Interplay between microRNAs, Serum Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9), and Lipid Parameters in Patients with Very High Lipoprotein(a) Treated with PCSK9 Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Biochemical Analysis
2.3. Measurement of Serum PCSK9 Levels
2.4. Extraction of Circulating RNAs from Plasma Samples and Reverse Transcription
2.5. Quantification of miRNA Expression by Quantitative PCR
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Subjects
3.2. Characteristics of Patients after Treatment with PCSK9 Inhibitors
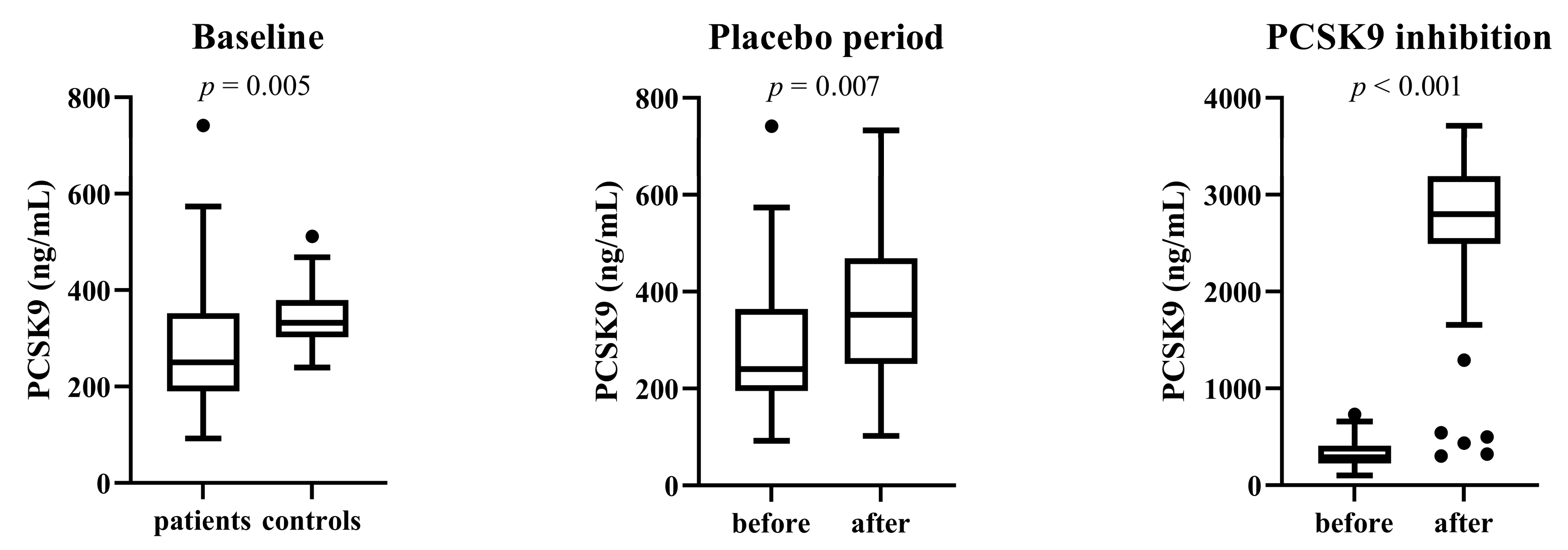
3.3. Total Serum PCSK9 Level
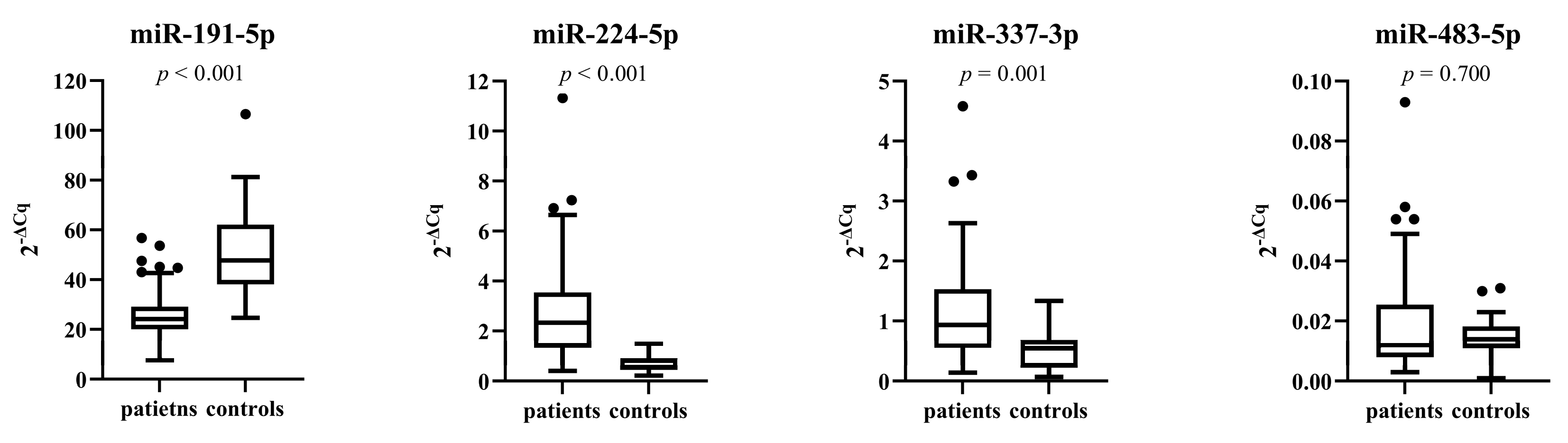
3.4. miRNA Expressions in Patients and Control Subjects before Treatment
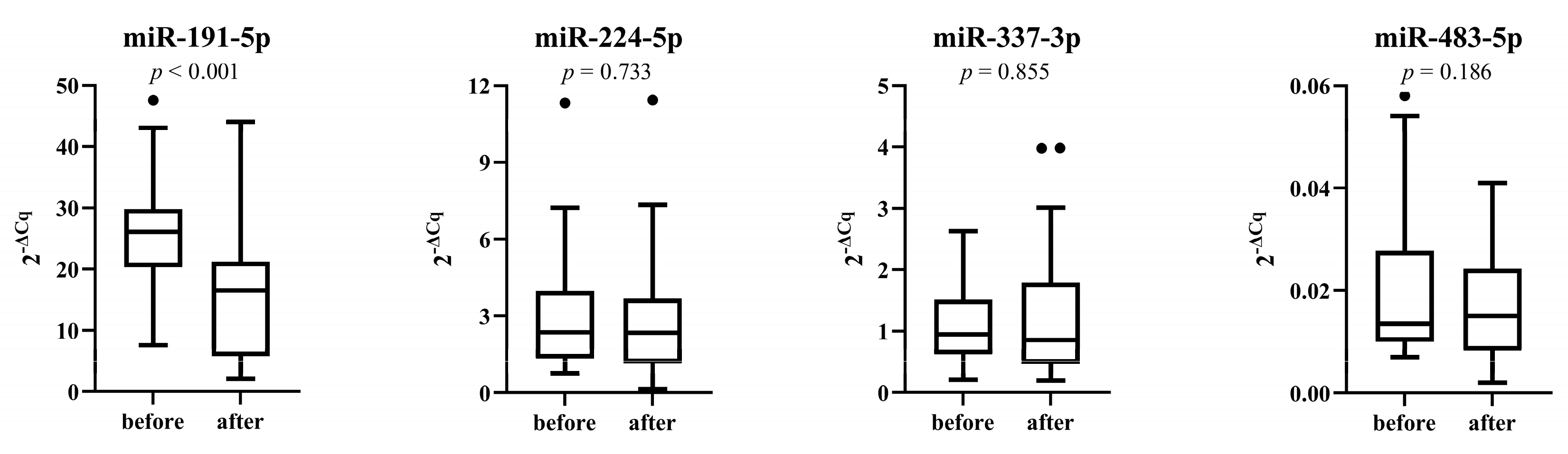
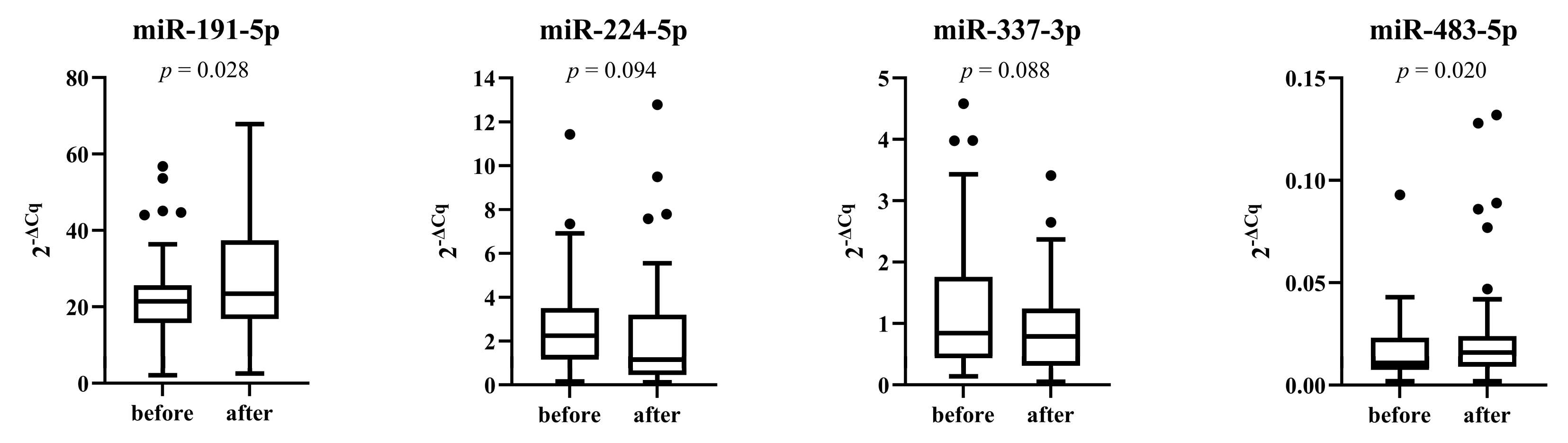
3.5. miRNAs Expression after Placebo and Treatment Period
3.6. Prediction of Serum PCSK9 Level by Circulating miRNAs
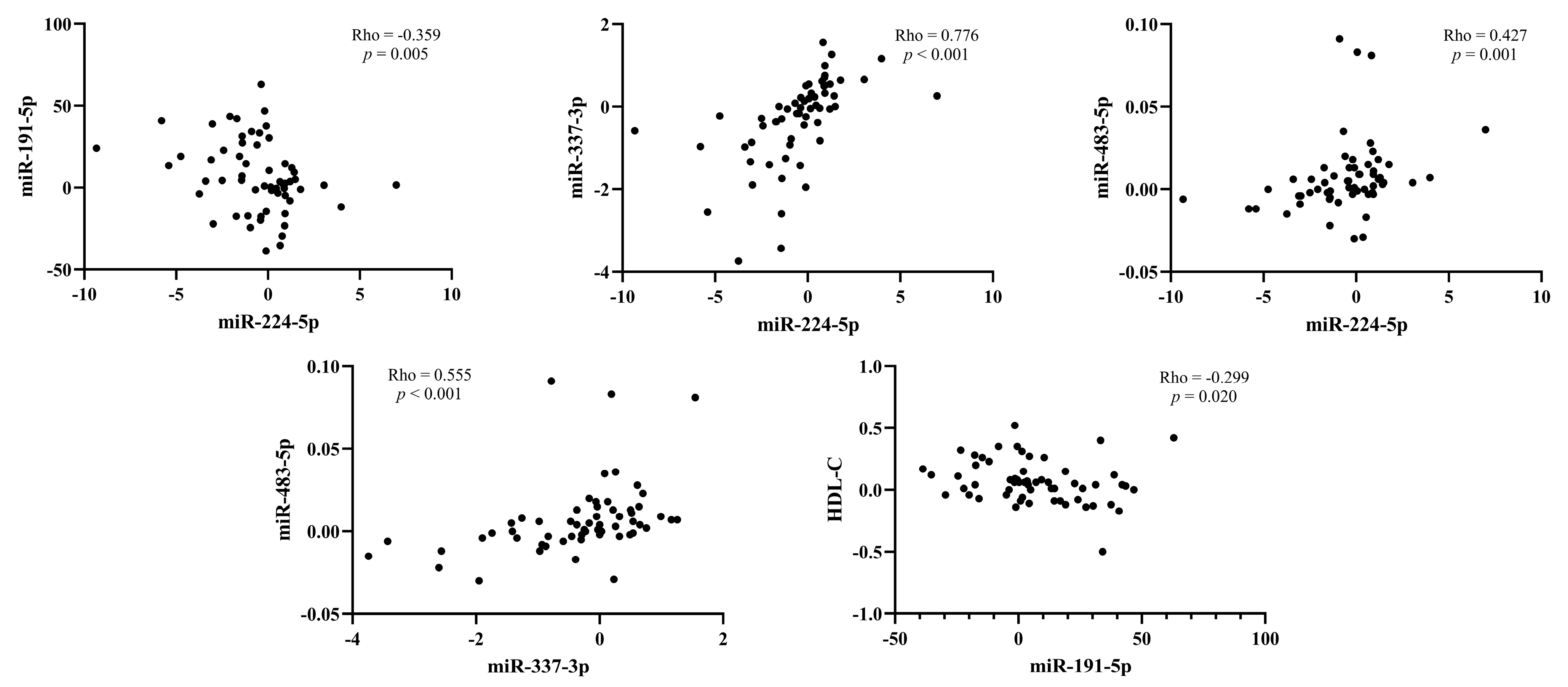
3.7. Correlation between the Change of miRNAs Expression and Lipids after Treatment with PCSK9 Inhibitors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Peden, J.F.; Hopewell, J.C.; Kyriakou, T.; Goel, A.; Heath, S.C.; Parish, S.; Barlera, S.; Franzosi, M.G.; Rust, S.; et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 2009, 361, 2518–2528. [Google Scholar] [CrossRef]
- Brunner, C.; Kraft, H.G.; Utermann, G.; Müller, H.J. Cys4057 of apolipoprotein(a) is essential for lipoprotein(a) assembly. Proc. Natl. Acad. Sci. USA 1993, 90, 11643–11647. [Google Scholar] [CrossRef]
- Utermann, G.; Weber, W. Protein composition of Lp(a) lipoprotein from human plasma. FEBS Lett. 1983, 154, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Boerwinkle, E.; Leffert, C.C.; Lin, J.; Lackner, C.; Chiesa, G.; Hobbs, H.H. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J. Clin. Investig. 1992, 90, 52–60. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabès, J.-P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Lo Surdo, P.; Bottomley, M.J.; Calzetta, A.; Settembre, E.C.; Cirillo, A.; Pandit, S.; Ni, Y.G.; Hubbard, B.; Sitlani, A.; Carfi, A. Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep. 2011, 12, 1300–1305. [Google Scholar] [CrossRef]
- Seidah, N.G.; Benjannet, S.; Wickham, L.; Marcinkiewicz, J.; Jasmin, S.B.; Stifani, S.; Basak, A.; Prat, A.; Chrétien, M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): Liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 928–933. [Google Scholar] [CrossRef]
- Benjannet, S.; Rhainds, D.; Essalmani, R.; Mayne, J.; Wickham, L.; Jin, W.; Asselin, M.C.; Hamelin, J.; Varret, M.; Allard, D.; et al. NARC-1/PCSK9 and its natural mutants: Zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 2004, 279, 48865–48875. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Fisher, E.A.; Breslow, J.L. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc. Natl. Acad. Sci. USA 2005, 102, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, J.; Chen, H.; Zhang, T.; He, D.; Luo, Q.; Chi, J.; Hong, Z.; Liao, Y.; Zhang, S.; et al. PCSK9 inhibition: From current advances to evolving future. Cells 2022, 11, 2972. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Boerwinkle, E.; Mosley Jr, T.H.; Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Karatasakis, A.; Danek, B.A.; Karacsonyi, J.; Rangan, B.V.; Roesle, M.K.; Knickelbine, T.; Miedema, M.D.; Khalili, H.; Ahmad, Z.; Abdullah, S.; et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: A meta-analysis of 35 randomized controlled trials. J. Am. Heart Assoc. 2017, 6, e006910. [Google Scholar] [CrossRef]
- Gaudet, D.; Watts, G.F.; Robinson, J.G.; Minini, P.; Sasiela, W.J.; Edelberg, J.; Louie, M.J.; Raal, F.J. Effect of alirocumab on lipoprotein(a) over >/=1.5 years (from the phase 3 ODYSSEY program). Am. J. Cardiol. 2017, 119, 40–46. [Google Scholar] [CrossRef]
