The Relevance of Telomerase and Telomere-Associated Proteins in B-Acute Lymphoblastic Leukemia
Abstract
1. Introduction
2. Materials and Methods
3. Discussion
3.1. Acute Lymphoblastic Leukemia
3.2. B-ALL and Its Molecular Markers
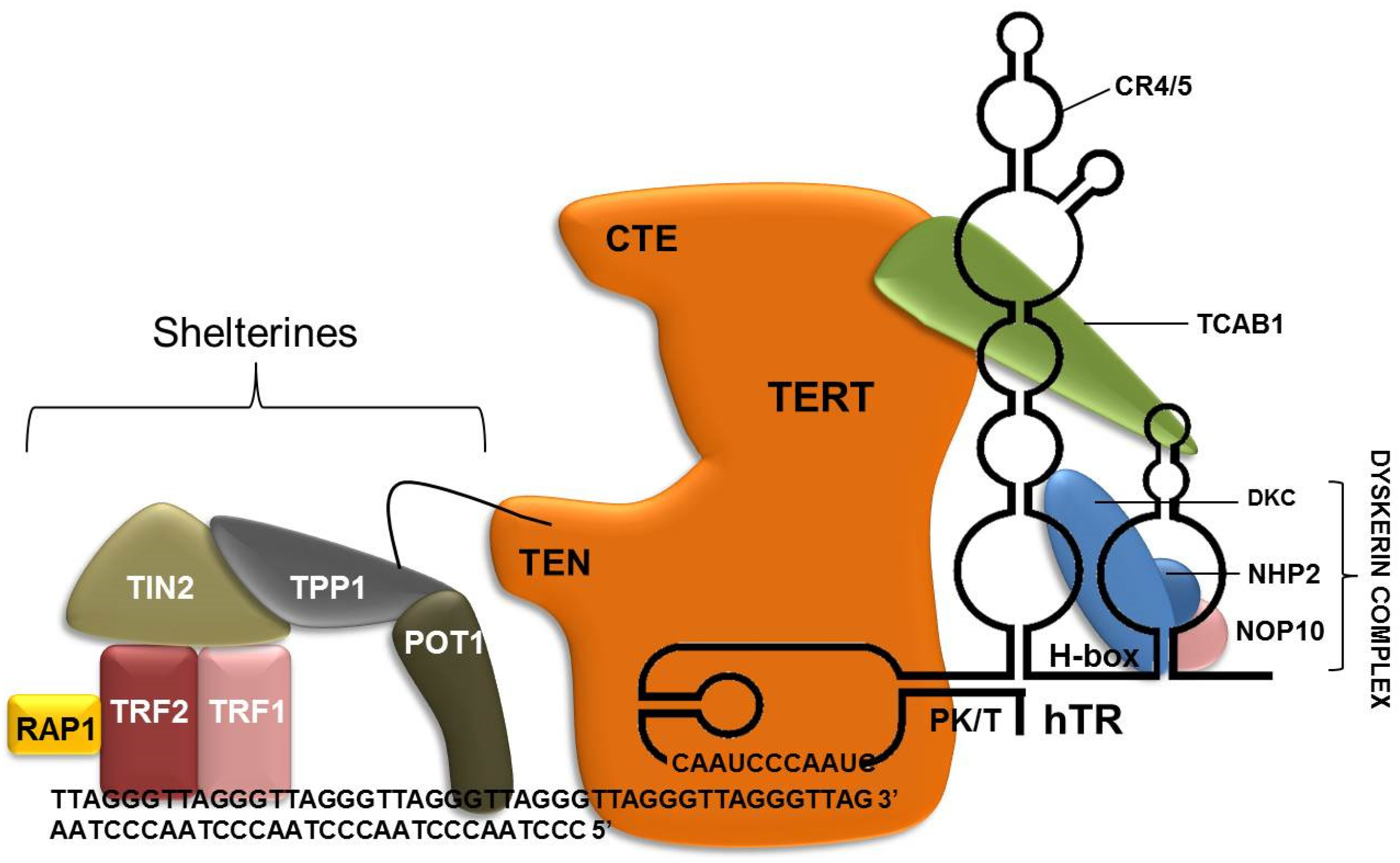
3.3. Telomeres, Shelterin Complex and Blood Cells
3.4. Telomerase and Cancer
3.5. Telomerses and Telomerase in B-Acute lymphoblastic leukemia
3.6. Shelterin in B Lymphoblastic Leukemia
3.7. Telomerase and Genetic Variation
3.8. Current Telomerase Inhibitors and Their Clinical Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whiteley, A.E.; Price, T.T.; Cantelli, G.; Sipkins, D.A. Leukaemia: A Model Metastatic Disease. Nat. Rev. Cancer 2021, 21, 461–475. [Google Scholar] [CrossRef]
- Lin, J.; Epel, E. Stress and Telomere Shortening: Insights from Cellular Mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef]
- Roussel, X.; Daguindau, E.; Berceanu, A.; Desbrosses, Y.; Warda, W.; Neto da Rocha, M.; Trad, R.; Deconinck, E.; Deschamps, M.; Ferrand, C. Acute Myeloid Leukemia: From Biology to Clinical Practices Through Development and Pre-Clinical Therapeutics. Front. Oncol. 2020, 10, 599933. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Hunger, S.P. The Genomic Landscape of Pediatric Acute Lymphoblastic Leukemia and Precision Medicine Opportunities. Semin. Cancer Biol. 2022, 84, 144–152. [Google Scholar] [CrossRef]
- Inaba, H.; Mullighan, C.G. Pediatric Acute Lymphoblastic Leukemia. Haematologica 2020, 105, 2524–2539. [Google Scholar] [CrossRef] [PubMed]
- Aberuyi, N.; Rahgozar, S.; Ghodousi, E.S.; Ghaedi, K. Drug Resistance Biomarkers and Their Clinical Applications in Childhood Acute Lymphoblastic Leukemia. Front. Oncol. 2020, 9, 1496. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.A. Leukemia in Children. Pediatr. Rev. 2019, 40, 319–331. [Google Scholar] [CrossRef]
- Waanders, E.; Gu, Z.; Dobson, S.M.; Antić, Ž.; Crawford, J.C.; Ma, X.; Edmonson, M.N.; Payne-Turner, D.; van de Vorst, M.; Jongmans, M.C.J.; et al. Mutational Landscape and Patterns of Clonal Evolution in Relapsed Pediatric Acute Lymphoblastic Leukemia. Blood Cancer Discov. 2020, 1, 96–111. [Google Scholar] [CrossRef]
- Samra, B.; Jabbour, E.; Ravandi, F.; Kantarjian, H.; Short, N.J. Evolving Therapy of Adult Acute Lymphoblastic Leukemia: State-of-the-Art Treatment and Future Directions. J. Hematol. Oncol. 2020, 13, 70. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Wang, B.-Y.; Zhang, W.-N.; Huang, J.-Y.; Li, B.-S.; Zhang, M.; Jiang, L.; Li, J.-F.; Wang, M.-J.; Dai, Y.-J.; et al. Genomic Profiling of Adult and Pediatric B-Cell Acute Lymphoblastic Leukemia. EBioMedicine 2016, 8, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Malczewska, M.; Kośmider, K.; Bednarz, K.; Ostapińska, K.; Lejman, M.; Zawitkowska, J. Recent Advances in Treatment Options for Childhood Acute Lymphoblastic Leukemia. Cancers 2022, 14, 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of Genome Editing Technology in the Targeted Therapy of Human Diseases: Mechanisms, Advances and Prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Kato, M.; Manabe, A. Treatment and Biology of Pediatric Acute Lymphoblastic Leukemia. Pediatr. Int. 2018, 60, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Chao, M.W.; Tu, H.J.; Chen, L.C.; Hsu, K.C.; Liou, J.P.; Yang, C.R.; Yen, S.C.; HuangFu, W.C.; Pan, S.L. A Novel Dual HDAC and HSP90 Inhibitor, MPT0G449, Downregulates Oncogenic Pathways in Human Acute Leukemia in Vitro and in Vivo. Oncogenesis 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M.M.; Aksentijevich, I.; Arber, D.A.; Barrett, J.; Brentjens, R.J.; Brufsky, J.; Cortes, J.; De Lima, M.; Forman, S.J.; Fuchs, E.J.; et al. The Society for Immunotherapy of Cancer (SITC) Clinical Practice Guideline on Immunotherapy for the Treatment of Acute Leukemia. J. Immunother. Cancer 2020, 8, e001235. [Google Scholar] [CrossRef]
- Autry, R.J.; Paugh, S.W.; Carter, R.; Shi, L.; Liu, J.; Daniel, C.; Lau, C.E.; Bonten, E.J.; Yang, W.; Mccorkle, J.R.; et al. Integrative Fenomic Analyses Reveal Mechanisms of Glucocorticoid Resistane in A. Nat. Cancer 2020, 1, 329–344. [Google Scholar] [CrossRef]
- Su, Q.; Fan, Z.; Huang, F.; Xu, N.; Nie, D.; Lin, D.; Guo, Z.; Shi, P.; Wang, Z.; Jiang, L.; et al. Comparison of Two Strategies for Prophylactic Donor Lymphocyte Infusion in Patients With Refractory/Relapsed Acute Leukemia. Front. Oncol. 2021, 11, 554503. [Google Scholar] [CrossRef]
- Iacobucci, I.; Mullighan, C.G. Genetic Basis of Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2017, 35, 975–983. [Google Scholar] [CrossRef]
- Maamari, D.; El-Khoury, H.; Saifi, O.; Muwakkit, S.A.; Zgheib, N.K. Implementation of Pharmacogenetics to Individualize Treatment Regimens for Children with Acute Lymphoblastic Leukemia. Pharmgenomic. Pers. Med. 2020, 13, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Rabin, K.R. Genetic Ancestry and Childhood Acute Lymphoblastic Leukemia Subtypes and Outcomes in the Genomic Era. JAMA Oncol. 2022, 8, 342. [Google Scholar] [CrossRef]
- Komorowski, L.; Fidyt, K.; Patkowska, E.; Firczuk, M. Philadelphia Chromosome-Positive Leukemia in the Lymphoid Lineage—Similarities and Differences with the Myeloid Lineage and Specific Vulnerabilities. Int. J. Mol. Sci. 2020, 21, 5776. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xiao, X.; Liu, P.; Liu, P.; Li, D.; Li, D.; Xia, Z.; Wang, P.; Wang, P.; Zhang, X.; et al. Combination Therapy of BCR-ABL-Positive B Cell Acute Lymphoblastic Leukemia by Tyrosine Kinase Inhibitor Dasatinib and c-JUN N-Terminal Kinase Inhibition. J. Hematol. Oncol. 2020, 13, 80. [Google Scholar] [CrossRef]
- Brown, L.M.; Hediyeh-zadeh, S.; Sadras, T.; Huckstep, H.; Sandow, J.J.; Bartolo, R.C.; Kosasih, H.J.; Davidson, N.M.; Schmidt, B.; Bjelosevic, S.; et al. SFPQ-ABL1 and BCR-ABL1 Use Different Signaling Networks to Drive B-Cell Acute Lymphoblastic Leukemia. Blood Adv. 2022, 6, 2373–2387. [Google Scholar] [CrossRef]
- Bakalova, R.; Ohba, H.; Zhelev, Z.; Kubo, T.; Fujii, M.; Ishikawa, M.; Shinohara, Y.; Baba, Y. Antisense Inhibition of Bcr-Abl/c-Abl Synthesis Promotes Telomerase Activity and Upregulates Tankyrase in Human Leukemia Cells. FEBS Lett. 2004, 564, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, Y.; Qin, M.; Li, D.; Odhiambo, W.O.; Yuan, M.; Lv, Z.; Liu, C.; Ma, Y.; Dong, Y.; et al. Involvement of Blnk and Foxo1 in Tumor Suppression in BCR-ABL1-Transformed pro-B Cells. Oncol. Rep. 2021, 45, 693–705. [Google Scholar] [CrossRef]
- Inaba, H.; Pui, C.-H. Advances in the Diagnosis and Treatment of Pediatric Acute Lymphoblastic Leukemia. J. Clin. Med. 2021, 10, 1926. [Google Scholar] [CrossRef]
- Tasian, S.K.; Loh, M.L.; Hunger, S.P. Philadelphia Chromosome–like Acute Lymphoblastic Leukemia. Blood 2017, 130, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, G.P. Advances in the Diagnosis and Treatment of Pediatric Acute Respiratory Distress Syndrome. Chin. J. Contemp. Pediatr. 2018, 20, 717–723. [Google Scholar] [CrossRef]
- Bei, Y. Targeted Therapy or Transplantation for Paediatric ABL-Class Ph-like Acute Lymphocytic Leukaemia? Physiol. Behav. 2020, 7, e858–e859. [Google Scholar] [CrossRef]
