 63, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Le-Niculescu, H.; Balaraman, Y.; Patel, S.; Tan, J.; Sidhu, K.; Jerome, R.E.; Edenberg, H.J.; Kuczenski, R.; Geyer, M.A.; Nurnberger, J.I.; et al. Towards understanding the schizophrenia code: An expanded convergent functional genomics approach. Am J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 129–158. [Google Scholar] [CrossRef] [PubMed]
- Folsom, T.D.; Fatemi, S.H. The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology 2013, 68, 122–135. [Google Scholar] [CrossRef]
- Bousman, C.A.; Luza, S.; Mancuso, S.G.; Kang, D.; Opazo, C.M.; Mostaid, M.S.; Cropley, V.; McGorry, P.; Shannon Weickert, C.; Pantelis, C.; et al. Elevated ubiquitinated proteins in brain and blood of individuals with schizophrenia. Sci. Rep. 2019, 9, 2307. [Google Scholar] [CrossRef]
- Huang, E.; Maciukiewicz, M.; Zai, C.C.; Tiwari, A.K.; Li, J.; Potkin, S.G.; Lieberman, J.A.; Meltzer, H.Y.; Müller, D.J.; Kennedy, J.L. Preliminary evidence for association of genome-wide significant DRD2 schizophrenia risk variant with clozapine response. Pharmacogenomics 2016, 17, 103–109. [Google Scholar] [CrossRef]
- Hwang, R.; Shinkai, T.; Deluca, V.; Macciardi, F.; Potkin, S.; Meltzer, H.Y.; Kennedy, J.L. Dopamine D2 receptor gene variants and quantitative measures of positive and negative symptom response following clozapine treatment. Eur. Neuropsychopharmacol. 2006, 16, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Scharfetter, J.; Chaudhry, H.R.; Hornik, K.; Fuchs, K.; Sieghart, W.; Kasper, S.; Aschauer, H.N. Dopamine D3 receptor gene polymorphism and response to clozapine in schizophrenic Pakastani patients. Eur. Neuropsychopharmacol. 1999, 10, 17–20. [Google Scholar] [CrossRef]
- Shaikh, S.; Collier, D.A.; Sham, P.C.; Ball, D.; Aitchison, K.; Vallada, H.; Smith, I.; Gill, M.; Kerwin, R.W. Allelic association between a Ser-9-Gly polymorphism in the dopamine D3 receptor gene and schizophrenia. Hum. Genet. 1996, 97, 714–719. [Google Scholar] [CrossRef]
- Hwang, R.; Tiwari, A.K.; Zai, C.C.; Felsky, D.; Remington, E.; Wallace, T.; Tong, R.P.; Souza, R.P.; Oh, G.; Potkin, S.G.; et al. Dopamine D4 and D5 receptor gene variant effects on clozapine response in schizophrenia: Replication and exploration. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 37, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, V.M.; Rajkumar, A.P.; Jacob, K.S.; Jacob, M. Gene-gene interaction between DRD4 and COMT modulates clinical response to clozapine in treatment-resistant schizophrenia. Pharmacogenet. Genomics 2018, 28, 31–35. [Google Scholar] [CrossRef]
- Xu, M.; Xing, Q.; Li, S.; Zheng, Y.; Wu, S.; Gao, R.; Yu, L.; Guo, T.; Yang, Y.; Liu, J.; et al. Pharacogenetic effects of dopamine transporter gene polymorphisms on response to chlorpromazine and clozapine and on extrapyramidal syndrome in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1026–1032. [Google Scholar] [CrossRef]
- Masellis, M.; Basile, V.; Meltzer, H.Y.; Lieberman, J.A.; Sevy, S.; Macciardi, F.M.; Cola, P.; Howard, A.; Badri, F.; Nothen, M.M.; et al. Serotonin subtype 2 receptor genes and clinical response to clozapine in schizophrenia patients. Neuropsychopharmacology 1998, 19, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, A.P.; Poonkuzhali, B.; Kuruvilla, A.; Srivastava, A.; Jacob, M.; Jacob, K.S. Outcome definitions and clinical predictors influence pharmacogenetic associations between HTR3A gene polymorphisms and response to clozapine in patients with schizophrenia. Psychopharmacology 2012, 224, 441–449. [Google Scholar] [CrossRef]