- Desai, N.R.; Kohli, P.; Giugliano, R.P.; O’Donoghue, M.L.; Somaratne, R.; Zhou, J.; Hoffman, E.B.; Huang, F.; Rogers, W.J.; Wasserman, S.M.; et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy. Circulation 2013, 128, 962–969. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as potential biomarkers in atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5547. [Google Scholar] [CrossRef]
- Fernández-Tussy, P.; Ruz-Maldonado, I.; Fernández-Hernando, C. MicroRNAs and circular RNAs in lipoprotein metabolism. Curr. Atheroscler. Rep. 2021, 23, 33. [Google Scholar] [CrossRef]
- Lu, Y.; Thavarajah, T.; Gu, W.; Cai, J.; Xu, Q. Impact of miRNA in atherosclerosis. Arterioscler. Thromb Vasc. Biol. 2018, 38, e159–e170. [Google Scholar] [CrossRef]
- Citrin, K.M.; Fernandez-Hernando, C.; Suarez, Y. MicroRNA regulation of cholesterol metabolism. Ann. N. Y. Acad. Sci. 2021, 1495, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Rehberger Likozar, A.; Blinc, A.; Trebušak Podkrajšek, K.; Šebeštjen, M. LPA genotypes and haplotypes are associated with lipoprotein(a) levels but not arterial wall properties in stable post-coronary event patients with very high lipoprotein(a) levels. J. Cardiovasc. Dev. Dis. 2021, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.; Levy, R.; Fredrickson, D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Zhelankin, A.V.; Stonogina, D.A.; Vasiliev, S.V.; Babalyan, K.A.; Sharova, E.I.; Doludin, Y.V.; Shchekochikhin, D.Y.; Generozov, E.V.; Akselrod, A.S. Circulating extracellular miRNA analysis in patients with stable CAD and acute coronary syndromes. Biomolecules 2021, 11, 962. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, W.; Peng, L.; Tang, J.; Yuan, Z. Identification and validation of microRNAs as endogenous controls for quantitative polymerase chain reaction in plasma for stable coronary artery disease. Cardiol. J. 2016, 23, 694–703. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wagschal, A.; Najafi-Shoushtari, S.H.; Wang, L.; Goedeke, L.; Sinha, S.; deLemos, A.S.; Black, J.C.; Ramirez, C.M.; Li, Y.; Tewhey, R.; et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat. Med. 2015, 21, 1290–1297. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Sabouri-Rad, S.; Gotto, A.M.; Pirro, M.; Banach, M.; Awan, Z.; Barreto, G.E.; Sahebkar, A. PCSK9 and inflammation: A review of experimental and clinical evidence. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 237–245. [Google Scholar] [CrossRef]
- Panahi, Y.; Ghahrodi, M.S.; Jamshir, M.; Safarpour, M.A.; Bianconi, V.; Pirro, M.; Farahani, M.M.; Sahebkar, A. PCSK9 and atherosclerosis burden in the coronary arteries of patients undergoing coronary angiography. Clin. Biochem. 2019, 74, 12–18. [Google Scholar] [CrossRef]
- Dubuc, G.; Chamberland, A.; Wassef, H.; Davignon, J.; Seidah, N.G.; Bernier, L.; Prat, A. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler. Thromb Vasc. Biol. 2004, 24, 1454–1459. [Google Scholar] [CrossRef]
- Afanasieva, O.; Ezhov, M.V.; Klesareva, E.; Razova, O.; Chubykina, U.; Egiazaryan, M.; Sherstyuk, E.; Afanasieva, M.; Utkina, E.; Pokrovsky, S. Effect of evolocumab on lipoprotein(a) and PCSK9 in healthy individuals with elevated lipoprotein(a) level. J. Cardiovasc. Dev. Dis. 2020, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Metzner, T.; Leitner, D.R.; Mellitzer, K.; Beck, A.; Sourij, H.; Stojakovic, T.; Reishofer, G.; Marz, W.; Landmesser, U.; Scharnagl, H.; et al. Effects of alirocumab on triglyceride metabolism: A fat-tolerance test and nuclear magnetic resonance spectroscopy study. Biomedicines 2022, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Farnier, M.; Kastelein, J.J.P.; Roth, E.M.; Taskinen, M.R.; Colhoun, H.M.; Brunet, A.; DiCioccio, A.T.; Lecorps, G.; Pordy, R.; et al. Relationship between alirocumab, PCSK9, and LDL-C levels in four phase 3 ODYSSEY trials using 75 and 150 mg doses. J. Clin. Lipidol. 