- Hurtz, C.; Wertheim, G.B.; Loftus, J.P.; Blumenthal, D.; Lehman, A.; Li, Y.; Bagashev, A.; Manning, B.; Cummins, K.D.; Burkhardt, J.K.; et al. Oncogene-Independent BCR-like Signaling Adaptation Confers Drug Resistance in Ph-like ALL. J. Clin. Invest. 2020, 130, 3637–3653. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, T.; Abdul-Hay, M. Acute Lymphoblastic Leukemia: A Comprehensive Review and 2017 Update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef] [PubMed]
- Lejman, M.; Chałupnik, A.; Chilimoniuk, Z.; Dobosz, M. Genetic Biomarkers and Their Clinical Implications in B-Cell Acute Lymphoblastic Leukemia in Children. Int. J. Mol. Sci. 2022, 23, 2755. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Yu, C.H.; Chen, Y.M.; Roberts, K.G.; Ni, Y.L.; Lin, K.H.; Jou, S.T.; Lu, M.Y.; Chen, S.H.; Wu, K.H.; et al. Philadelphia Chromosome-Negative B-Cell Acute Lymphoblastic Leukaemia with Kinase Fusions in Taiwan. Sci. Rep. 2021, 11, 5802. [Google Scholar] [CrossRef] [PubMed]
- Tosi, M.; Spinelli, O.; Leoncin, M.; Cavagna, R.; Pavoni, C.; Lussana, F.; Intermesoli, T.; Frison, L.; Perali, G.; Carobolante, F.; et al. Mrd-based Therapeutic Decisions in Genetically Defined Subsets of Adolescents and Young Adult Philadelphia-negative All. Cancers 2021, 13, 2108. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.C.; Tasian, S.K. Clinical Diagnostics and Treatment Strategies for Philadelphia Chromosome-like Acute Lymphoblastic Leukemia. Blood Adv. 2020, 4, 218–228. [Google Scholar] [CrossRef]
- Jenkins, T.W.; Kopyscinski, S.L.D.; Fields, J.L.; Rahme, G.J.; Colley, W.C.; Israel, M.A. Activity of Immunoproteasome Inhibitor ONX-0914 in Acute Lymphoblastic Leukemia Expressing MLL-AF4 Fusion Protein. Sci. Rep. 2021, 11, 10883. [Google Scholar] [CrossRef]
- Antunes, E.T.B.; Ottersbach, K. The MLL/SET Family and Haematopoiesis. Biochim. Biophys. Acta-Gene Regul. Mech. 2020, 1863, 194579. [Google Scholar] [CrossRef]
- Cao, L.; Mitra, P.; Gonda, T.J. The Mechanism of MYB Transcriptional Regulation by MLL-AF9 Oncoprotein. Sci. Rep. 2019, 9, 20084. [Google Scholar] [CrossRef]
- Wen, J.; Zhou, M.; Shen, Y.; Long, Y.; Guo, Y.; Song, L.; Xiao, J. Poor Treatment Responses Were Related to Poor Outcomes in Pediatric B Cell Acute Lymphoblastic Leukemia with KMT2A Rearrangements. BMC Cancer 2022, 22, 859. [Google Scholar] [CrossRef]
- Gessner, A.; Thomas, M.; Garrido Castro, P.; Büchler, L.; Scholz, A.; Brümmendorf, T.H.; Martinez Soria, N.; Vormoor, J.; Greil, J.; Heidenreich, O. Leukemic Fusion Genes MLL/AF4 and AML1/MTG8 Support Leukemic Self-Renewal by Controlling Expression of the Telomerase Subunit TERT. Leukemia 2010, 24, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, M.; Ozkaynak, M.; Drullinsky, P.; Sandoval, C.; Tugal, O.; Jayabose, S.; Moore, M. Telomerase Activity and Telomere Length in Pediatric Patients with Malignancies Undergoing Chemotherapy. Leukemia 1998, 12, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.S.; de Moraes, L.S.; da Rocha, C.A.M.; Ferreira-Fernandes, H.; Yoshioka, F.K.N.; Rey, J.A.; Pinto, G.R.; Burbano, R.R. Telomere Length and Telomerase Activity of Leukocytes as Biomarkers of Selective Serotonin Reuptake Inhibitor Responses in Patients with Major Depressive Disorder. Psychiatr. Genet. 2022, 32, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Afshari, N.; Al-Gazally, M.E.; Rasulova, I.; Jalil, A.T.; Matinfar, S.; Momeninejad, M. Sensitive Bioanalytical Methods for Telomerase Activity Detection: A Cancer Biomarker. Anal. Methods 2022, 14, 4174–4184. [Google Scholar] [CrossRef]
- Karow, A.; Haubitz, M.; Oppliger Leibundgut, E.; Helsen, I.; Preising, N.; Steiner, D.; Dantonello, T.M.; Ammann, R.A.; Roessler, J.; Kartal-Kaess, M.; et al. Targeting Telomere Biology in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021, 22, 6653. [Google Scholar] [CrossRef]
- Bashash, D.; Ghaffari, S.H.; Mirzaee, R.; Alimoghaddam, K.; Ghavamzadeh, A. Telomerase Inhibition by Non-Nucleosidic Compound BIBR1532 Causes Rapid Cell Death in Pre-B Acute Lymphoblastic Leukemia Cells. Leuk. Lymphoma 2013, 54, 561–568. [Google Scholar] [CrossRef]
- Nogueira, B.M.D.; da Costa Pantoja, L.; da Silva, E.L.; Mello Júnior, F.A.R.; Teixeira, E.B.; Wanderley, A.V.; da Silva Maués, J.H.; de Moraes Filho, M.O.; de Moraes, M.E.A.; Montenegro, R.C.; et al. Telomerase (HTERT) Overexpression Reveals a Promising Prognostic Biomarker and Therapeutical Target in Different Clinical Subtypes of Pediatric Acute Lymphoblastic Leukaemia. Genes 2021, 12, 1632. [Google Scholar] [CrossRef]
- Caitlin, M.; Roake and Steven, E. Artandi Regulation of Human Telomerase in Homeostasis and Disease. Physiol. Behav. 2020, 21, 384–397. [Google Scholar] [CrossRef]
- Tomita, K. How Long Does Telomerase Extend Telomeres? Regulation of Telomerase Release and Telomere Length Homeostasis. Curr. Genet. 2018, 64, 1177–1181. [Google Scholar] [CrossRef]
- Mei, Y.; Deng, Z.; Vladimirova, O.; Gulve, N.; Johnson, F.B.; Drosopoulos, W.C.; Schildkraut, C.L.; Lieberman, P.M. TERRA G-Quadruplex RNA Interaction with TRF2 GAR Domain Is Required for Telomere Integrity. Sci. Rep. 2021, 11, 3509. [Google Scholar] [CrossRef]
- Sieverling, L.; Hong, C.; Koser, S.D.; Ginsbach, P.; Kleinheinz, K.; Hutter, B.; Braun, D.M.; Cortés-Ciriano, I.; Xi, R.; Kabbe, R.; et al. Genomic Footprints of Activated Telomere Maintenance Mechanisms in Cancer. Nat. Commun. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 630186. [Google Scholar] [CrossRef]
- Von Zglinicki, T.; Wan, T.; Miwa, S. Senescence in Post-Mitotic Cells: A Driver of Aging? Antioxid. Redox Signal. 2021, 34, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Kaur, P.; Barnes, R.; Detwiler, A.C.; Sanford, S.L.; Liu, M.; Xu, P.; Mahn, C.; Tang, Q.; Hao, P.; et al. Structure, Dynamics, and Regulation of TRF1-TIN2-Mediated Trans- And Cis-Interactions on Telomeric DNA. J. Biol. Chem. 2021, 297, 101080. [Google Scholar] [CrossRef]
- Erdel, F.; Kratz, K.; Willcox, S.; Griffith, J.D.; Greene, E.C.; de Lange, T. Telomere Recognition and Assembly Mechanism of Mammalian Shelterin. Cell Rep. 2017, 18, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Liu, J.; Hu, X.; Yu, C.; Roskamp, K.; Sankaran, B.; Huang, L.; Komives, E.A.; Qiao, F. Structural Basis for Shelterin Bridge Assembly. Mol. Cell 2017, 68, 698–714.e5. [Google Scholar] [CrossRef] [PubMed]
- Sekne, Z.; Ghanim, G.E.; van Roon, A.-M.M.; Nguyen, T.H.D. Structural Basis of Human Telomerase Recruitment by TPP1-POT1. Science 2022, 375, 1173–1176. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; Bao, K.; Kim, J.K.; Zhang, C.; Jia, S.; Qiao, F. The Cooperative Assembly of Shelterin Bridge Provides a Kinetic Gateway That Controls Telomere Length Homeostasis. Nucleic Acids Res. 2021, 49, 8110–8119. [Google Scholar] [CrossRef]
- Semeraro, M.D.; Smith, C.; Kaiser, M.; Levinger, I.; Duque, G.; Gruber, H.-J.; Herrmann, M. Physical Activity, a Modulator of Aging through Effects on Telomere Biology. Aging 2020, 12, 13803–13823. [Google Scholar] [CrossRef]
- Krasnienkov, D.S.; Khalangot, M.D.; Kravchenko, V.I.; Kovtun, V.A.; Guryanov, V.G.; Chizhova, V.P.; Korkushko, O.V.; Shatilo, V.B.; Kukharsky, V.M.; Vaiserman, A.M. Hyperglycemia Attenuates the Association between Telomere Length and Age in Ukrainian Population. Exp. Gerontol. 2018, 110, 247–252. [Google Scholar] [CrossRef]