- Ji, X.; Takahashi, N.; Saito, S.; Ishihara, R.; Maeno, N.; Inada, T.; Ozaki, N. Relationship between three serotonin receptor subtypes (HTR3A, HTR2A and HTR4) and treatment-resistant schizophrenia in the Japanese population. Neurosci. Lett. 2008, 435, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Takahashi, N.; Branko, A.; Ishihara, R.; Nagai, T.; Mouri, A.; Saito, S.; Maeno, N.; Inada, T.; Ozaki, N. An association between serotonin receptor 3B gene (HTR3B) and treatment-resistant schizophrenia (TRS) in a Japanese population. Nagoya J. Med. Sci. 2008, 70, 11–17. [Google Scholar]
- Kohlrausch, F.B.; Salatino-Oliveira, A.; Gama, C.S.; Lobato, M.I.; Belmonte-de-Abreu, P.; Hutz, M.H. Influence of serotonin transporter gene polymorphisms on clozapine response in Brazilian schizophrenics. J. Psychiatr. Res. 2010, 44, 1158–1162. [Google Scholar] [CrossRef]
- Sershen, H.; Guidotti, A.; Auta, J.; Drnevich, J.; Grayson, D.R.; Veldic, M.; Meyers, J.; Youseff, M.; Zhubi, A.; Faurot, K.; et al. Gene Expression Of Methylation Cycle And Related Genes In Lymphocytes And Brain Of Patients With Schizophrenia And Non-Psychotic Controls. Biomark. Neuropsychiatry 2021, 5, 100038. [Google Scholar] [CrossRef]
- Akamine, Y.; Sugawara-Kikuchi, Y.; Uno, T.; Shimizu, T.; Miura, M. Quantification of the steady-state plasma concentrations of clozapine and N-desmethylclozapine in Japanese patients with schizophrenia using a novel HPLC method and the effects of CYPs and ABC transporters polymorphisms. Ann. Clin. Biochem. 2017, 54, 677–685. [Google Scholar] [CrossRef]
- Akkouh, I.A.; Hribkova, H.; Grabiec, M.; Budinska, E.; Szabo, A.; Kasparek, T.; Andreassen, O.A.; Sun, Y.M.; Djurovic, S. Derivation and Molecular Characterization of a Morphological Subpopulation of Human iPSC Astrocytes Reveal a Potential Role in Schizophrenia and Clozapine Response. Schizophr. Bull. 2022, 48, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.J.; Yu, Y.W.; Lin, C.H.; Tsai, S.J. An association study of a brain-derived neurotrophic factor Val66Met polymorphism and clozapine response of schizophrenic patients. Neurosci. Lett. 2003, 349, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Numata, S.; Tajima, A.; Yamamori, H.; Yasuda, Y.; Fujimoto, M.; Watanabe, S.; Umehara, H.; Shimodera, S.; Nakazawa, T.; et al. Effect of Clozapine on DNA Methylation in Peripheral Leukocytes from Patients with Treatment-Resistant Schizophrenia. Int. J. Mol. Sci. 2017, 18, 632. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.; Chadli, Z.; Mhalla, A.; Khouadja, S.; Hannachi, I.; Alshaikheid, M.; Slama, A.; Ben Fredj, N.; Ben Fadhel, N.; Ben Romdhane, H.; et al. Clinical and genetic influencing factors on clozapine pharmacokinetics in Tunisian schizophrenic patients. Pharm. J. 2021, 21, 551–558. [Google Scholar] [CrossRef]
- Huang, H.C.; Lua, A.C.; Wu, L.S.; Wu, B.J.; Lee, S.M.; Liu, C.Z. Cigarette smoking has a differential effect on the plasma level of clozapine in Taiwanese schizophrenic patients associated with the CYP1A2 gene -163A/C single nucleotide polymorphism. Psychiatr. Genet. 2016, 26, 172–177. [Google Scholar] [CrossRef]
- Okhuijsen-Pfeifer, C.; van der Horst, M.Z.; Bousman, C.A.; Lin, B.; van Eijk, K.R.; Ripke, S.; Ayhan, Y.; Babaoglu, M.O.; Bak, M.; Alink, W.; et al. Genome-wide association analyses of symptom severity among clozapine-treated patients with schizophrenia spectrum disorders. Transl. Psychiatry 2022, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Mouaffak, F.; Kebir, O.; Chayet, M.; Tordjman, S.; Vacheron, M.N.; Millet, B.; Jaafari, N.; Bellon, A.; Olié, J.P.; Krebs, M.O. Association of Disrupted in Schizophrenia 1 (DISC1) missense variants with ultra-resistant schizophrenia. Pharm. J. 2011, 11, 267–273. [Google Scholar] [CrossRef]
