Identification of Circular RNA Profiles in the Liver of Diet-Induced Obese Mice and Construction of the ceRNA Network
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Biochemical and Histological Analysis
2.3. Identification of circRNAs in the Liver
2.4. Bioinformatic Analysis and Construction of the ceRNA Network
2.5. Validation of RNA-Seq Results
2.6. Statistical Analysis
3. Results
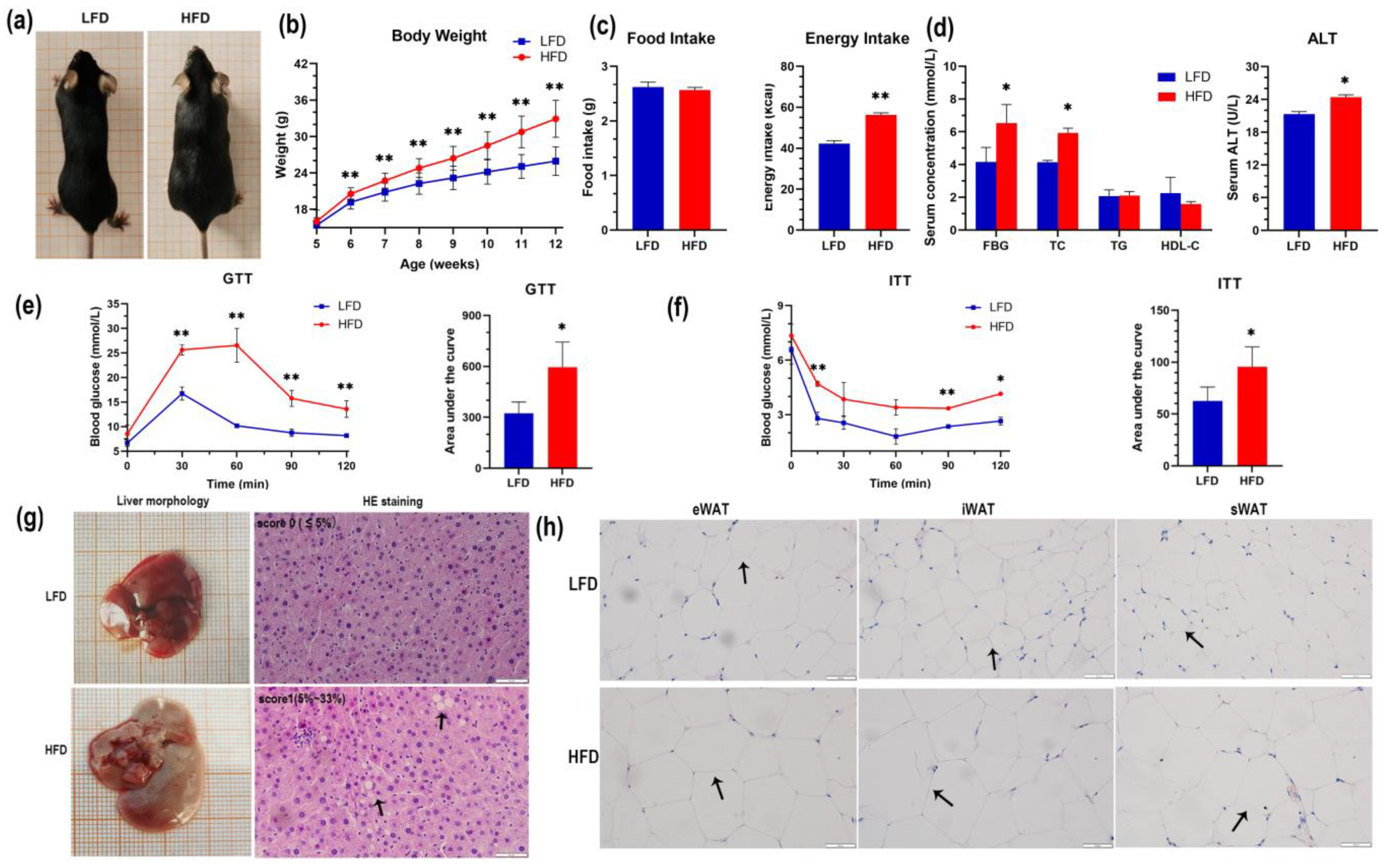
3.1. Effects of a High-Fat Diet on Mice
3.2. Overview of RNA-Seq
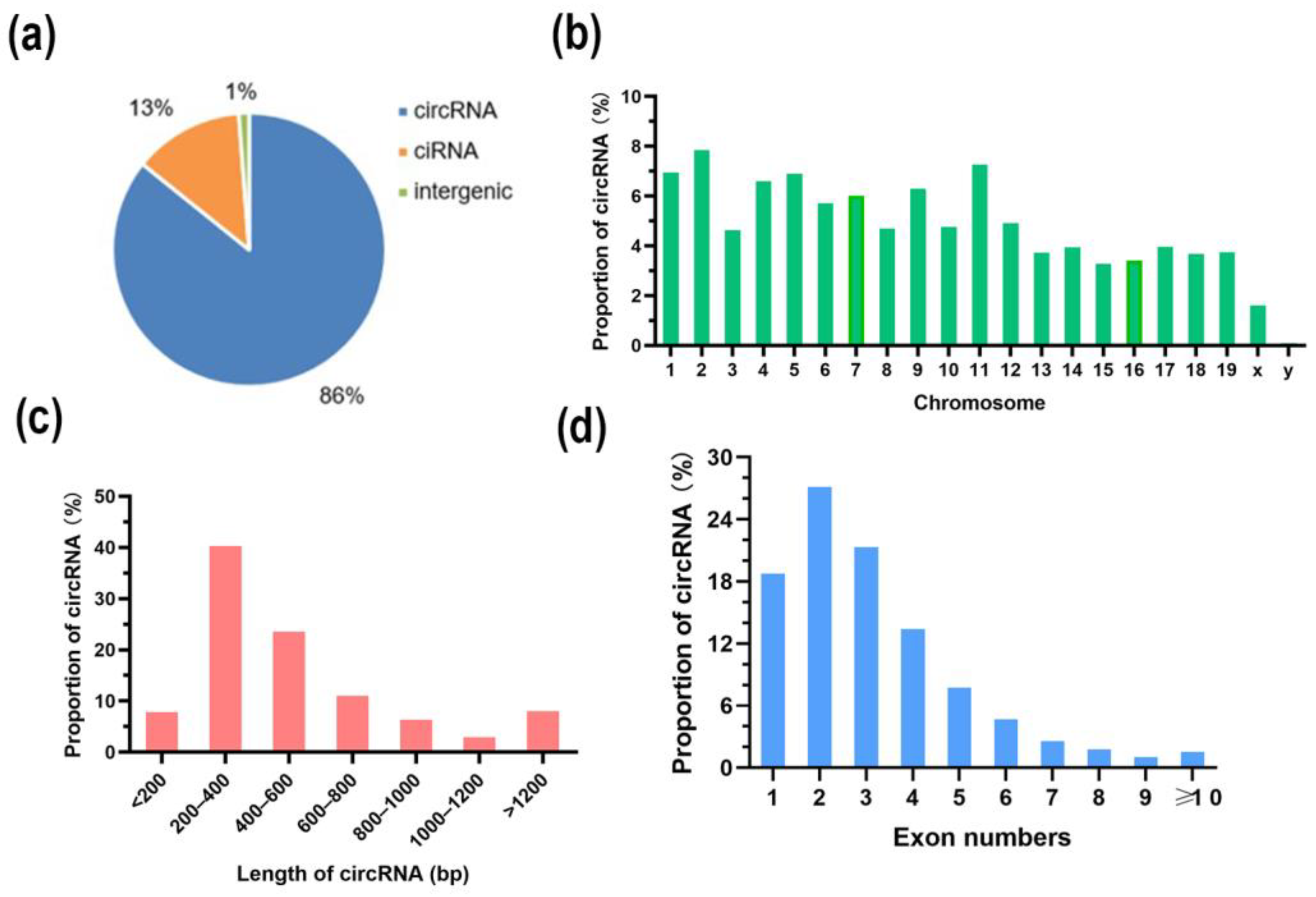
3.3. Differentially Expressed circRNAs
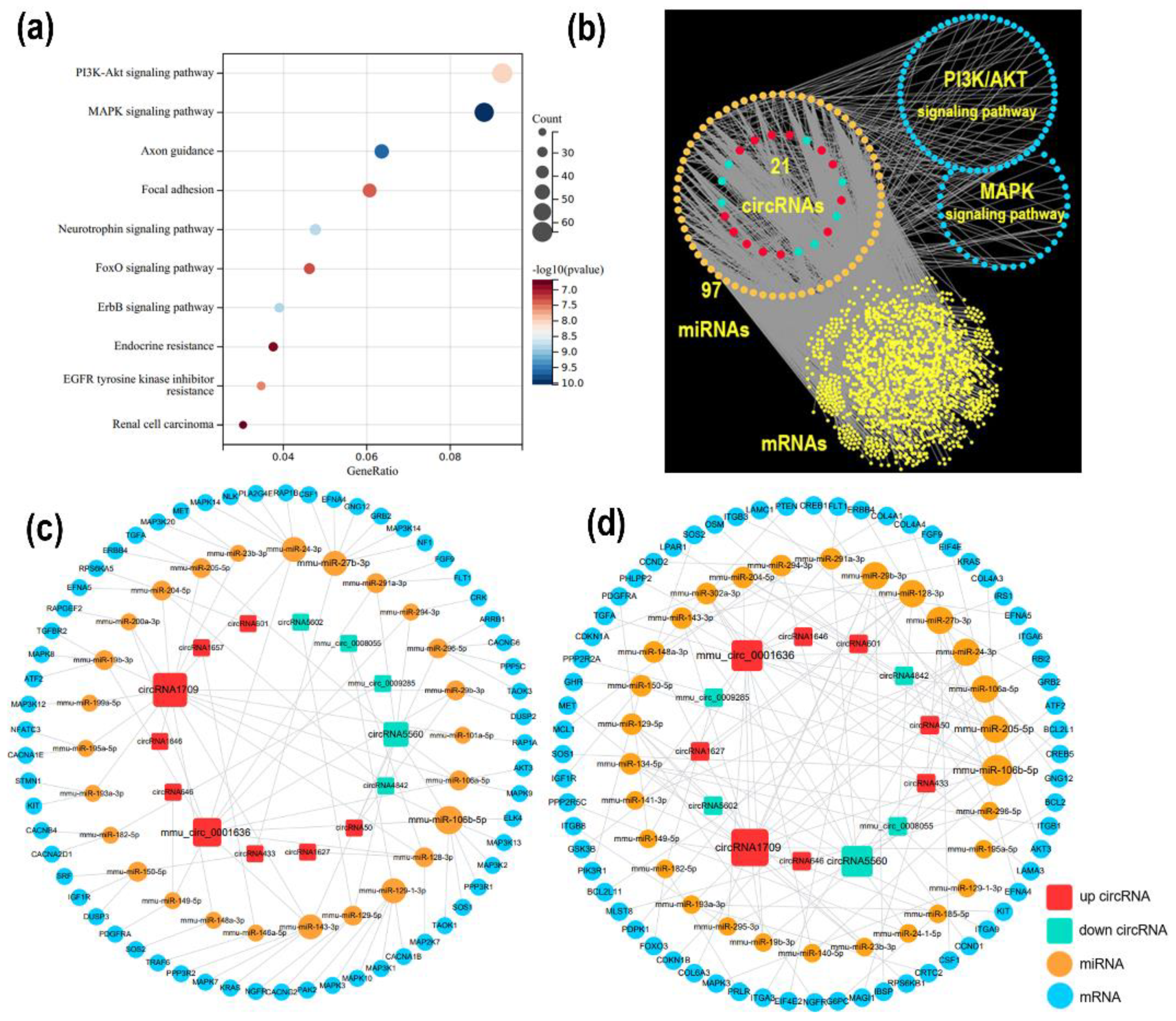
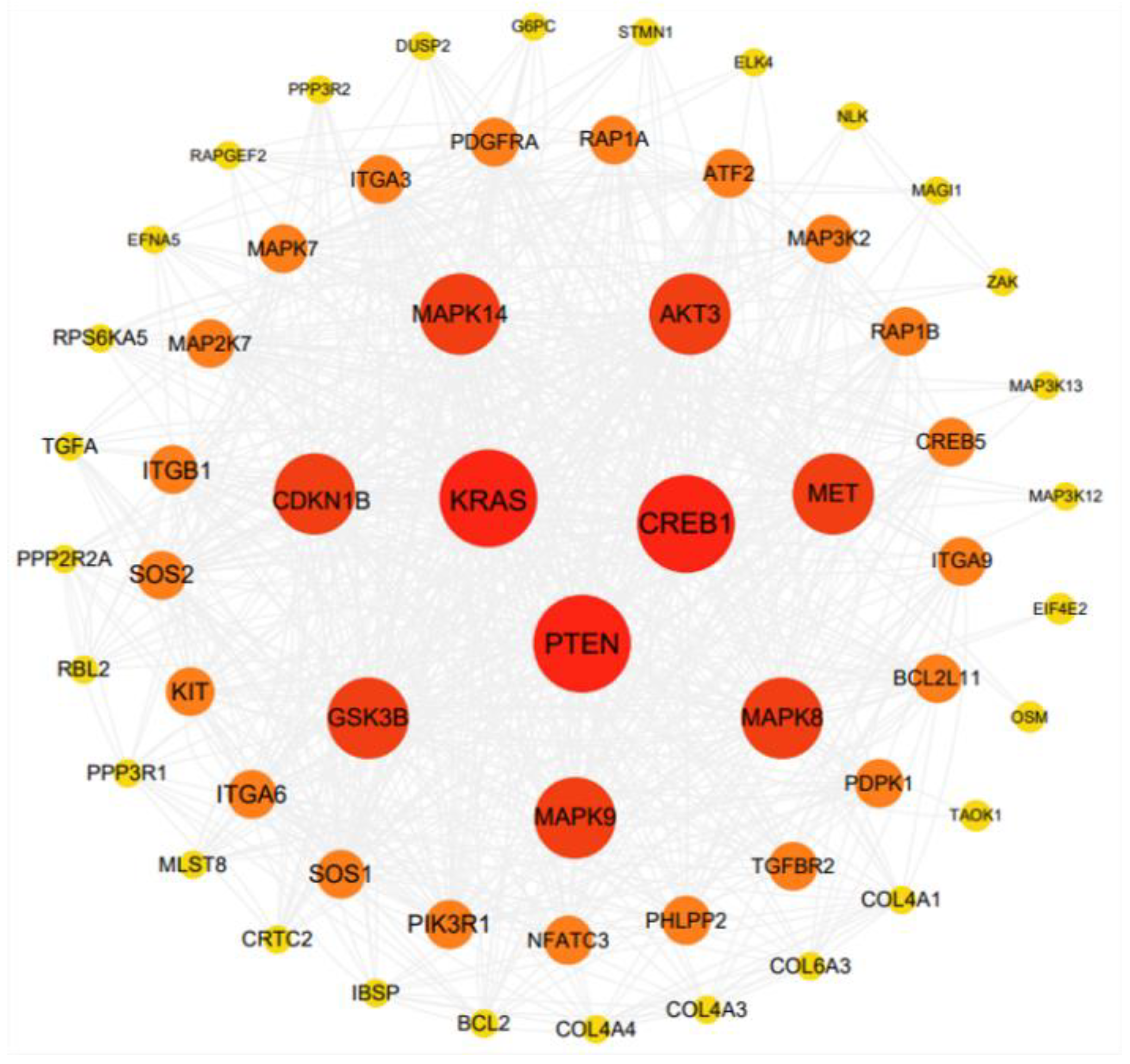
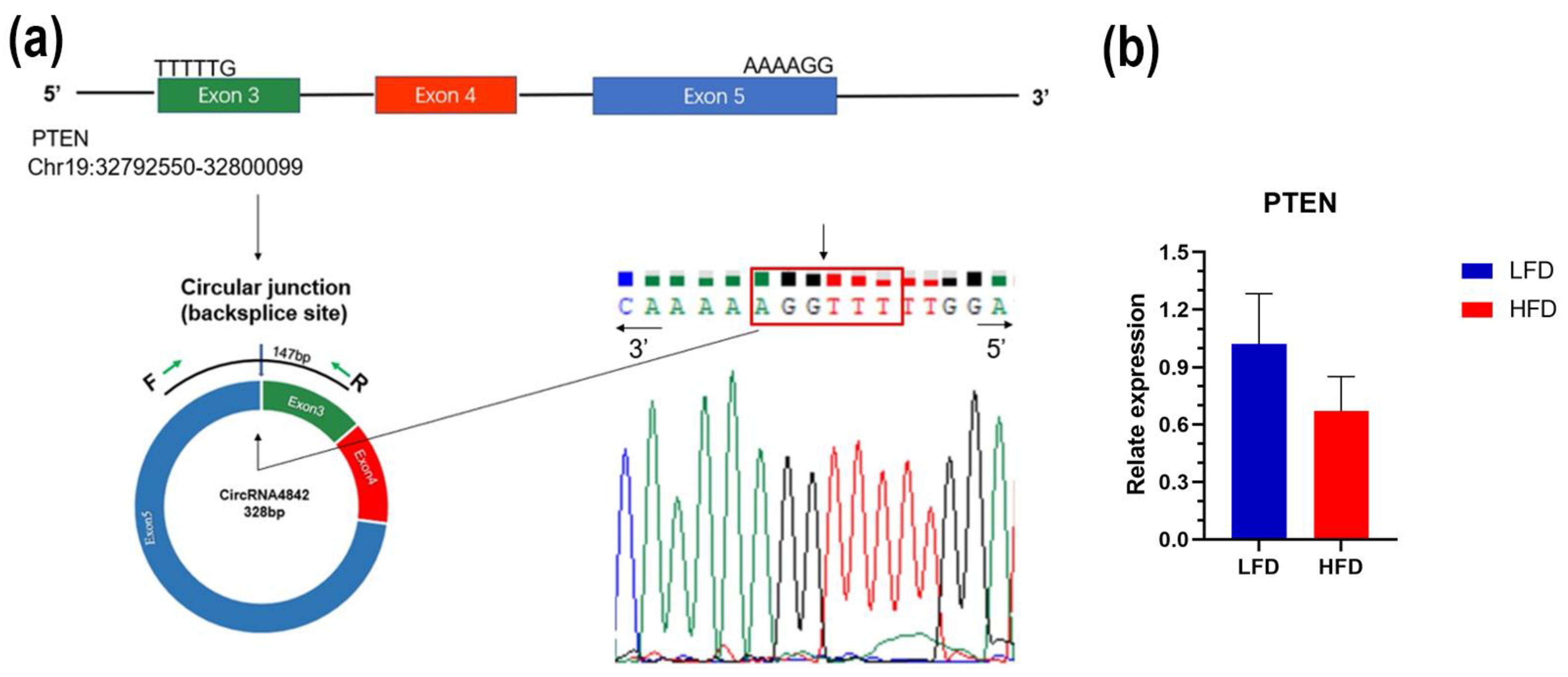
3.4. Construction of the ceRNA Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Lu, F.-B.; Hu, E.-D.; Xu, L.-M.; Chen, L.; Wu, J.-L.; Li, H.; Chen, D.-Z.; Chen, Y.-P. The Relationship between Obesity and the Severity of Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 491–502. [Google Scholar] [CrossRef]
- Scapaticci, S.; D’Adamo, E.; Mohn, A.; Chiarelli, F.; Giannini, C. Non-Alcoholic Fatty Liver Disease in Obese Youth with Insulin Resistance and Type 2 Diabetes. Front. Endocrinol. 2021, 12, 639548. [Google Scholar] [CrossRef]
- Ji, C.; Guo, X. The Clinical Potential of Circulating MicroRNAs in Obesity. Nat. Rev. Endocrinol. 2019, 15, 731–743. [Google Scholar] [CrossRef]