- Hastings, W.J.; Shalev, I.; Belsky, D.W. Translating Measures of Biological Aging to Test Effectiveness of Geroprotective Interventions: What Can We Learn from Research on Telomeres? Front. Genet. 2017, 8, 164. [Google Scholar] [CrossRef]
- Sharma, S.; Chowdhury, S. Emerging Mechanisms of Telomerase Reactivation in Cancer. Trends Cancer 2022, 8, 632–641. [Google Scholar] [CrossRef]
- Jacczak, B.; Rubiś, B.; Totoń, E. Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging. Int. J. Mol. Sci. 2021, 22, 6381. [Google Scholar] [CrossRef]
- Dogan, F.; Forsyth, N.R. Telomerase Regulation: A Role for Epigenetics. Cancers 2021, 13, 1213. [Google Scholar] [CrossRef]
- Udroiu, I.; Marinaccio, J.; Sgura, A. Many Functions of Telomerase Components: Certainties, Doubts, and Inconsistencies. Int. J. Mol. Sci. 2022, 23, 15189. [Google Scholar] [CrossRef]
- Thompson, C.A.H.; Wong, J.M.Y. Non-Canonical Functions of Telomerase Reverse Transcriptase: Emerging Roles and Biological Relevance. Curr. Top. Med. Chem. 2020, 20, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Jakobs, P.; Ale-Agha, N.; Altschmied, J.; Haendeler, J. Non-Canonical Functions of Telomerase Reverse Transcriptase–Impact on Redox Homeostasis. Redox Biol. 2020, 34, 101543. [Google Scholar] [CrossRef]
- Demerath, E.W.; Cameron, N.; Gillman, M.W.; Towne, B.; Siervogel, R.M. Telomeres and Telomerase in the Fetal Origins of Cardiovascular Disease: A Review. Hum. Biol. 2004, 76, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Celtikci, B.; Erkmen, G.K.; Dikmen, Z.G. Regulation and Effect of Telomerase and Telomeric Length in Stem Cells. Curr. Stem Cell Res. Ther. 2021, 16, 809–823. [Google Scholar] [CrossRef]
- Schaich, M.A.; Sanford, S.L.; Welfer, G.A.; Johnson, S.A.; Khoang, T.H.; Opresko, P.L.; Freudenthal, B.D. Mechanisms of Nucleotide Selection by Telomerase. eLife 2020, 9, e55438. [Google Scholar] [CrossRef] [PubMed]
- Radu, L.E.; Colita, A.; Pasca, S.; Tomuleasa, C.; Popa, C.; Serban, C.; Gheorghe, A.; Serbanica, A.; Jercan, C.; Marcu, A.; et al. Day 15 and Day 33 Minimal Residual Disease Assessment for Acute Lymphoblastic Leukemia Patients Treated According to the BFM ALL IC 2009 Protocol: Single-Center Experience of 133 Cases. Front. Oncol. 2020, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.F.; Coughlan, H.D.; Zhou, J.H.S.; Keenan, C.R.; Bediaga, N.G.; Hodgkin, P.D.; Smyth, G.K.; Johanson, T.M.; Allan, R.S. Pre-Mitotic Genome Re-Organisation Bookends the B Cell Differentiation Process. Nat. Commun. 2021, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Bryan, T.M.; Reddel, R.R. Increased Copy Number of the TERT and TERC Telomerase Subunit Genes in Cancer Cells. Cancer Sci. 2008, 99, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic Analysis of Telomere Length and Somatic Alterations in 31 Cancer Types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Zhang, L.; Ma, L.; Jiang, K.; Yao, G.; Zhu, L. TERT Promoter Mutations and Telomerase in Melanoma. J. Oncol. 2022, 2022, 6300329. [Google Scholar] [CrossRef]
- Heidenreich, B.; Kumar, R. TERT Promoter Mutations in Telomere Biology. Mutat. Res.-Rev. Mutat. Res. 2017, 771, 15–31. [Google Scholar] [CrossRef]
- Kyo, S.; Takakura, M.; Fujiwara, T.; Inoue, M. Understanding and Exploiting HTERT Promoter Regulation for Diagnosis and Treatment of Human Cancers. Cancer Sci. 2008, 99, 1528–1538. [Google Scholar] [CrossRef]
- Chiba, K.; Lorbeer, F.K.; Shain, A.H.; McSwiggen, D.T.; Schruf, E.; Oh, A.; Ryu, J.; Darzacq, X.; Bastian, B.C.; Hockemeyer, D. Mutations in the Promoter of the Telomerase Gene TERT Contribute to Tumorigenesis by a Two-Step Mechanism. Science 2017, 357, 1416–1420. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Li, D.; Li, J.; Luo, Y.; Dan, J. Roles of Telomeres and Telomerase in Age-Related Renal Diseases (Review). Mol. Med. Rep. 2021, 23, 96. [Google Scholar] [CrossRef]
- Robinson, N.J.; Schiemann, W.P. Telomerase in Cancer: Function, Regulation, and Clinical Translation. Cancers 2022, 14, 808. [Google Scholar] [CrossRef]
- Liu, X.; Zou, L.; Zhu, L.; Zhang, H.; Du, C.; Li, Z.; Gao, C.; Zhao, X.; Bao, S.; Zheng, H. MiRNA Mediated Up-Regulation of Cochaperone P23 Acts as an Anti-Apoptotic Factor in Childhood Acute Lymphoblastic Leukemia. Leuk. Res. 2012, 36, 1098–1104. [Google Scholar] [CrossRef]
- Sheikh-Zeineddini, N.; Bashash, D.; Safaroghli-Azar, A.; Riyahi, N.; Shabestari, R.M.; Janzamin, E.; Safa, M. Suppression of C-Myc Using 10058-F4 Exerts Caspase-3-dependent Apoptosis and Intensifies the Antileukemic Effect of Vincristine in Pre-B Acute Lymphoblastic Leukemia Cells. J. Cell. Biochem. 2019, 120, 14004–14016. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, H.; Deb, S.; Liu, J.P. TERT Regulates Cell Survival Independent of Telomerase Enzymatic Activity. Oncogene 2002, 21, 3130–3138. [Google Scholar] [CrossRef]
- Lin, S.Y.; Elledge, S.J. Multiple Tumor Suppressor Pathways Negatively Regulate Telomerase. Cell 2003, 113, 881–889. [Google Scholar] [CrossRef]
- Zhang, Y.; Toh, L.; Lau, P.; Wang, X. Human Telomerase Reverse Transcriptase (HTERT) Is a Novel Target of the Wnt/β-Catenin Pathway in Human Cancer. J. Biol. Chem. 2012, 287, 32494–32511. [Google Scholar] [CrossRef] [PubMed]
- Linne, H.; Yasaei, H.; Marriott, A.; Harvey, A.; Mokbel, K.; Newbold, R.; Roberts, T. Functional Role of SETD2, BAP1, PARP-3 and PBRM1 Candidate Genes on the Regulation of HTERT Gene Expression. Oncotarget 2017, 8, 61890–61900. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Qin, R.; Shu, Y.; Liu, Z.; Zhang, P.; Duan, C.; Hong, D.; Yu, J.; Zou, L. The Cellular Senescence of Leukemia-Initiating Cells from Acute Lymphoblastic Leukemia Is Postponed by β-Arrestin1 Binding with P300-Sp1 to Regulate HTERT Transcription. Cell Death Dis. 2017, 8, e2756. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Kumar, R. Bcl-2 Modulates Telomerase Activity. J. Biol. Chem. 1997, 272, 14183–14187. [Google Scholar] [CrossRef]
- Jin, Y.; You, L.; Kim, H.J.; Lee, H.-W. Telomerase Reverse Transcriptase Contains a BH3-Like Motif and Interacts with BCL-2 Family Members. Mol. Cells 2018, 41, 684–694. [Google Scholar] [CrossRef]
- Ding, X.; Nie, Z.; She, Z.; Bai, X.; Yang, Q.; Wang, F.; Wang, F.; Geng, X. The Regulation of ROS- and BECN1-Mediated Autophagy by Human Telomerase Reverse Transcriptase in Glioblastoma. Oxid. Med. Cell. Longev. 2021, 2021, 6636510. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, F.; Li, Y.; Hao, J.; Tang, Z.; Tian, C.; Yang, Q.; Zhu, T.; Diao, C.; Zhang, C.; et al. BPTF Promotes Hepatocellular Carcinoma Growth by Modulating HTERT Signaling and Cancer Stem Cell Traits. Redox Biol. 2019, 20, 427–441. [Google Scholar] [CrossRef]
- Park, J.-I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase Modulates Wnt Signalling by Association with Target Gene Chromatin. Nature 2009, 460, 66–72. [Google Scholar] [CrossRef]