2019, 13, 979–988.e10. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Fan, L.; Dong, Y.; Xu, X.; Yu, C.; Chen, J.; Ren, J. New PCSK9 inhibitor miR-552-3p reduces LDL-C via enhancing LDLR in high fat diet-fed mice. Pharmacol. Res. 2021, 167, 105562. [Google Scholar] [CrossRef]
- Salerno, A.G.; van Solingen, C.; Scotti, E.; Wanschel, A.; Afonso, M.S.; Oldebeken, S.R.; Spiro, W.; Tontonoz, P.; Rayner, K.J.; Moore, K.J. LDL receptor pathway regulation by miR-224 and miR-520d. Front. Cardiovasc. Med. 2020, 7, 81. [Google Scholar] [CrossRef]
- Dong, J.; He, M.; Li, J.; Pessentheiner, A.; Wang, C.; Zhang, J.; Sun, Y.; Wang, W.T.; Zhang, Y.; Liu, J.; et al. microRNA-483 ameliorates hypercholesterolemia by inhibiting PCSK9 production. JCI Insight 2020, 5, e143812. [Google Scholar] [CrossRef]
- Xu, X.; Dong, Y.; Ma, N.; Kong, W.; Yu, C.; Gong, L.; Chen, J.; Ren, J. MiR-337-3p lowers serum LDL-C level through targeting PCSK9 in hyperlipidemic mice. Metabolism 2021, 119, 154768. [Google Scholar] [CrossRef]
- Naeli, P.; Mirzadeh Azad, F.; Malakootian, M.; Seidah, N.G.; Mowla, S.J. Post-transcriptional regulation of PCSK9 by miR-191, miR-222, and miR-224. Front. Genet. 2017, 8, 189. [Google Scholar] [CrossRef]
- van Solingen, C.; Oldebeken, S.R.; Salerno, A.G.; Wanschel, A.; Moore, K.J. High-throughput screening identifies microRNAs regulating human PCSK9 and hepatic low-density lipoprotein receptor expression. Front. Cardiovasc. Med. 2021, 8, 667298. [Google Scholar] [CrossRef]
- Willeit, P.; Zampetaki, A.; Dudek, K.; Kaudewitz, D.; King, A.; Kirkby, N.S.; Crosby-Nwaobi, R.; Prokopi, M.; Drozdov, I.; Langley, S.R.; et al. Circulating microRNAs as novel biomarkers for platelet activation. Circ. Res. 2013, 112, 595–600. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Huang, J.; Sun, Q.; Wang, L. Clinical impact of circulating miR-26a, miR-191, and miR-208b in plasma of patients with acute myocardial infarction. Eur. J. Med. Res. 2015, 20, 58. [Google Scholar] [CrossRef]