- Lu, Q.; Guo, P.; Liu, A.; Ares, I.; Martínez-Larrañaga, M.; Wang, X.; Anadón, A.; Martínez, M. The Role of Long Noncoding RNA in Lipid, Cholesterol, and Glucose Metabolism and Treatment of Obesity Syndrome. Med. Res. Rev. 2021, 41, 1751–1774. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Taheri, M. The Expression Profile and Role of Non-Coding RNAs in Obesity. Eur. J. Pharmacol. 2021, 892, 173809. [Google Scholar] [CrossRef]
- Chen, L.-L. The Expanding Regulatory Mechanisms and Cellular Functions of Circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef]
- Misir, S.; Wu, N.; Yang, B.B. Specific Expression and Functions of Circular RNAs. Cell Death Differ. 2022, 29, 481–491. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Jakobsen, T.; Hager, H.; Kjems, J. The Emerging Roles of CircRNAs in Cancer and Oncology. Nat. Rev. Clin. Oncol. 2022, 19, 188–206. [Google Scholar] [CrossRef]
- Mehta, S.L.; Dempsey, R.J.; Vemuganti, R. Role of Circular RNAs in Brain Development and CNS Diseases. Prog. Neurobiol. 2020, 186, 101746. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, C.-Y.; Mai, T.-L.; Chuang, C.-F.; Chen, Y.-C.; Gupta, S.K.; Yen, L.; Wang, Y.-D.; Chuang, T.-J. Genome-Wide, Integrative Analysis of Circular RNA Dysregulation and the Corresponding Circular RNA-MicroRNA-MRNA Regulatory Axes in Autism. Genome Res. 2020, 30, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The Biogenesis, Biology and Characterization of Circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Prats, A.-C.; David, F.; Diallo, L.H.; Roussel, E.; Tatin, F.; Garmy-Susini, B.; Lacazette, E. Circular RNA, the Key for Translation. Int. J. Mol. Sci. 2020, 18, 8591. [Google Scholar] [CrossRef]