- Zuo, L.; Luo, X.; Krystal, J.H.; Cramer, J.; Charney, D.S.; Gelernter, J. The efficacies of clozapine and haloperidol in refractory schizophrenia are related to DTNBP1 variation. Pharmacogenet. Genomics 2009, 19, 437–446. [Google Scholar] [CrossRef]
- Souza, R.P.; Romano-Silva, M.A.; Lieberman, J.A.; Meltzer, H.Y.; MacNeil, L.T.; Culotti, J.G.; Kennedy, J.L.; Wong, A.H. Genetic association of the GDNF alpha-receptor genes with schizophrenia and clozapine response. J. Psychiatr. Res. 2010, 44, 700–706. [Google Scholar] [CrossRef]
- Kohlrausch, F.B.; Salatino-Oliveira, A.; Gama, C.S.; Lobato, M.I.; Belmonte-de-Abreu, P.; Hutz, M.H. G-protein gene 825C>T polymorphism is associated with response to clozapine in Brazilian schizophrenics. Pharmacogenomics 2008, 9, 1429–1436. [Google Scholar] [CrossRef]
- Smith, R.L.; O’Connell, K.; Athanasiu, L.; Djurovic, S.; Kringen, M.K.; Andreassen, O.A.; Molden, E. Identification of a novel polymorphism associated with reduced clozapine concentration in schizophrenia patients-a genome-wide association study adjusting for smoking habits. Transl. Psychiatry 2020, 10, 198. [Google Scholar] [CrossRef]
- Lett, T.A.; Tiwari, A.K.; Meltzer, H.Y.; Lieberman, J.A.; Potkin, S.G.; Voineskos, A.N.; Kennedy, J.L.; Müller, D.J. The putative functional rs1045881 marker of neurexin-1 in schizophrenia and clozapine response. Schizophr. Res. 2011, 132, 121–124. [Google Scholar] [CrossRef]
- Mouaffak, F.; Kebir, O.; Bellon, A.; Gourevitch, R.; Tordjman, S.; Viala, A.; Millet, B.; Jaafari, N.; Olié, J.P.; Krebs, M.O. Association of an UCP4 (SLC25A27) haplotype with ultra-resistant schizophrenia. Pharmacogenomics 2011, 12, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.J.; Yu, Y.W.; Lin, C.H.; Song, H.L.; Lai, H.C.; Yang, K.H.; Tsai, S.J. Association study of apolipoprotein E epsilon4 with clinical phenotype and clozapine response in schizophrenia. Neuropsychobiology 2000, 42, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Tsai, S.J.; Yu, Y.W.; Song, H.L.; Tu, P.C.; Sim, C.B.; Hsu, C.P.; Yang, K.H.; Hong, C.J. No evidence for association of serotonin-2A receptor variant (102T/C) with schizophrenia or clozapine response in a Chinese population. Neuroreport 1999, 10, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Collier, D.A.; Sham, P.; Pilowsky, L.; Sharma, T.; Lin, L.K.; Crocq, M.A.; Gill, M.; Kerwin, R. Analysis of clozapine response and polymorphisms of the dopamine D4 receptor gene (DRD4) in schizophrenic patients. Am. J. Med. Genet. 1995, 60, 541–545. [Google Scholar] [CrossRef]
- Tsai, S.J.; Hong, C.J.; Yu, Y.W.; Lin, C.H.; Song, H.L.; Lai, H.C.; Yang, K.H. Association study of a functional serotonin transporter gene polymorphism with schizophrenia, psychopathology and clozapine response. Schizophr. Res. 2000, 44, 177–181. [Google Scholar] [CrossRef]
- Tsai, S.J.; Wang, Y.C.; Yu Younger, W.Y.; Lin, C.H.; Yang, K.H.; Hong, C.J. Association analysis of polymorphism in the promoter region of the alpha2a-adrenoceptor gene with schizophrenia and clozapine response. Schizophr. Res. 2001, 49, 53–58. [Google Scholar] [CrossRef]
- Tsai, S.J.; Hong, C.J.; Yu, Y.W.; Lin, C.H.; Liu, L.L. No association of tumor necrosis factor alpha gene polymorphisms with schizophrenia or response to clozapine. Schizophr. Res. 2003, 65, 27–32. [Google Scholar] [CrossRef]