- Sanyal, S.; Mondal, P.; Sen, S.; Sengupta, S.; Das, C. SUMO E3 Ligase CBX4 Regulates HTERT-Mediated Transcription of CDH1 and Promotes Breast Cancer Cell Migration and Invasion. Biochem. J. 2020, 477, 3803–3818. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Zhang, R.; Sun, L.; Yu, W.; Guo, W.; Gao, Y.; Li, M.; Liu, W.; Liang, P.; et al. CDC5L Promotes HTERT Expression and Colorectal Tumor Growth. Cell. Physiol. Biochem. 2017, 41, 2475–2488. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, M.; Ando, Y.; Yamashita, T.; Matsuda, Y.; Shoji, S.; Morioka, M.S.; Kawaji, H.; Shiozawa, K.; Machitani, M.; Abe, T.; et al. CDK1 Dependent Phosphorylation of HTERT Contributes to Cancer Progression. Nat. Commun. 2020, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-J.; Zeng, S.; Xie, R.; Hu, C.-J.; Wang, S.-M.; Wu, Y.-Y.; Xiao, Y.-F.; Yang, S.-M. HTERT Promotes Gastric Intestinal Metaplasia by Upregulating CDX2 via NF-ΚB Signaling Pathway. Oncotarget 2017, 8, 26969–26978. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-J.; Grandori, C.; Amacker, M.; Simon-Vermot, N.; Polack, A.; Lingner, J.; Dalla-Favera, R. Direct Activation of TERT Transcription by C-MYC. Nat. Genet. 1999, 21, 220–224. [Google Scholar] [CrossRef]
- Eldholm, V.; Haugen, A.; Zienolddiny, S. CTCF Mediates the TERT Enhancer-Promoter Interactions in Lung Cancer Cells: Identification of a Novel Enhancer Region Involved in the Regulation of TERT Gene. Int. J. Cancer 2014, 134, 2305–2313. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Y.; Xu, H.; Xie, X.; He, Z.; Lin, S.; Li, R.; Jin, S.; Cui, J.; Hu, H.; et al. An Inducible CRISPR/Cas9 Screen Identifies DTX2 as a Transcriptional Regulator of Human Telomerase. iScience 2022, 25, 103813. [Google Scholar] [CrossRef]
- Crowe, D.L.; Nguyen, D.C. Rb and E2F-1 Regulate Telomerase Activity in Human Cancer Cells. Biochim. Biophys. Acta-Gene Struct. Expr. 2001, 1518, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, A.; Shen, C.; Zhang, B.; Rao, Z.; Wang, R.; Yang, S.; Ning, S.; Mao, G.; Fang, D. E2F1 Acts as a Negative Feedback Regulator of C-Myc-Induced HTERT Transcription during Tumorigenesis. Oncol. Rep. 2014, 32, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Qin, Y.; Zhang, H.; Gao, M.; Wang, Y. EGF Upregulates RFPL3 and HTERT via the MEK Signaling Pathway in Non-small Cell Lung Cancer Cells. Oncol. Rep. 2018, 40, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, D.D.; Vasina, D.A.; Grachev, V.A.; Orlova, E.V.; Orlova, V.S.; Pokrovskaya, M.V.; Alexandrova, S.S.; Sokolov, N.N. Alternative Splicing of Telomerase Catalytic Subunit HTERT Generated by Apoptotic Endonuclease EndoG Induces Human CD4+ T Cell Death. Eur. J. Cell Biol. 2017, 96, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, A.; Nanni, S.; Colussi, C.; Aiello, A.; Benvenuti, V.; Ragone, G.; Moretti, F.; Sacchi, A.; Bacchetti, S.; Gaetano, C.; et al. Estrogen Receptor-α and Endothelial Nitric Oxide Synthase Nuclear Complex Regulates Transcription of Human Telomerase. Circ. Res. 2008, 103, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Akutagawa, O.; Nishi, H.; Kyo, S.; Higuma, C.; Inoue, M.; Isaka, K. Early Growth Response-1 Mediates Up-Regulation of Telomerase in Placenta. Placenta 2007, 28, 920–927. [Google Scholar] [CrossRef]
- Yuseran, H.; Hartoyo, E.; Nurseta, T.; Kalim, H. Molecular Docking of Genistein on Estrogen Receptors, Promoter Region of BCLX, Caspase-3, Ki-67, Cyclin D1, and Telomere Activity. J. Taibah Univ. Med. Sci. 2019, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, T.; Chen, S.; Song, C.; Shan, D.; Xu, S.; Xu, S. Case Report: Identification of Multiple TERT and FGFR2 Gene Fusions in a Pineal Region Glioblastoma Case. Front. Oncol. 2021, 11, 739309. [Google Scholar] [CrossRef]
- Hu, C.; Ni, Z.; Li, B.; Yong, X.; Yang, X.; Zhang, J.; Zhang, D.; Qin, Y.; Jie, M.; Dong, H.; et al. HTERT Promotes the Invasion of Gastric Cancer Cells by Enhancing FOXO3a Ubiquitination and Subsequent ITGB1 Upregulation. Gut 2017, 66, 31–42. [Google Scholar] [CrossRef]
- Xing, X.; Mu, N.; Yuan, X.; Wang, N.; Juhlin, C.C.; Strååt, K.; Larsson, C.; Neo, S.Y.; Xu, D. Downregulation and Hypermethylation of GABPB1 Is Associated with Aggressive Thyroid Cancer Features. Cancers 2022, 14, 1385. [Google Scholar] [CrossRef]
- Wang, D.-X.; Zhu, X.-D.; Ma, X.-R.; Wang, L.-B.; Dong, Z.-J.; Lin, R.-R.; Cao, Y.-N.; Zhao, J.-W. Loss of Growth Differentiation Factor 11 Shortens Telomere Length by Downregulating Telomerase Activity. Front. Physiol. 2021, 12, 726345. [Google Scholar] [CrossRef]
- Wu, L.; Wang, S.; Tang, B.; Tang, L.; Lei, Y.; Liu, Y.; Yang, M.; Yang, G.; Zhang, D.; Liu, E. Human Telomerase Reverse Transcriptase (HTERT) Synergistic with Sp1 Upregulate Gli1 Expression and Increase Gastric Cancer Invasion and Metastasis. J. Mol. Histol. 2021, 52, 1165–1175. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, S.; Huang, A.; Gao, Y.; Peng, B.; Li, Z.; Ma, W.; Songyang, Z.; Zhang, S.; He, M.; et al. GOLPH3 Promotes Cancer Growth by Interacting with STIP1 and Regulating Telomerase Activity in Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2020, 10, 575358. [Google Scholar] [CrossRef]
- Lu, H.; Lyu, Y.; Tran, L.; Lan, J.; Xie, Y.; Yang, Y.; Murugan, N.L.; Wang, Y.J.; Semenza, G.L. HIF-1 Recruits NANOG as a Coactivator for TERT Gene Transcription in Hypoxic Breast Cancer Stem Cells. Cell Rep. 2021, 36, 109757. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Cho, K.J.; Yun, S.H.; Jin, B.; Lee, H.Y.; Ro, S.W.; Kim, D.Y.; Ahn, S.H.; Han, K.; Park, J.Y. HKR3 Regulates Cell Cycle through the Inhibition of HTERT in Hepatocellular Carcinoma Cell Lines. J. Cancer 2020, 11, 2442–2452. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Ooi, W.F.; Qamra, A.; Cheung, A.; Ma, D.; Sundaram, G.M.; Xu, C.; Xing, M.; Poon, L.; Wang, J.; et al. HoxC5 and MiR-615-3p Target Newly Evolved Genomic Regions to Repress HTERT and Inhibit Tumorigenesis. Nat. Commun. 2018, 9, 100. [Google Scholar] [CrossRef]
- Sharma, G.G.; Hwang, K.; Pandita, R.K.; Gupta, A.; Dhar, S.; Parenteau, J.; Agarwal, M.; Worman, H.J.; Wellinger, R.J.; Pandita, T.K. Human Heterochromatin Protein 1 Isoforms HP1 Hsα and HP1 Hsβ Interfere with HTERT-Telomere Interactions and Correlate with Changes in Cell Growth and Response to Ionizing Radiation. Mol. Cell. Biol. 2003, 23, 8363–8376. [Google Scholar] [CrossRef]
- Chung, S.S.; Wu, Y.; Okobi, Q.; Adekoya, D.; Atefi, M.; Clarke, O.; Dutta, P.; Vadgama, J.V. Proinflammatory Cytokines IL-6 and TNF-α Increased Telomerase Activity through NF-κ B/STAT1/STAT3 Activation, and Withaferin A Inhibited the Signaling in Colorectal Cancer Cells. Med. Inflamm. 2017, 2017, 5958429. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Mizuguchi, M.; Fujii, M.; Nakamura, M. Krüppel-like Factor 2 Represses Transcription of the Telomerase Catalytic Subunit Human Telomerase Reverse Transcriptase (HTERT) in Human T Cells. J. Biol. Chem. 2015, 290, 8758–8763. [Google Scholar] [CrossRef]
- Wong, C.-W.; Hou, P.-S.; Tseng, S.-F.; Chien, C.-L.; Wu, K.-J.; Chen, H.-F.; Ho, H.-N.; Kyo, S.; Teng, S.-C. Krüppel-Like Transcription Factor 4 Contributes to Maintenance of Telomerase Activity in Stem Cells. Stem Cells 2010, 28, 1510–1517. [Google Scholar] [CrossRef]
- Zhang, C.; Song, C.; Liu, T.; Tang, R.; Chen, M.; Gao, F.; Xiao, B.; Qin, G.; Shi, F.; Li, W.; et al. KMT2A Promotes Melanoma Cell Growth by Targeting HTERT Signaling Pathway. Cell Death Dis. 2017, 8, e2940. [Google Scholar] [CrossRef]