- Yang, X.; Du, X.; Ma, K.; Li, G.; Liu, Z.; Rong, W.; Miao, H.; Zhu, F.; Cui, Q.; Wu, S.; et al. Circulating miRNAs related to long-term adverse cardiovascular events in STEMI patients: A nested case-control study. Can. J. Cardiol. 2021, 37, 77–85. [Google Scholar] [CrossRef]
- Gallo, W.; Ottosson, F.; Kennback, C.; Jujic, A.; Esguerra, J.L.S.; Eliasson, L.; Melander, O. Replication study reveals miR-483-5p as an important target in prevention of cardiometabolic disease. BMC Cardiovasc. Disord. 2021, 21, 162. [Google Scholar] [CrossRef] [PubMed]
- Gallo, W.; Esguerra, J.L.S.; Eliasson, L.; Melander, O. miR-483-5p associates with obesity and insulin resistance and independently associates with new onset diabetes mellitus and cardiovascular disease. PLoS ONE 2018, 13, e0206974. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, L.; Wang, X. Aberrant expression of miR-483-5p in patients with asymptomatic carotid artery stenosis and its predictive value for cerebrovascular event occurrence. Exp. Ther. Med. 2021, 22, 1101. [Google Scholar] [CrossRef]
- Peng, Y.; Xiang, H.; Chen, C.; Zheng, R.; Chai, J.; Peng, J.; Jiang, S. MiR-224 impairs adipocyte early differentiation and regulates fatty acid metabolism. Int. J. Biochem. Cell Biol. 2013, 45, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Baruch, A.; Mosesova, S.; Davis, J.D.; Budha, N.; Vilimovskij, A.; Kahn, R.; Peng, K.; Cowan, K.J.; Harris, L.P.; Gelzleichter, T.; et al. Effects of RG7652, a monoclonal antibody against PCSK9, on LDL-C, LDL-C subfractions, and inflammatory biomarkers in patients at high risk of or with established coronary heart disease (from the Phase 2 EQUATOR Study). Am. J. Cardiol. 2017, 119, 1576–1583. [Google Scholar] [CrossRef]