- Zai, G.; Müller, D.J.; Volavka, J.; Czobor, P.; Lieberman, J.A.; Meltzer, H.Y.; Kennedy, J.L. Family and case-control association study of the tumor necrosis factor-alpha (TNF-alpha) gene with schizophrenia and response to antipsychotic medication. Psychopharmacology 2006, 188, 171–182. [Google Scholar] [CrossRef]
- Bolonna, A.A.; Arranz, M.J.; Munro, J.; Osborne, S.; Petouni, M.; Martinez, M.; Kerwin, R.W. No influence of adrenergic receptor polymorphisms on schizophrenia and antipsychotic response. Neurosci. Lett. 2000, 280, 65–68. [Google Scholar] [CrossRef]
- Hong, C.J.; Yu, Y.W.; Lin, C.H.; Cheng, C.Y.; Tsai, S.J. Association analysis for NMDA receptor subunit 2B (GRIN2B) genetic variants and psychopathology and clozapine response in schizophrenia. Psychiatr. Genet. 2001, 11, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.K.; Goldman, D.; Buchanan, R.W.; Rooney, W.; Clifton, A.; Kosmidis, M.H.; Breier, A.; Pickar, D. The dopamine D3 receptor (DRD3) Ser9Gly polymorphism and schizophrenia: A haplotype relative risk study and association with clozapine response. Mol. Psychiatry. 1998, 3, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Masellis, M.; Basile, V.S.; Meltzer, H.Y.; Lieberman, J.A.; Sevy, S.; Goldman, D.A.; Hamblin, M.W.; Macciardi, F.M.; Kennedy, J.L. Lack of association between the T-->C 267 serotonin 5-HT6 receptor gene (HTR6) polymorphism and prediction of response to clozapine in schizophrenia. Schizophr. Res. 2001, 47, 49–58. [Google Scholar] [CrossRef]
- Souza, R.P.; Romano-Silva, M.A.; Lieberman, J.A.; Meltzer, H.Y.; Wong, A.H.; Kennedy, J.L. Association study of GSK3 gene polymorphisms with schizophrenia and clozapine response. Psychopharmacology 2008, 200, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Haddley, K.; Vasiliou, A.S.; Ali, F.R.; Paredes, U.M.; Bubb, V.J.; Quinn, J.P. Molecular genetics of monoamine transporters: Relevance to brain disorders. Neurochem. Res. 2008, 33, 652–667. [Google Scholar] [CrossRef]
- Mehler-Wex, C.; Riederer, P.; Gerlach, M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: Implications for the pathophysiology of Parkinson’s disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox. Res. 2006, 10, 167–179. [Google Scholar] [CrossRef]
- Zhang, A.; Xing, Q.; Wang, L.; Du, J.; Yu, L.; Lin, Z.; Li, X.; Feng, G.; He, L. Dopamine transporter polymorphisms and risperidone response in Chinese schizophrenia patients: An association study. Pharmacogenomics 2007, 8, 1337–1345. [Google Scholar] [CrossRef]
- Guidotti, A.; Auta, J.; Chen, Y.; Davis, J.M.; Dong, E.; Gavin, D.P.; Grayson, D.R.; Matrisciano, F.; Pinna, G.; Satta, R.; et al. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology 2011, 60, 1007–1016. [Google Scholar] [CrossRef]
- Consoli, G.; Lastella, M.; Ciapparelli, A.; Catena Dell’Osso, M.; Ciofi, L.; Guidotti, E.; Danesi, R.; Dell’Osso, L.; Del Tacca, M.; Di Paolo, A. ABCB1 polymorphisms are associated with clozapine plasma levels in psychotic patients. Pharmacogenomics 2009, 10, 1267–1276. [Google Scholar] [CrossRef]
- Wang, J.S.; Zhu, H.J.; Markowitz, J.S.; Donovan, J.L.; Yuan, H.J.; Devane, C.L. Antipsychotic drugs inhibit the function of breast cancer resistance protein. Basic Clin. Pharmacol. Toxicol. 2008, 103, 336–341. [Google Scholar] [CrossRef]
- Einoch, R.; Weinreb, O.; Mandiuk, N.; Youdim, M.B.H.; Bilker, W.; Silver, H. The involvement of BDNF-CREB signaling pathways in the pharmacological mechanism of combined SSRI- antipsychotic treatment in schizophrenia. Eur. Neuropsychopharmacol. 2017, 27, 470–483. [Google Scholar] [CrossRef]