- Arcinas, C.; Tan, W.; Fang, W.; Desai, T.P.; Teh, D.C.S.; Degirmenci, U.; Xu, D.; Foo, R.; Sun, L. Adipose Circular RNAs Exhibit Dynamic Regulation in Obesity and Functional Role in Adipogenesis. Nat. Metab. 2019, 1, 688–703. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Li, Y.; Mao, R.; Yang, H.; Zhang, Y.; Zhang, Y.; Guo, P.; Zhan, D.; Zhang, T. Circular RNA SAMD4A Controls Adipogenesis in Obesity through the MiR-138-5p/EZH2 Axis. Theranostics 2020, 10, 4705–4719. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, M.; Luo, Y.; Yu, H.; Zhang, J.; Li, D.; He, Q. Maternal Obesity Alters CircRNA Expression and the Potential Role of Mmu_circRNA_0000660 via Sponging MiR_693 in Offspring Liver at Weaning Age. Gene 2020, 731, 144354. [Google Scholar] [CrossRef]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome Analysis by Strand-Specific Sequencing of Complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate Alignment of Transcriptomes in the Presence of Insertions, Deletions and Gene Fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Kim, D.; Salzberg, S.L. TopHat-Fusion: An Algorithm for Discovery of Novel Fusion Transcripts. Genome Biol. 2011, 12, R72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.O.; Dong, R.; Zhang, Y.; Zhang, J.L.; Luo, Z.; Zhang, J.; Chen, L.L.; Yang, L. Diverse Alternative Back-Splicing and Alternative Splicing Landscape of Circular RNAs. Genome Res. 2016, 26, 1277–1287. [Google Scholar] [CrossRef]