- Bittner, V.A.; Szarek, M.; Aylward, P.E.; Bhatt, D.L.; Diaz, R.; Edelberg, J.M.; Fras, Z.; Goodman, S.G.; Halvorsen, S.; Hanotin, C.; et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J. Am. Coll. Cardiol. 2020, 75, 133–144. [Google Scholar] [CrossRef]
| microRNA | Sequence | Accession Number | GeneGlobe ID |
|---|---|---|---|
| hsa-miR-191-5p | 5′CAACGGAAUCCCAAAAGCAGCUG | MIMAT0000440 | YP00204306 |
| hsa-miR-224-5p | 5′UCAAGUCACUAGUGGUUCCGUUUAG | MIMAT0000281 | YP02119313 |
| hsa-miR-337-3p | 5′CUCCUAUAUGAUGCCUUUCUUC | MIMAT0000754 | YP00205938 |
| hsa-miR-483-5p | 5′AAGACGGGAGGAAAGAAGGGAG | MIMAT0004761 | YP00205693 |
| hsa-miR-552-3p | 5′AACAGGUGACUGGUUAGACAA | MIMAT0003215 | YP0020603 |
| hsa-miR-16-5p | 5′UAGCAGCACGUAAAUAUUGGCG | MIMAT0000069 | YP00203906 |
| hsa-miR-4516 | 5′GGGAGAAGGGUCGGGGC | MIMAT0019053 | YP02112882 |
| Parameter | Patients | Control Subjects | p |
|---|---|---|---|
| Age at inclusion (years) | 52.4 (45.7–56.8) | 50.9 (43.5–52.0) | 0.207 |
| Gender (% male) | 89.1 | 87.5 | 1.000 |
| Body mass index (kg/m2) | 28.7 ± 4.1 | 26.6 ± 3.2 | 0.034 |
| Systolic blood pressure (mmHg) | 129 (120–136) | 128 (110–145) | 0.813 |
| Diastolic blood pressure (mmHg) | 79 (70–82) | 77 (68–88) | 0.786 |
| Cholesterol (mmol/L) | 4.22 ± 0.83 | 5.46 ± 0.41 | <0.001 |
| HDL-C (mmol/L) | 1.16 ± 0.25 | 1.52 ± 0.44 | 0.005 |
| Non-HDL-C (mmol/L) | 3.07 ± 0.83 | 3.93 ± 0.55 | <0.001 |
| LDL-C (mmol/L) | 2.29 (1.70–2.66) | 3.30 (3.03–3.48) | <0.001 |
| Triglycerides (mmol/L) | 1.40 (1.00–2.08) | 1.28 (0.95–1.51) | 0.215 |
| ApoB (g/L) | 0.82 ± 0.21 | 1.07 ± 0.13 | <0.001 |
| ApoA1 (g/L) | 1.28 (1.17–1.44) | 1.39 (1.27–1.59) | 0.021 |
| Lp(a) (mg/L) | 1463 (1207–1769) | 82 (62–138) | <0.001 |
| Parameter | Before Treatment | After Treatment | p |
|---|---|---|---|
| Cholesterol (mmol/L) | 4.27 (3.72–4.84) | 2.54 (2.10–3.07) | <0.001 |