- Chu, A.C.; Ho, P.W.; Kwok, K.H.; Ho, J.W.; Chan, K.H.; Liu, H.F.; Kung, M.H.; Ramsden, D.B.; Ho, S.L. Mitochondrial UCP4 attenuates MPP+—And dopamine-induced oxidative stress, mitochondrial depolarization, and ATP deficiency in neurons and is interlinked with UCP2 expression. Free Radic. Biol. Med. 2009, 46, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Liu, D.; Kyriazis, G.A.; Bagsiyao, P.; Ouyang, X.; Mattson, M.P. Mitochondrial uncoupling protein-4 regulates calcium homeostasis and sensitivity to store depletion-induced apoptosis in neural cells. J. Biol. Chem. 2006, 281, 37391–37403. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, D.; Laifenfeld, D. Mitochondria, synaptic plasticity, and schizophrenia. Int. Rev. Neurobiol. 2004, 59, 273–296. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef]
- Poliak, S.; Gollan, L.; Martinez, R.; Custer, A.; Einheber, S.; Salzer, J.L.; Trimmer, J.S.; Shrager, P.; Peles, E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 1999, 24, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Consortium, I.S. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 2008, 455, 237–241. [Google Scholar] [CrossRef]
- Lemonde, S.; Du, L.; Bakish, D.; Hrdina, P.; Albert, P.R. Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int. J. Neuropsychopharmacol. 2004, 7, 501–506. [Google Scholar] [CrossRef]
- Zill, P.; Baghai, T.C.; Zwanzger, P.; Schüle, C.; Minov, C.; Riedel, M.; Neumeier, K.; Rupprecht, R.; Bondy, B. Evidence for an association between a G-protein beta3-gene variant with depression and response to antidepressant treatment. Neuroreport 2000, 11, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cha, J.H.; Ham, B.J.; Han, C.S.; Kim, Y.K.; Lee, S.H.; Ryu, S.H.; Kang, R.H.; Choi, M.J.; Lee, M.S. Association between a G-protein beta 3 subunit gene polymorphism and the symptomatology and treatment responses of major depressive disorders. Pharm. J. 2004, 4, 29–33. [Google Scholar] [CrossRef]
- Brandon, N.J.; Sawa, A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat. Rev. Neurosci. 2011, 12, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Boksa, P. Abnormal synaptic pruning in schizophrenia: Urban myth or reality? J. Psychiatry Neurosci. 2012, 37, 75–77. [Google Scholar] [CrossRef]
- Feinberg, I. Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res. 1982, 17, 319–334. [Google Scholar] [CrossRef]
- Cheah, S.Y.; Lawford, B.R.; Young, R.M.; Morris, C.P.; Voisey, J. Dysbindin (DTNBP1) variants are associated with hallucinations in schizophrenia. Eur. Psychiatry 2015, 30, 486–491. [Google Scholar] [CrossRef]
- Burke, R.E. GDNF as a candidate striatal target-derived neurotrophic factor for the development of substantia nigra dopamine neurons. J. Neural. Transm. Suppl. 2006, 41–45. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Kikuchi, M.; Saito, K.; Watanabe, A.; Yamada, K.; Shibuya, H.; Nankai, M.; Kurumaji, A.; Hattori, E.; Ishiguro, H.; et al. Evidence for association of the myo-inositol monophosphatase 2 (IMPA2) gene with schizophrenia in Japanese samples. Mol. Psychiatry 2001, 6, 202–210. [Google Scholar] [CrossRef]
- Neusch, C.; Rozengurt, N.; Jacobs, R.E.; Lester, H.A.; Kofuji, P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J. Neurosci. 2001, 21, 5429–5438. [Google Scholar] [CrossRef] [PubMed]