- Zhang, X.O.; Wang, H.B.; Zhang, Y.; Lu, X.; Chen, L.L.; Yang, L. Complementary Sequence-Mediated Exon Circularization. Cell 2014, 159, 134–147. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Zhao, F. CIRI: An Efficient and Unbiased Algorithm for de Novo Circular RNA Identification. Genome Biol. 2015, 16, 4. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Song, Z.; Zhong, X.; Huang, M.; Shen, D.; Gao, P.; Qian, X.; Wang, M.; He, X.; Wang, T.; et al. Sangerbox: A Comprehensive, Interaction-friendly Clinical Bioinformatics Analysis Platform. iMeta 2022, 1, e36. [Google Scholar] [CrossRef]
- Mohammed, M.S.; Sendra, S.; Lloret, J.; Bosch, I. Systems and WBANs for Controlling Obesity. J. Health Eng. 2018, 2018, 1564748. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and Nonalcoholic Fatty Liver Disease: From Pathophysiology to Therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal Models of Obesity and Diabetes Mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High Fat Diet Induced Obesity Model Using Four Strainsof Mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and Pregnancy: Mechanisms of Short Term and Long Term Adverse Consequences for Mother and Child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- Endalifer, M.L.; Diress, G. Epidemiology, Predisposing Factors, Biomarkers, and Prevention Mechanism of Obesity: A Systematic Review. J. Obes. 2020, 2020, 6134362. [Google Scholar] [CrossRef]