- Briatore, F.; Barrera, G.; Pizzimenti, S.; Toaldo, C.; Della Casa, C.; Laurora, S.; Pettazzoni, P.; Dianzani, M.U.; Ferrero, D. Increase of Telomerase Activity and HTERT Expression in Myelodysplastic Syndromes. Cancer Biol. Ther. 2009, 8, 883–889. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Ge, Y.; Zhao, Q.; Zheng, X.; Zhao, Y. HTERT Promotes Cell Adhesion and Migration Independent of Telomerase Activity. Sci. Rep. 2016, 6, 22886. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Sun, X.; Huang, L.-B.; Liu, X.-J.; Qin, G.; Wang, L.-N.; Zhang, X.-L.; Ke, Z.-Y.; Luo, J.-S.; Liang, C.; et al. Melatonin Inhibits MLL-Rearranged Leukemia via RBFOX3/HTERT and NF-ΚB/COX-2 Signaling Pathways. Cancer Lett. 2019, 443, 167–178. [Google Scholar] [CrossRef]
- Karlsen, T.R.; Olsen, M.B.; Kong, X.Y.; Yang, K.; Quiles-Jiménez, A.; Kroustallaki, P.; Holm, S.; Lines, G.T.; Aukrust, P.; Skarpengland, T.; et al. NEIL3-Deficient Bone Marrow Displays Decreased Hematopoietic Capacity and Reduced Telomere Length. Biochem. Biophys. Rep. 2022, 29, 101211. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, N.; Veeraraghavan, J.; Madhusoodhanan, R.; Herman, T.S.; Natarajan, M. Curcumin Regulates Low-Linear Energy Transfer γ-Radiation-Induced NFκB-Dependent Telomerase Activity in Human Neuroblastoma Cells. Int. J. Radiat. Oncol. 2011, 79, 1206–1215. [Google Scholar] [CrossRef]
- Gizard, F.; Heywood, E.B.; Findeisen, H.M.; Zhao, Y.; Jones, K.L.; Cudejko, C.; Post, G.R.; Staels, B.; Bruemmer, D. Telomerase Activation in Atherosclerosis and Induction of Telomerase Reverse Transcriptase Expression by Inflammatory Stimuli in Macrophages. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Gewin, L. Identification of a Novel Telomerase Repressor That Interacts with the Human Papillomavirus Type-16 E6/E6-AP Complex. Genes Dev. 2004, 18, 2269–2282. [Google Scholar] [CrossRef]
- Saha, D.; Singh, A.; Hussain, T.; Srivastava, V.; Sengupta, S.; Kar, A.; Dhapola, P.; Dhople, V.; Ummanni, R.; Chowdhury, S. Epigenetic Suppression of Human Telomerase (HTERT) Is Mediated by the Metastasis Suppressor NME2 in a G-Quadruplex–Dependent Fashion. J. Biol. Chem. 2017, 292, 15205–15215. [Google Scholar] [CrossRef]
- Sayed, M.E.; Yuan, L.; Robin, J.D.; Tedone, E.; Batten, K.; Dahlson, N.; Wright, W.E.; Shay, J.W.; Ludlow, A.T. NOVA1 Directs PTBP1 to HTERT Pre-MRNA and Promotes Telomerase Activity in Cancer Cells. Oncogene 2019, 38, 2937–2952. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, C.; Lu, Y.; Lin, J.; Tian, Y.; Ma, Y.; Li, J.; Zhang, H.; Yan, W.; Jiao, P.; et al. Noxa and Puma Genes Regulated by HTERT Promoter Can Mitigate Growth and Induce Apoptosis in Hepatocellular Carcinoma Mouse Model. J. Cancer 2022, 13, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xia, Y.; Jin, S.; Xue, C.; Wang, Y.; Hu, R.; Jiang, H. Nrf2 Attenuates Ferroptosis-Mediated IIR-ALI by Modulating TERT and SLC7A11. Cell Death Dis. 2021, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Romanova, L.; Kellner, S.; Katoku-Kikyo, N.; Kikyo, N. Novel Role of Nucleostemin in the Maintenance of Nucleolar Architecture and Integrity of Small Nucleolar Ribonucleoproteins and the Telomerase Complex. J. Biol. Chem. 2009, 284, 26685–26694. [Google Scholar] [CrossRef] [PubMed]
- Beitzinger, M.; Oswald, C.; Beinoraviciute-Kellner, R.; Stiewe, T. Regulation of Telomerase Activity by the P53 Family Member P73. Oncogene 2006, 25, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Bougel, S.; Renaud, S.; Braunschweig, R.; Loukinov, D.; Morse, H.C., III; Bosman, F.T.; Lobanenkov, V.; Benhattar, J. PAX5 Activates the Transcription of the Human Telomerase Reverse Transcriptase Gene in B Cells. J. Pathol. 2010, 220, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Campbell, H.G.; Wiles, A.K.; Eccles, M.R.; Reddel, R.R.; Braithwaite, A.W.; Royds, J.A. PAX8 Regulates Telomerase Reverse Transcriptase and Telomerase RNA Component in Glioma. Cancer Res. 2008, 68, 5724–5732. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hwang, S.S.; Liesa, M.; Gan, B.; Sahin, E.; Jaskelioff, M.; Ding, Z.; Ying, H.; Boutin, A.T.; Zhang, H.; et al. Antitelomerase Therapy Provokes ALT and Mitochondrial Adaptive Mechanisms in Cancer. Cell 2012, 148, 651–663. [Google Scholar] [CrossRef]
- Ho, S.-T.; Jin, R.; Cheung, D.H.-C.; Huang, J.-J.; Shaw, P.-C. The PinX1/NPM Interaction Associates with HTERT in Early-S Phase and Facilitates Telomerase Activation. Cell Biosci. 2019, 9, 47. [Google Scholar] [CrossRef]
- Ohira, T.; Kojima, H.; Kuroda, Y.; Aoki, S.; Inaoka, D.; Osaki, M.; Wanibuchi, H.; Okada, F.; Oshimura, M.; Kugoh, H. PITX1 Protein Interacts with ZCCHC10 to Regulate HTERT MRNA Transcription. PLoS ONE 2019, 14, e0217605. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, W.; Wang, Q.; Wang, J.; Chai, L.; Chen, Y.; Wang, Y.; Liu, J.; Li, M.; Xie, X. PPARγ Activation Inhibits PDGF-Induced Pulmonary Artery Smooth Muscle Cell Proliferation and Migration by Modulating TERT. Biomed. Pharmacother. 2022, 152, 113233. [Google Scholar] [CrossRef]
- Luo, C.; Zhu, X.; Luo, Q.; Bu, F.; Huang, C.; Zhu, J.; Zhao, J.; Zhang, W.; Lin, K.; Hu, C.; et al. RBFOX3 Promotes Gastric Cancer Growth and Progression by Activating HTERT Signaling. Front. Oncol. 2020, 10, 1044. [Google Scholar] [CrossRef]
- Liu, T.; Li, W.; Lu, W.; Chen, M.; Luo, M.; Zhang, C.; Li, Y.; Qin, G.; Shi, D.; Xiao, B.; et al. RBFOX3 Promotes Tumor Growth and Progression via HTERT Signaling and Predicts a Poor Prognosis in Hepatocellular Carcinoma. Theranostics 2017, 7, 3138–3154. [Google Scholar] [CrossRef]
- Zohud, B.A.; Guo, P.; Zohud, B.A.; Li, F.; Hao, J.J.; Shan, X.; Yu, W.; Guo, W.; Qin, Y.; Cai, X. Importin 13 Promotes NSCLC Progression by Mediating RFPL3 Nuclear Translocation and HTERT Expression Upregulation. Cell Death Dis. 2020, 11, 879. [Google Scholar] [CrossRef]
- Liu, Y.-B.; Mei, Y.; Long, J.; Zhang, Y.; Hu, D.-L.; Zhou, H.-H. RIF1 Promotes Human Epithelial Ovarian Cancer Growth and Progression via Activating Human Telomerase Reverse Transcriptase Expression. J. Exp. Clin. Cancer Res. 2018, 37, 182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Veisaga, M.L.; Barbieri, M.A. Role of RIN1 on Telomerase Activity Driven by EGF-Ras Mediated Signaling in Breast Cancer. Exp. Cell Res. 2020, 396, 112318. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Sun, X.; Xiao, J.; Chen, Y.; Chen, X.; Pang, J.; Mi, J.; Tang, Y.; Liu, Q.; Ling, W. Inhibition of S-Adenosylhomocysteine Hydrolase Induces Endothelial Senescence via HTERT Downregulation. Atherosclerosis 2022, 353, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Parulekar, A.; Choksi, A.; Taye, N.; Totakura, K.V.S.; Firmal, P.; Kundu, G.C.; Chattopadhyay, S. SMAR1 Suppresses the Cancer Stem Cell Population via HTERT Repression in Colorectal Cancer Cells. Int. J. Biochem. Cell Biol. 2021, 141, 106085. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Yim, J.; Kim, T.K. Sp1 and Sp3 Recruit Histone Deacetylase to Repress Transcription of Human Telomerase Reverse Transcriptase (HTERT) Promoter in Normal Human Somatic Cells. J. Biol. Chem. 2002, 277, 38230–38238. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczanska, B.; Brankiewicz, W.; Stachowicz-Suhs, M.; Wietrzyk, J.; Matkowski, R.; Ekiert, M.; Szelachowska, J.; Maciejczyk, A.; Szajewski, M.; et al. Relationship between Telomere Length, TERT Genetic Variability and TERT, TP53, SP1, MYC Gene Co-Expression in the Clinicopathological Profile of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 5164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhao, Y.; Wang, S.; Jia, W.; Kang, J.; Zhu, J. Human Telomerase Reverse Transcriptase (HTERT) Transcription Requires Sp1/Sp3 Binding to the Promoter and a Permissive Chromatin Environment. J. Biol. Chem. 2015, 290, 30193–30203. [Google Scholar] [CrossRef]