- Dho, S.E.; French, M.B.; Woods, S.A.; McGlade, C.J. Characterization of four mammalian numb protein isoforms. Identification of cytoplasmic and membrane-associated variants of the phosphotyrosine binding domain. J. Biol. Chem.. 1999, 274, 33097–33104. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.; Barry, G.; Harvey, T.J.; McLeay, R.; Smith, A.G.; Harris, L.; Mason, S.; Stringer, B.W.; Day, B.W.; Wray, N.R.; et al. NFIB-mediated repression of the epigenetic factor Ezh2 regulates cortical development. J. Neurosci. 2014, 34, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Steele-Perkins, G.; Plachez, C.; Butz, K.G.; Yang, G.; Bachurski, C.J.; Kinsman, S.L.; Litwack, E.D.; Richards, L.J.; Gronostajski, R.M. The transcription factor gene Nfib is essential for both lung maturation and brain development. Mol. Cell Biol. 2005, 25, 685–698. [Google Scholar] [CrossRef]
- Etherton, M.R.; Blaiss, C.A.; Powell, C.M.; Südhof, T.C. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc. Natl. Acad. Sci. USA 2009, 106, 17998–18003. [Google Scholar] [CrossRef]
- Kehrer, C.; Maziashvili, N.; Dugladze, T.; Gloveli, T. Altered Excitatory-Inhibitory Balance in the NMDA-Hypofunction Model of Schizophrenia. Front. Mol. Neurosci. 2008, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Almonte, A.G.; Sweatt, J.D. Serine proteases, serine protease inhibitors, and protease-activated receptors: Roles in synaptic function and behavior. Brain Res. 2011, 1407, 107–122. [Google Scholar] [CrossRef]
- Moyon, S.; Frawley, R.; Marechal, D.; Huang, D.; Marshall-Phelps, K.L.H.; Kegel, L.; Bøstrand, S.M.K.; Sadowski, B.; Jiang, Y.H.; Lyons, D.A.; et al. TET1-mediated DNA hydroxymethylation regulates adult remyelination in mice. Nat. Commun. 2021, 12, 3359. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, J.; Ma, Y.; Mi, Y.; Cui, D.; Ju, G.; Macklin, W.B.; Jin, W. Dynamics of ten-eleven translocation hydroxylase family proteins and 5-hydroxymethylcytosine in oligodendrocyte differentiation. Glia 2014, 62, 914–926. [Google Scholar] [CrossRef]
- Pinto, J.A.F.; Freitas, P.H.B.; Nunes, F.D.D.; Granjeiro, P.A.; Santos, L.L.D.; Machado, R.M. Prevalence of polymorphisms in the ANKK1, DRD2, DRD3 genes and metabolic syndrome in refractory schizophrenia. Rev. Lat. Am. Enfermagem. 2018, 26, e2983. [Google Scholar] [CrossRef]
- Fehsel, K.; Loeffler, S.; Krieger, K.; Henning, U.; Agelink, M.; Kolb-Bachofen, V.; Klimke, A. Clozapine induces oxidative stress and proapoptotic gene expression in neutrophils of schizophrenic patients. J. Clin. Psychopharmacol. 2005, 25, 419–426. [Google Scholar] [CrossRef]
- Dettling, M.; Cascorbi, I.; Opgen-Rhein, C.; Schaub, R. Clozapine-induced agranulocytosis in schizophrenic Caucasians: Confirming clues for associations with human leukocyte class I and II antigens. Pharm. J. 2007, 7, 325–332. [Google Scholar] [CrossRef]
- Amar, A.; Segman, R.H.; Shtrussberg, S.; Sherman, L.; Safirman, C.; Lerer, B.; Brautbar, C. An association between clozapine-induced agranulocytosis in schizophrenics and HLA-DQB1*0201. Int. J. Neuropsychopharmacol. 1998, 1, 41–44. [Google Scholar] [CrossRef]
- Valevski, A.; Klein, T.; Gazit, E.; Meged, S.; Stein, D.; Elizur, A.; Narinsky, E.R.; Kutzuk, D.; Weizman, A. HLA-B38 and clozapine-induced agranulocytosis in Israeli Jewish schizophrenic patients. Eur. J. Immunogenet. 1998, 25, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Yunis, J.; Egea, E.; Canoso, R.T.; Kane, J.M.; Yunis, E.J. HLA-B38, DR4, DQw3 and clozapine-induced agranulocytosis in Jewish patients with schizophrenia. Arch. Gen. Psychiatry 1990, 47, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Lee, J.I.; Han, H.R.; Soh, M.; Hong, J.P. Polymorphisms of the leptin and HTR2C genes and clozapine-induced weight change and baseline BMI in patients with chronic schizophrenia. Psychiatr. Genet. 2014, 24, 249–256. [Google Scholar] [CrossRef]