- Ali, S.A.; Peffers, M.J.; Ormseth, M.J.; Jurisica, I.; Kapoor, M. The Non-Coding RNA Interactome in Joint Health and Disease. Nat. Rev. Rheumatol. 2021, 17, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Basu, J. The Role of MicroRNAs and Long Non-Coding RNAs in the Regulation of the Immune Response to Mycobacterium Tuberculosis Infection. Front. Immunol. 2021, 12, 687962. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, J.; Shi, H.; Qian, H.; Zhang, X.; Xu, W. CircRNA: A Rising Star in Gastric Cancer. Cell. Mol. Life Sci. 2020, 77, 1661–1680. [Google Scholar] [CrossRef]
- Yang, Y.; Yujiao, W.; Fang, W.; Linhui, Y.; Ziqi, G.; Zhichen, W.; Zirui, W.; Shengwang, W. The Roles of MiRNA, LncRNA and CircRNA in the Development of Osteoporosis. Biol. Res. 2020, 53, 40. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, X.; Xu, J. The New Function of CircRNA: Translation. Clin. Transl. Oncol. 2020, 22, 2162–2169. [Google Scholar] [CrossRef]
- Wu, P.; Mo, Y.; Peng, M.; Tang, T.; Zhong, Y.; Deng, X.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; et al. Emerging Role of Tumor-Related Functional Peptides Encoded by LncRNA and CircRNA. Mol. Cancer 2020, 19, 22. [Google Scholar] [CrossRef]
- Shao, T.; Pan, Y.H.; Xiong, X.D. Circular RNA: An Important Player with Multiple Facets to Regulate Its Parental Gene Expression. Mol. Ther. Nucleic Acids 2021, 23, 369–376. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Yu, S.; Wang, W.; Liu, G.; Jiang, X.; Sun, D. Circular RNAs Regulate Parental Gene Expression: A New Direction for Molecular Oncology Research. Front. Oncol. 2022, 12, 947775. [Google Scholar] [CrossRef]