- Diao, C.; Guo, P.; Yang, W.; Sun, Y.; Liao, Y.; Yan, Y.; Zhao, A.; Cai, X.; Hao, J.; Hu, S.; et al. SPT6 Recruits SND1 to Co-activate Human Telomerase Reverse Transcriptase to Promote Colon Cancer Progression. Mol. Oncol. 2021, 15, 1180–1202. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Aroh, C.; Vadgama, J.V. Constitutive Activation of STAT3 Signaling Regulates HTERT and Promotes Stem Cell-Like Traits in Human Breast Cancer Cells. PLoS ONE 2013, 8, e83971. [Google Scholar] [CrossRef]
- Chang, W.-T.; Lin, Y.-C.; Hong, C.-S.; Huang, P.-S.; Lin, Y.-W.; Chen, Z.-C.; Lin, T.-H.; Chao, T.-H. Effects of STAT3 on Aging-Dependent Neovascularization Impairment Following Limb Ischemia: From Bedside to Bench. Aging 2022, 14, 4897–4913. [Google Scholar] [CrossRef]
- Yamada, O.; Ozaki, K.; Akiyama, M.; Kawauchi, K. JAK–STAT and JAK–PI3K–MTORC1 Pathways Regulate Telomerase Transcriptionally and Posttranslationally in ATL Cells. Mol. Cancer Ther. 2012, 11, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Endoh, T.; Tsuji, N.; Asanuma, K.; Yagihashi, A.; Watanabe, N. Survivin Enhances Telomerase Activity via Up-Regulation of Specificity Protein 1- and c-Myc-Mediated Human Telomerase Reverse Transcriptase Gene Transcription. Exp. Cell Res. 2005, 305, 300–311. [Google Scholar] [CrossRef]
- Miao, B.; Zhang, C.; Stroh, N.; Brenner, L.; Hufnagel, K.; Hoheisel, J.D.; Bandapalli, O.R. Transcription Factor TFE3 Enhances Cell Cycle and Cancer Progression by Binding to the HTERT Promoter. Cancer Commun. 2021, 41, 1423–1426. [Google Scholar] [CrossRef]
- Burgess, J.K.; Ketheson, A.; Faiz, A.; Limbert Rempel, K.A.; Oliver, B.G.; Ward, J.P.T.; Halayko, A.J. Phenotype and Functional Features of Human Telomerase Reverse Transcriptase Immortalized Human Airway Smooth Muscle Cells from Asthmatic and Non-Asthmatic Donors. Sci. Rep. 2018, 8, 805. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, N.; Ma, C.; Xu, W.; Jin, G.; Zheng, Y.; Zhang, L.; Liu, B.; Gao, C.; Liu, S. The Overexpression of Tipe2 in CRC Cells Suppresses Survival While Endogenous Tipe2 Accelerates AOM/DSS Induced-Tumor Initiation. Cell Death Dis. 2021, 12, 1001. [Google Scholar] [CrossRef]
- Deng, T.; Huang, Y.; Weng, K.; Lin, S.; Li, Y.; Shi, G.; Chen, Y.; Huang, J.; Liu, D.; Ma, W.; et al. TOE1 Acts as a 3′ Exonuclease for Telomerase RNA and Regulates Telomere Maintenance. Nucleic Acids Res. 2019, 47, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Olbertova, H.; Plevova, K.; Pavlova, S.; Malcikova, J.; Kotaskova, J.; Stranska, K.; Spunarova, M.; Trbusek, M.; Navrkalova, V.; Dvorackova, B.; et al. Evolution of TP53 Abnormalities during CLL Disease Course Is Associated with Telomere Length Changes. BMC Cancer 2022, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Rinaldetti, S.; Cheikh, B.B.; Zhou, Q.; Hass, E.P.; Jones, R.T.; Joshi, M.; LaBarbera, D.V.; Knott, S.R.V.; Cech, T.R.; et al. TRIM28 Is a Transcriptional Activator of the Mutant TERT Promoter in Human Bladder Cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2102423118. [Google Scholar] [CrossRef]
- Che, Y.; Li, Y.; Zheng, F.; Zou, K.; Li, Z.; Chen, M.; Hu, S.; Tian, C.; Yu, W.; Guo, W.; et al. TRIP4 Promotes Tumor Growth and Metastasis and Regulates Radiosensitivity of Cervical Cancer by Activating MAPK, PI3K/AKT, and HTERT Signaling. Cancer Lett. 2019, 452, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Goueli, B.S.; Janknecht, R. Regulation of Telomerase Reverse Transcriptase Gene Activity by Upstream Stimulatory Factor. Oncogene 2003, 22, 8042–8047. [Google Scholar] [CrossRef] [PubMed]
- Yago, M.; Ohki, R.; Hatakeyama, S.; Fujita, T.; Ishikawa, F. Variant Forms of Upstream Stimulatory Factors (USFs) Control the Promoter Activity of HTERT, the Human Gene Encoding the Catalytic Subunit of Telomerase. FEBS Lett. 2002, 520, 40–46. [Google Scholar] [CrossRef]
- Chang, J.T.-C.; Yang, H.-T.; Wang, T.-C.V.; Cheng, A.-J. Upstream Stimulatory Factor (USF) as a Transcriptional Suppressor of Human Telomerase Reverse Transcriptase (HTERT) in Oral Cancer Cells. Mol. Carcinog. 2005, 44, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Shen, X.; Yang, W.; Zhang, Y.; Liu, C.; Huang, T. ZEB1 Stimulates Breast Cancer Growth by Up-Regulating HTERT Expression. Biochem. Biophys. Res. Commun. 2018, 495, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Asfour, I.A.; Fayek, M.H.; El-kourashy, S.A.-E.A.; Youssef, S.R.; El-Gohary, G.M.T.; Mohamed, O.F. Correlation of Telomerase Activity to Apoptosis and Survival in Adult Acute Lymphoblastic Leukemia: An Egyptian Single-Center Study. Ann. Hematol. 2008, 87, 213–221. [Google Scholar] [CrossRef]
- Rafat, A.; Dizaji Asl, K.; Mazloumi, Z.; Movassaghpour, A.A.; Farahzadi, R.; Nejati, B.; Nozad Charoudeh, H. Telomerase-based Therapies in Haematological Malignancies. Cell Biochem. Funct. 2022, 40, 127–140. [Google Scholar] [CrossRef]
- Porika, M.; Tippani, R.; Saretzki, G.C. CRISPR/Cas: A New Tool in the Research of Telomeres and Telomerase as Well as a Novel Form of Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 3002. [Google Scholar] [CrossRef]
- Yik, M.Y.; Azlan, A.; Rajasegaran, Y.; Rosli, A.; Yusoff, N.M.; Moses, E.J. Mechanism of Human Telomerase Reverse Transcriptase (HTERT) Regulation and Clinical Impacts in Leukemia. Genes 2021, 12, 1188. [Google Scholar] [CrossRef]
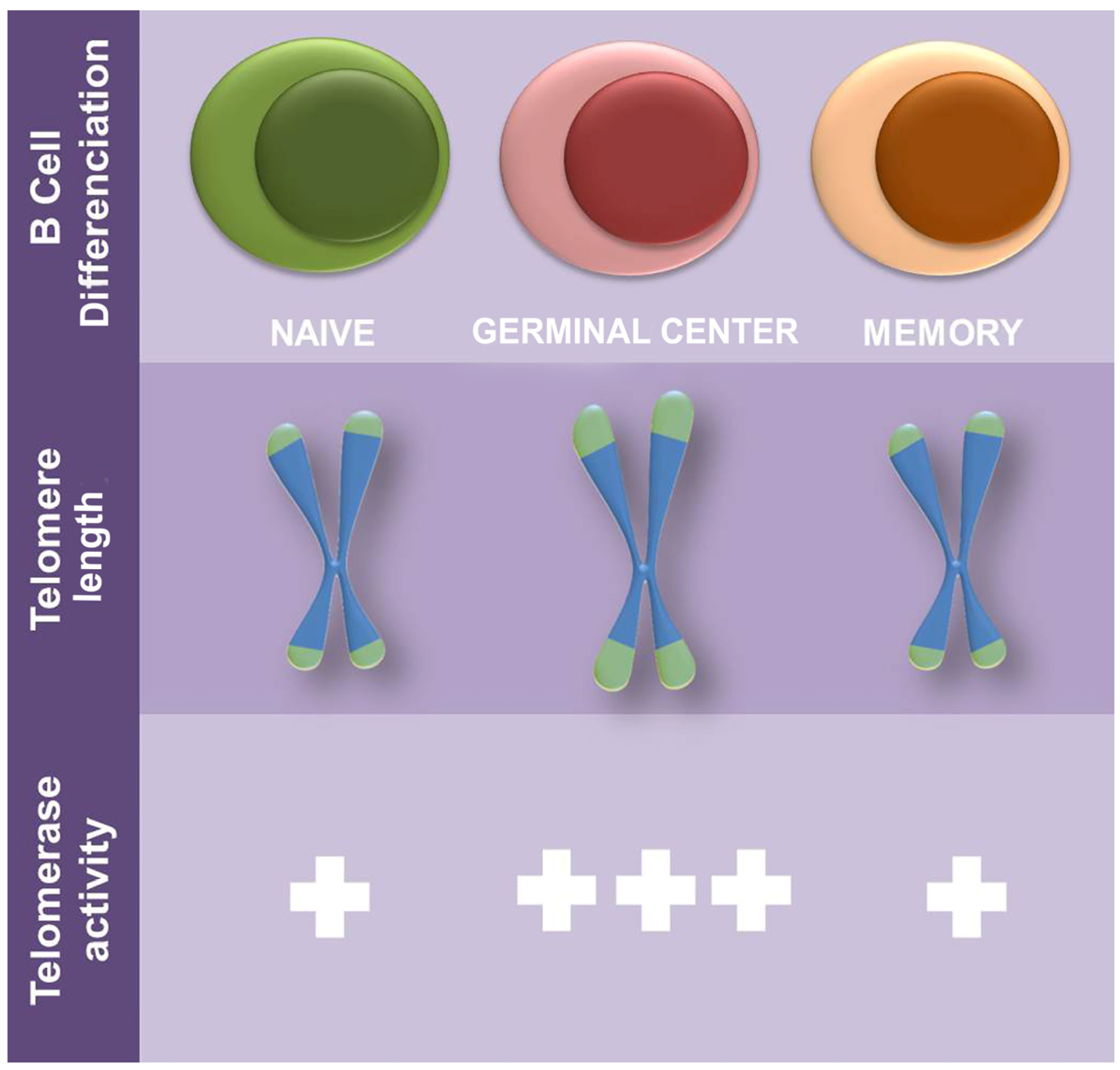
- Weng, N.-P.; Granger, L.; Hodes, R.J. Telomere Lengthening and Telomerase Activation during Human B Cell Differentiation. Proc. Natl. Acad. Sci. USA 1997, 94, 10827–10832. [Google Scholar] [CrossRef]
- Son, N.H.; Joyce, B.; Hieatt, A.; Chrest, F.J.; Yanovski, J.; Weng, N. Stable Telomere Length and Telomerase Expression from Naive to Memory B-Lymphocyte Differentiation. Mech. Ageing Dev. 2003, 124, 427–432. [Google Scholar] [CrossRef]
- Igarashi, H.; Sakaguchi, N. Telomerase Activity Is Induced in Human Peripheral B Lymphocytes by the Stimulation to Antigen Receptor. Blood 1997, 89, 1299–1307. [Google Scholar] [CrossRef]
- Hu, B.T.; Insel, R.A. Up-Regulation of Telomerase in Human B Lymphocytes Occurs Independently of Cellular Proliferation and with Expression of the Telomerase Catalytic Subunit. Eur. J. Immunol. 1999, 29, 3745–3753. [Google Scholar] [CrossRef]
- Weng, N. Regulation of Telomerase Expression in Human Lymphocytes. Springer Semin. Immunopathol. 2002, 24, 23–33. [Google Scholar] [CrossRef]
- Bienz, M.; Ramdani, S.; Knecht, H. Molecular Pathogenesis of Hodgkin Lymphoma: Past, Present, Future. Int. J. Mol. Sci. 2020, 21, 6623. [Google Scholar] [CrossRef]