- Chen, P.Y.; Lu, M.L.; Huang, M.C.; Kao, C.F.; Kuo, P.H.; Chiu, C.C.; Lin, S.K.; Chen, C.H. The Relationships of Obesity-Related Genetic Variants With Metabolic Profiles and Response to Metformin in Clozapine-Treated Patients with Schizophrenia. J. Clin. Psychopharmacol. 2015, 35, 574–578. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Liu, D.; Yu, S.; Cong, E.; Li, Y.; Wu, H.; Yue, Y.; Zuo, S.; Wang, Y.; et al. Association between SREBF2 gene polymorphisms and metabolic syndrome in clozapine-treated patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 56, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Philipp, M.; Brede, M.; Hein, L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: One receptor is not enough. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R287–R295. [Google Scholar] [CrossRef]
- Suzuki, T.; Kanahara, N.; Yamanaka, H.; Takase, M.; Kimura, H.; Watanabe, H.; Iyo, M. Dopamine supersensitivity psychosis as a pivotal factor in treatment-resistant schizophrenia. Psychiatry Res. 2015, 227, 278–282. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. A neurobiological hypothesis for the classification of schizophrenia: Type A (hyperdopaminergic) and type B (normodopaminergic). Br. J. Psychiatry 2014, 205, 1–3. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Mailman, R.B.; Duncan, G.; Sikich, L.; Chakos, M.; Nichols, D.E.; Kraus, J.E. Serotonergic basis of antipsychotic drug effects in schizophrenia. Biol. Psychiatry 1998, 44, 1099–1117. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Espejo, E.; Viveros, M.P.; Núñez, L.; Ellenbroek, B.A.; Rodriguez de Fonseca, F. Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology 2009, 206, 531–549. [Google Scholar] [CrossRef] [PubMed]
- Ferretjans, R.; Moreira, F.A.; Teixeira, A.L.; Salgado, J.V. The endocannabinoid system and its role in schizophrenia: A systematic review of the literature. Braz. J. Psychiatry 2012, 34 (Suppl. S2), S163–S177. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Dwivedi, Y. Non-Coding RNAs in Psychiatric Disorders and Suicidal Behavior. Front. Psychiatry 2020, 11, 543893. [Google Scholar] [CrossRef]
- Sriretnakumar, V.; Huang, E.; Müller, D.J. Pharmacogenetics of clozapine treatment response and side-effects in schizophrenia: An update. Expert. Opin. Drug Metab. Toxicol. 2015, 11, 1709–1731. [Google Scholar] [CrossRef]
- Bosia, M.; Lorenzi, C.; Pirovano, A.; Guglielmino, C.; Cocchi, F.; Spangaro, M.; Bramanti, P.; Smeraldi, E.; Cavallaro, R. COMT Val158Met and 5-HT1A-R -1019 C/G polymorphisms: Effects on the negative symptom response to clozapine. Pharmacogenomics 2015, 16, 35–44. [Google Scholar] [CrossRef]
- Galling, B.; Vernon, J.A.; Pagsberg, A.K.; Wadhwa, A.; Grudnikoff, E.; Seidman, A.J.; Tsoy-Podosenin, M.; Poyurovsky, M.; Kane, J.M.; Correll, C.U. Efficacy and safety of antidepressant augmentation of continued antipsychotic treatment in patients with schizophrenia. Acta Psychiatr. Scand. 2018, 137, 187–205. [Google Scholar] [CrossRef]
- Doude van Troostwijk, L.J.; Koopmans, R.P.; Vermeulen, H.D.; Guchelaar, H.J. CYP1A2 activity is an important determinant of clozapine dosage in schizophrenic patients. Eur. J. Pharm. Sci. 2003, 20, 451–457. [Google Scholar] [CrossRef]