- Chen, L.; Huang, C.; Shan, G. Circular RNAs in Physiology and Non-Immunological Diseases. Trends Biochem. Sci. 2022, 47, 250–264. [Google Scholar] [CrossRef]
- Liang, Z.Z.; Guo, C.; Zou, M.M.; Meng, P.; Zhang, T.T. CircRNA-MiRNA-MRNA Regulatory Network in Human Lung Cancer: An Update. Cancer Cell Int. 2020, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, F.; Wilkinson, M.; Baxter, E.; Brennan, D.J. Mitogen-Activated Protein Kinase (MAPK) and Obesity-Related Cancer. Int. J. Mol. Sci. 2020, 21, 1241. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling Pathway of MAPK/ERK in Cell Proliferation, Differentiation, Migration, Senescence and Apoptosis. J. Recept. Signal. Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling Pathways in Obesity: Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef]
- Sun, F.; Wang, J.; Sun, Q.; Li, F.; Gao, H.; Xu, L.; Zhang, J.; Sun, X.; Tian, Y.; Zhao, Q.; et al. Interleukin-8 Promotes Integrin Β3 Upregulation and Cell Invasion through PI3K/Akt Pathway in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 449. [Google Scholar] [CrossRef] [PubMed]
- Corti, F.; Nichetti, F.; Raimondi, A.; Niger, M.; Prinzi, N.; Torchio, M.; Tamborini, E.; Perrone, F.; Pruneri, G.; Di Bartolomeo, M.; et al. Targeting the PI3K/AKT/MTOR Pathway in Biliary Tract Cancers: A Review of Current Evidences and