- Ackermann, S.; Fischer, M. Telomere Maintenance in Pediatric Cancer. Int. J. Mol. Sci. 2019, 20, 5836. [Google Scholar] [CrossRef]
- Capraro, V.; Zane, L.; Poncet, D.; Perol, D.; Galia, P.; Preudhomme, C.; Bonnefoy-Berard, N.; Gilson, E.; Thomas, X.; El-Hamri, M. Telomere Deregulations Possess Cytogenetic, Phenotype, and Prognostic Specificities in Acute Leukemias. Exp. Hematol. 2011, 39, 195–202.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, M.; Sun, X.; Sun, J. Telomerase Activity and Telomere Length in Acute Leukemia: Correlations with Disease Progression, Subtypes and Overall Survival. Int. J. Lab. Hematol. 2010, 32, 230–238. [Google Scholar] [CrossRef]
- Eskandari, E.; Hashemi, M.; Naderi, M.; Bahari, G.; Safdari, V.; Taheri, M. Leukocyte Telomere Length Shortening, HTERT Genetic Polymorphisms and Risk of Childhood Acute Lymphoblastic Leukemia. Asian Pac. J. Cancer Prev. 2018, 19, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Chien, W.W.; Catallo, R.; Chebel, A.; Baranger, L.; Thomas, X.; Béné, M.-C.; Gerland, L.M.; Schmidt, A.; Beldjord, K.; Klein, N.; et al. The P16INK4A/PRb Pathway and Telomerase Activity Define a Subgroup of Ph+ Adult Acute Lymphoblastic Leukemia Associated with Inferior Outcome. Leuk. Res. 2015, 39, 453–461. [Google Scholar] [CrossRef]
- Chai, J.H.; Zhang, Y.; Tan, W.H.; Chng, W.J.; Li, B.; Wang, X. Regulation of HTERT by BCR-ABL at Multiple Levels in K562 Cells. BMC Cancer 2011, 11, 512. [Google Scholar] [CrossRef]
- Cogulu, O.; Kosova, B.; Gunduz, C.; Karaca, E.; Aksoylar, S.; Erbay, A.; Karapinar, D.; Vergin, C.; Vural, F.; Tombuloglu, M.; et al. The Evaluation of HTERT MRNA Expression in Acute Leukemia Children and 2 Years Follow-up of 40 Cases. Int. J. Hematol. 2008, 87, 276–283. [Google Scholar] [CrossRef]
- Borssén, M.; Cullman, I.; Norén-Nyström, U.; Sundström, C.; Porwit, A.; Forestier, E.; Roos, G. HTERT Promoter Methylation and Telomere Length in Childhood Acute Lymphoblastic Leukemia—Associations with Immunophenotype and Cytogenetic Subgroup. Exp. Hematol. 2011, 39, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Zobeck, M.; Bernhardt, M.B.; Kamdar, K.Y.; Rabin, K.R.; Lupo, P.J.; Scheurer, M.E. Novel and Replicated Clinical and Genetic Risk Factors for Toxicity from High-dose Methotrexate in Pediatric Acute Lymphoblastic Leukemia. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2023, 00, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Tsaur, G.; Permikin, Z.; Henze, G.; Verzhbitskaya, T.; Plekhanova, O.; Nokhrina, E.; Valochnik, A.; Sibiryakov, P.; Zerkalenkova, E.; et al. Genetic Characteristics and Treatment Outcome in Infants with KMT2A Germline B-cell Precursor Acute Lymphoblastic Leukemia: Results of MLL-Baby Protocol. Pediatr. Blood Cancer 2023, 70, e30204. [Google Scholar] [CrossRef]
- Safavi, S.; Olsson, L.; Biloglav, A.; Veerla, S.; Blendberg, M.; Tayebwa, J.; Behrendtz, M.; Castor, A.; Hansson, M.; Johansson, B.; et al. Genetic and Epigenetic Characterization of Hypodiploid Acute Lymphoblastic Leukemia. Oncotarget 2015, 6, 42793–42802. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Chen, X.; Liu, S.; Wen, R.; Yuan, X.; Xu, D.; Liu, G.; Wen, F. Methylation of CDKN2B CpG Islands Is Associated with Upregulated Telomerase Activity in Children with Acute Lymphoblastic Leukemia. Oncol. Lett. 2017, 13, 2115–2120. [Google Scholar] [CrossRef]
- Assi, R.; Mahfouz, R.; Owen, R.; Gunthorpe, M.; Chehab, F.F.; Bazarbachi, A. PAX5, NOTCH3, CBFB, and ACD Drive an Activated RAS Pathway and Monosomy 7 to B-ALL and AML in Donor Cell Leukemia. Bone Marrow Transplant. 2019, 54, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Spinella, J.-F.; Cassart, P.; Garnier, N.; Rousseau, P.; Drullion, C.; Richer, C.; Ouimet, M.; Saillour, V.; Healy, J.; Autexier, C.; et al. A Novel Somatic Mutation in ACD Induces Telomere Lengthening and Apoptosis Resistance in Leukemia Cells. BMC Cancer 2015, 15, 621. [Google Scholar] [CrossRef]
- Zia, S.; Khan, N.; Tehreem, K.; Rehman, N.; Sami, R.; Baty, R.S.; Tayeb, F.J.; Almashjary, M.N.; Alsubhi, N.H.; Alrefaei, G.I.; et al. Transcriptomic Analysis of Conserved Telomere Maintenance Component 1 (CTC1) and Its Association with Leukemia. J. Clin. Med. 2022, 11, 5780. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhang, L.; Luo, D.; Tong, N.; Wang, M.; Fang, Y.; Li, J.; Zhang, Z. A Common Variant near TERC and Telomere Length Are Associated with Susceptibility to Childhood Acute Lymphoblastic Leukemia in Chinese. Leuk. Lymphoma 2012, 53, 1688–1692. [Google Scholar] [CrossRef]
- Reddy, A.; Espinoza, I.; Cole, D.; Schallheim, J.; Poosarla, T.; Bhanat, E.; Zhou, Y.; Zabaleta, J.; Megason, G.; Gomez, C.R. Genetic Mutations in B-Acute Lymphoblastic Leukemia Among African American and European American Children. Clin. Lymphoma Myeloma Leuk. 2018, 18, e501–e508. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, M.I.; Zatsepin, T.S.; Azhibek, D.M.; Shubernetskaya, O.S.; Shpanchenko, O.V.; Dontsova, O.A. Oligonucleotide Inhibitors of Telomerase: Prospects for Anticancer Therapy and Diagnostics. Biochemistry 2015, 80, 251–259. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, S. Evaluation of the Efficacy of MST-312, as a Telomerase Inhibitor, in the Treatment of Patients with Multiple Myeloma after Stem Cell Transplantation. Cell. Mol. Biol. 2022, 67, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Andrade da Mota, T.H.; Reis Guimarães, A.F.; Silva de Carvalho, A.É.; Saldanha-de Araujo, F.; Pinto de Faria Lopes, G.; Pittella-Silva, F.; do Amaral Rabello, D.; Madureira de Oliveira, D. Effects of in Vitro Short- and Long-Term Treatment with Telomerase Inhibitor in U-251 Glioma Cells. Tumor Biol. 2021, 43, 327–340. [Google Scholar] [CrossRef]
- Gurung, R.L.; Lim, S.N.; Low, G.K.M.; Hande, M.P. MST-312 Alters Telomere Dynamics, Gene Expression Profiles and Growth in Human Breast Cancer Cells. Lifestyle Genom. 2014, 7, 283–298. [Google Scholar] [CrossRef]
- Sajed, H.; Sahebkar, A.; Iranshahi, M. Zataria multiflora Boiss. (Shirazi Thyme)—An Ancient Condiment with Modern Pharmaceutical Uses. J. Ethnopharmacol. 2013, 145, 686–698. [Google Scholar] [CrossRef]
- Lashkari, M.; Fatemi, A.; Valandani, H.M.; Khalilabadi, R.M. Promising Anti-Leukemic Effect of Zataria multiflora Extract in Combination with Doxorubicin to Combat Acute Lymphoblastic Leukemia Cells (Nalm-6) (In Vitro and In Silico). Sci. Rep. 2022, 12, 12657. [Google Scholar] [CrossRef]
- Habibi, E.; Shokrzadeh, M.; Ahmadi, A.; Chabra, A.; Naghshvar, F.; Haghi-Aminjan, H.; Salehi, F. Pulmonoprotective Action of Zataria multiflora Ethanolic Extract on Cyclophosphamide-Induced Oxidative Lung Toxicity in Mice. Chin. J. Integr. Med. 2020, 26, 754–761. [Google Scholar] [CrossRef]
- Altamura, G.; degli Uberti, B.; Galiero, G.; De Luca, G.; Power, K.; Licenziato, L.; Maiolino, P.; Borzacchiello, G. The Small Molecule BIBR1532 Exerts Potential Anti-Cancer Activities in Preclinical Models of Feline Oral Squamous Cell Carcinoma Through Inhibition of Telomerase Activity and Down-Regulation of TERT. Front. Vet. Sci. 2021, 7, 620776. [Google Scholar] [CrossRef] [PubMed]