| HDL-C (mmol/L) | 1.14 (1.01–1.32) | 1.17 (1.02–1.50) | 0.004 |
| Non-HDL-C (mmol/L) | 3.10 (2.40–3.70) | 1.20 (0.90–1.78) | <0.001 |
| LDL-C (mmol/L) | 2.40 (1.75–2.66) | 0.63 (0.40–1.15) | <0.001 |
| Triglycerides (mmol/L) | 1.46 (1.06–2.08) | 1.20 (0.78–1.88) | <0.001 |
| ApoB (g/L) | 0.82 (0.62–0.98) | 0.35 (0.35–0.49) | <0.001 |
| ApoA1 (g/L) | 1.28 (1.19–1.44) | 1.35 (1.22–1.54) | 0.002 |
| Lp(a) (mg/L) | 1431 (1219–1776) | 1133 (821–1664) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levstek, T.; Karun, T.; Rehberger Likozar, A.; Šebeštjen, M.; Trebušak Podkrajšek, K. Interplay between microRNAs, Serum Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9), and Lipid Parameters in Patients with Very High Lipoprotein(a) Treated with PCSK9 Inhibitors. Genes 2023, 14, 632. https://doi.org/10.3390/genes14030632
Levstek T, Karun T, Rehberger Likozar A, Šebeštjen M, Trebušak Podkrajšek K. Interplay between microRNAs, Serum Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9), and Lipid Parameters in Patients with Very High Lipoprotein(a) Treated with PCSK9 Inhibitors. Genes. 2023; 14(3):632. https://doi.org/10.3390/genes14030632
Chicago/Turabian StyleLevstek, Tina, Tina Karun, Andreja Rehberger Likozar, Miran Šebeštjen, and Katarina Trebušak Podkrajšek. 2023. "Interplay between microRNAs, Serum Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9), and Lipid Parameters in Patients with Very High Lipoprotein(a) Treated with PCSK9 Inhibitors" Genes 14, no. 3: 632. https://doi.org/10.3390/genes14030632
APA StyleLevstek, T., Karun, T., Rehberger Likozar, A., Šebeštjen, M., & Trebušak Podkrajšek, K. (2023). Interplay between microRNAs, Serum Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9), and Lipid Parameters in Patients with Very High Lipoprotein(a) Treated with PCSK9 Inhibitors. Genes, 14(3), 632. https://doi.org/10.3390/genes14030632