Future Perspectives. Cancer Treat. Rev. 2019, 72, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical Nodes in Signalling Pathways: Insights into Insulin Action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F.M. The PTEN/PI3K/AKT Signalling Pathway in Cancer, Therapeutic Implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.O.; Kim, J.H.; Kim, S.J.; You, G.Y.; Moon, J.W.; Jung, J.H.; Park, S.H.; Uhm, K.-O.; Park, J.M.; et al. Metformin Sensitizes Insulin Signaling through AMPK-Mediated PTEN down-Regulation in Preadipocyte 3T3-L1 Cells. J. Cell. Biochem. 2011, 112, 1259–1267. [Google Scholar] [CrossRef]
- Li, Y.Z.; Di Cristofano, A.; Woo, M. Metabolic Role of PTEN in Insulin Signaling and Resistance. Cold Spring Harb. Perspect. Med. 2020, 10, a036137. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Qiu, M.; Zhou, H.; Wang, T.; Guo, W. PTEN, Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3667–3676. [Google Scholar] [CrossRef] [PubMed]

| Sample | Raw Data | Valid Data | Valid Ratio | Mapped Reads | Unique Mapped Reads | Q20% | Q30% | GC Contents% |
|---|---|---|---|---|---|---|---|---|
| HFD1 | 55,704,582 | 40,656,388 | 72.99 | 28,545,064 (70.21%) | 17,168,081 (42.23%) | 99.95 | 96.33 | 59 |
| HFD2 | 55,388,836 | 42,390,104 | 76.53 | 25,915,631 (61.14%) | 11,418,281 (26.94%) | 99.86 | 90.81 | 59.5 |
| HFD3 | 51,100,520 | 40,360,256 | 78.98 | 24,590,954 (60.93%) | 10,049,723 (24.90%) | 99.90 | 90.85 | 61.5 |