- El-Daly, H.; Kull, M.; Zimmermann, S.; Pantic, M.; Waller, C.F.; Martens, U.M. Selective Cytotoxicity and Telomere Damage in Leukemia Cells Using the Telomerase Inhibitor BIBR1532. Blood 2005, 105, 1742–1749. [Google Scholar] [CrossRef]
- Pourbagheri-Sigaroodi, A.; Bashash, D.; Safaroghli-Azar, A.; Farshi-Paraasghari, M.; Momeny, M.; Mansoor, F.N.; Ghaffari, S.H. Contributory Role of MicroRNAs in Anti-Cancer Effects of Small Molecule Inhibitor of Telomerase (BIBR1532) on Acute Promyelocytic Leukemia Cell Line. Eur. J. Pharmacol. 2019, 846, 49–62. [Google Scholar] [CrossRef]
- Bashash, D.; Ghaffari, S.H.; Zaker, F.; Hezave, K.; Kazerani, M.; Ghavamzadeh, A.; Alimoghaddam, K.; Mosavi, S.A.; Gharehbaghian, A.; Vossough, P. Direct Short-Term Cytotoxic Effects of BIBR 1532 on Acute Promyelocytic Leukemia Cells Through Induction of P21 Coupled with Downregulation of c-Myc and HTERT Transcription. Cancer Invest. 2012, 30, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Barwe, S.P.; Huang, F.; Kolb, E.A.; Gopalakrishnapillai, A. Imetelstat Induces Leukemia Stem Cell Death in Pediatric Acute Myeloid Leukemia Patient-Derived Xenografts. J. Clin. Med. 2022, 11, 1923. [Google Scholar] [CrossRef] [PubMed]
- Marian, C.O.; Cho, S.K.; Mcellin, B.M.; Maher, E.A.; Hatanpaa, K.J.; Madden, C.J.; Mickey, B.E.; Wright, W.E.; Shay, J.W.; Bachoo, R.M. The Telomerase Antagonist, Imetelstat, Efficiently Targets Glioblastoma Tumor-Initiating Cells Leading to Decreased Proliferation and Tumor Growth. Clin. Cancer Res. 2010, 16, 154–163. [Google Scholar] [CrossRef]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of Doxorubicin on Apoptosis and Oxidative Stress in Breast Cancer Cell Lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-Induced Cardiotoxicity: An Update on the Molecular Mechanism and Novel Therapeutic Strategies for Effective Management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Ghasemimehr, N.; Farsinejad, A.; Mirzaee Khalilabadi, R.; Yazdani, Z.; Fatemi, A. The Telomerase Inhibitor MST-312 Synergistically Enhances the Apoptotic Effect of Doxorubicin in Pre-B Acute Lymphoblastic Leukemia Cells. Biomed. Pharmacother. 2018, 106, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fidan, K.; Um, J.-Y.; Ahn, K.S. Telomerase: Key Regulator of Inflammation and Cancer. Pharmacol. Res. 2020, 155, 104726. [Google Scholar] [CrossRef] [PubMed]
- Pascolo, E.; Wenz, C.; Lingner, J.; Hauel, N.; Priepke, H.; Kauffmann, I.; Garin-Chesa, P.; Rettig, W.J.; Damm, K.; Schnapp, A. Mechanism of Human Telomerase Inhibition by BIBR1532, a Synthetic, Non-Nucleosidic Drug Candidate. J. Biol. Chem. 2002, 277, 15566–15572. [Google Scholar] [CrossRef]
- Bashash, D.; Zareii, M.; Safaroghli-Azar, A.; Omrani, M.D.; Ghaffari, S.H. Inhibition of Telomerase Using BIBR1532 Enhances Doxorubicin-Induced Apoptosis in Pre-B Acute Lymphoblastic Leukemia Cells. Hematology 2017, 22, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Mengual Gomez, D.L.; Armando, R.G.; Cerrudo, C.S.; Ghiringhelli, P.D.; Gomez, D.E. Telomerase as a Cancer Target. Development of New Molecules. Curr. Top. Med. Chem. 2016, 16, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Relitti, N.; Saraswati, A.P.; Federico, S.; Khan, T.; Brindisi, M.; Zisterer, D.; Brogi, S.; Gemma, S.; Butini, S.; Campiani, G. Telomerase-Based Cancer Therapeutics: A Review on Their Clinical Trials. Curr. Top. Med. Chem. 2020, 20, 433–457. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Kaneko, S. Telomerase-Targeted Cancer Immunotherapy. Int. J. Mol. Sci. 2019, 20, 1823. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Chemical Structures | Type of Therapy | Main Findings | References |
|---|---|---|---|---|
| N,N′-1,3-Phenylenebis-[2,3-dihydroxy-benzamide] (MST-312) |  | Combined with doxorubicin | MST-312 inhibits the progress of multiple myeloma by inhibiting the telomerase activity of this cells. Monotherapy long-term exposure to the MST-312 in U251 cells resulted in the induction of cell adaptations with possible negative clinical implications. MST-312 alters telomere dynamics, gene expression profiles and growth in human breast cancer cells | [194,195,196] |
| Zataria multiflora extract (ZME) | Chemical structures of main volatile and non-volatile constituents are in Sajed, Sahebkar and Iranshahi works [197] | Combined with doxorubicin | Pulmonoprotective action of Zataria multiflora ethanolic extract on cyclophosphamide-induced oxidative lung toxicity in mice Anti-leukemic effect of Zataria multiflora extract in combination with doxorubicin to combat acute lymphoblastic leukemia cells. | [198,199] |
| 2-[(E)-3-naphtalen-2-yl-but-2-enoylamino]-benzoic acid (BIBR1532) | 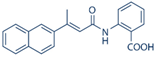 | Monotherapy and combined with doxorubicin | BIBR1532 exerts a series of anti-cancer activities linked to the inhibition of the canonical telomerase pathway and the TERT extra-telomeric functions in feline oral squamous cell carcinoma. BIBR1532 exhibits a selective cytotoxicity against primary leukemia cells from acute myeloid leukemia and chronic lymphocytic leukemia patients. Telomerase inhibition by BIBR1532 causes rapid cell death in pre-B-acute lymphoblastic leukemia cells BIBR1532 exerted potent cytotoxic effects on a panel of human cancer cells in a dose-dependent manner in leukemic cells which were more sensitive to the inhibitor BIBR 1532, exerts a direct short-term growth suppressive effect in a concentration-dependent manner possibly through the downregulation of c-Myc and hTERT expression | [46,200,201,202,203] |
| lipid-conjugated N30-P50 thiophosphoramidate GRN163L (Imetelstat) | 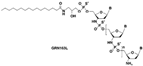 | Monotherapy | Imetelstat induces leukemia stem cell death in pediatric acute myeloid leukemia. The telomerase antagonist imetelstat efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and tumor growth The inhibition of telomerase with imetelstat ex vivo led to significant dose-dependent apoptosis of B-ALL cells. Thus, imeteostat can be usefull in the standard treatment of B-ALL | [45,204,205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Mota, T.H.A.; Camargo, R.; Biojone, E.R.; Guimarães, A.F.R.; Pittella-Silva, F.; de Oliveira, D.M. The Relevance of Telomerase and Telomere-Associated Proteins in B-Acute Lymphoblastic Leukemia. Genes 2023, 14, 691. https://doi.org/10.3390/genes14030691
da Mota THA, Camargo R, Biojone ER, Guimarães AFR, Pittella-Silva F, de Oliveira DM. The Relevance of Telomerase and Telomere-Associated Proteins in B-Acute Lymphoblastic Leukemia. Genes. 2023; 14(3):691. https://doi.org/10.3390/genes14030691
Chicago/Turabian Styleda Mota, Tales Henrique Andrade, Ricardo Camargo, Estefânia Rodrigues Biojone, Ana Flávia Reis Guimarães, Fabio Pittella-Silva, and Diêgo Madureira de Oliveira. 2023. "The Relevance of Telomerase and Telomere-Associated Proteins in B-Acute Lymphoblastic Leukemia" Genes 14, no. 3: 691. https://doi.org/10.3390/genes14030691
APA Styleda Mota, T. H. A., Camargo, R., Biojone, E. R., Guimarães, A. F. R., Pittella-Silva, F., & de Oliveira, D. M. (2023). The Relevance of Telomerase and Telomere-Associated Proteins in B-Acute Lymphoblastic Leukemia. Genes, 14(3), 691. https://doi.org/10.3390/genes14030691