- Olesen, O.V.; Linnet, K. Contributions of five human cytochrome P450 isoforms to the N-demethylation of clozapine in vitro at low and high concentrations. J. Clin. Pharmacol. 2001, 41, 823–832. [Google Scholar] [CrossRef]
- Eiermann, B.; Engel, G.; Johansson, I.; Zanger, U.M.; Bertilsson, L. The involvement of CYP1A2 and CYP3A4 in the metabolism of clozapine. Br. J. Clin. Pharmacol. 1997, 44, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Hägg, S.; Söderberg, S.; Ahrén, B.; Olsson, T.; Mjörndal, T. Leptin concentrations are increased in subjects treated with clozapine or conventional antipsychotics. J. Clin. Psychiatry 2001, 62, 843–848. [Google Scholar] [CrossRef]
- Illing, P.T.; Vivian, J.P.; Dudek, N.L.; Kostenko, L.; Chen, Z.; Bharadwaj, M.; Miles, J.J.; Kjer-Nielsen, L.; Gras, S.; Williamson, N.A.; et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 2012, 486, 554–558. [Google Scholar] [CrossRef]
- Lisoway, A.J.; Chen, C.C.; Zai, C.C.; Tiwari, A.K.; Kennedy, J.L. Toward personalized medicine in schizophrenia: Genetics and epigenetics of antipsychotic treatment. Schizophr. Res. 2021, 232, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Mohit, B. Cost-effectiveness of screening for HLA-B*1502 prior to initiation of carbamazepine in epilepsy patients of Asian ancestry in the United States. Epilepsia 2019, 60, 1472–1481. [Google Scholar] [CrossRef]
- Farrell, M.S.; Werge, T.; Sklar, P.; Owen, M.J.; Ophoff, R.A.; O’Donovan, M.C.; Corvin, A.; Cichon, S.; Sullivan, P.F. Evaluating historical candidate genes for schizophrenia. Mol. Psychiatry 2015, 20, 555–562. [Google Scholar] [CrossRef]
- Border, R.; Johnson, E.C.; Evans, L.M.; Smolen, A.; Berley, N.; Sullivan, P.F.; Keller, M.C. No Support for Historical Candidate Gene or Candidate Gene-by-Interaction Hypotheses for Major Depression Across Multiple Large Samples. Am. J. Psychiatry 2019, 176, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.R.R.; Brentani, H.; Powell, T.R. Ditching candidate gene association studies: Lessons from psychiatric genetics. Braz. J. Psychiatry 2021, 43, 342–344. [Google Scholar] [CrossRef]
- Wiström, E.D.; O’Connell, K.S.; Karadag, N.; Bahrami, S.; Hindley, G.F.L.; Lin, A.; Cheng, W.; Steen, N.E.; Shadrin, A.; Frei, O.; et al. Genome-wide analysis reveals genetic overlap between alcohol use behaviours, schizophrenia and bipolar disorder and identifies novel shared risk loci. Addiction 2022, 117, 600–610. [Google Scholar] [CrossRef]
- Zhu, Z.; Anttila, V.; Smoller, J.W.; Lee, P.H. Statistical power and utility of meta-analysis methods for cross-phenotype genome-wide association studies. PLoS ONE 2018, 13, e0193256. [Google Scholar] [CrossRef] [PubMed]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef]
- Legge, S.E.; Santoro, M.L.; Periyasamy, S.; Okewole, A.; Arsalan, A.; Kowalec, K. Genetic architecture of schizophrenia: A review of major advancements. Psychol. Med. 2021, 51, 2168–2177. [Google Scholar] [CrossRef]
- Lewis, C.M.; Vassos, E. Polygenic risk scores: From research tools to clinical instruments. Genome. Med. 2020, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, C.; Squassina, A. Treatment-Resistant Schizophrenia: Insights From Genetic Studies and Machine Learning Approaches. Front. Pharmacol. 2019, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Cao, T.; Cai, H. Peripheral biomarkers of treatment-resistant schizophrenia: Genetic, inflammation and stress perspectives. Front. Pharmacol. 2022, 13, 1005702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).