| LFD1 | 50,753,286 | 37,071,822 | 73.04 | 24,760,292 (66.79%) | 6,777,698 (18.28%) | 99.89 | 92.22 | 62.5 |
| LFD2 | 51,741,522 | 37,645,092 | 72.76 | 23,792,149 (63.20%) | 9,708,540 (25.79%) | 99.90 | 92.09 | 60 |
| LFD3 | 49,576,072 | 34,475,702 | 69.54 | 24,803,033 (71.94%) | 9,635,483 (27.95%) | 99.95 | 96.50 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Gu, S.; Shen, S.; Luo, T.; Zhao, H.; Liu, S.; Feng, J.; Yang, M.; Yi, L.; Fan, Z.; et al. Identification of Circular RNA Profiles in the Liver of Diet-Induced Obese Mice and Construction of the ceRNA Network. Genes 2023, 14, 688. https://doi.org/10.3390/genes14030688
Zhang X, Gu S, Shen S, Luo T, Zhao H, Liu S, Feng J, Yang M, Yi L, Fan Z, et al. Identification of Circular RNA Profiles in the Liver of Diet-Induced Obese Mice and Construction of the ceRNA Network. Genes. 2023; 14(3):688. https://doi.org/10.3390/genes14030688
Chicago/Turabian StyleZhang, Xiaoxiao, Shuhua Gu, Shunyi Shen, Tao Luo, Haiyi Zhao, Sijia Liu, Jingjie Feng, Maosheng Yang, Laqi Yi, Zhaohan Fan, and et al. 2023. "Identification of Circular RNA Profiles in the Liver of Diet-Induced Obese Mice and Construction of the ceRNA Network" Genes 14, no. 3: 688. https://doi.org/10.3390/genes14030688
APA StyleZhang, X., Gu, S., Shen, S., Luo, T., Zhao, H., Liu, S., Feng, J., Yang, M., Yi, L., Fan, Z., Liu, Y., & Han, R. (2023). Identification of Circular RNA Profiles in the Liver of Diet-Induced Obese Mice and Construction of the ceRNA Network. Genes, 14(3), 688. https://doi.org/10.3390/genes14030688






