MiRNA Profiling and Its Potential Roles in Rapid Growth of Velvet Antler in Gansu Red Deer (Cervus elaphus kansuensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. RNA Extraction, Library Preparation, and Sequencing
2.3. Data Processing and Analysis
2.4. Real-Time PCR to Verify Differentially Expressed microRNAs
3. Results
3.1. Sequencing Data Processing
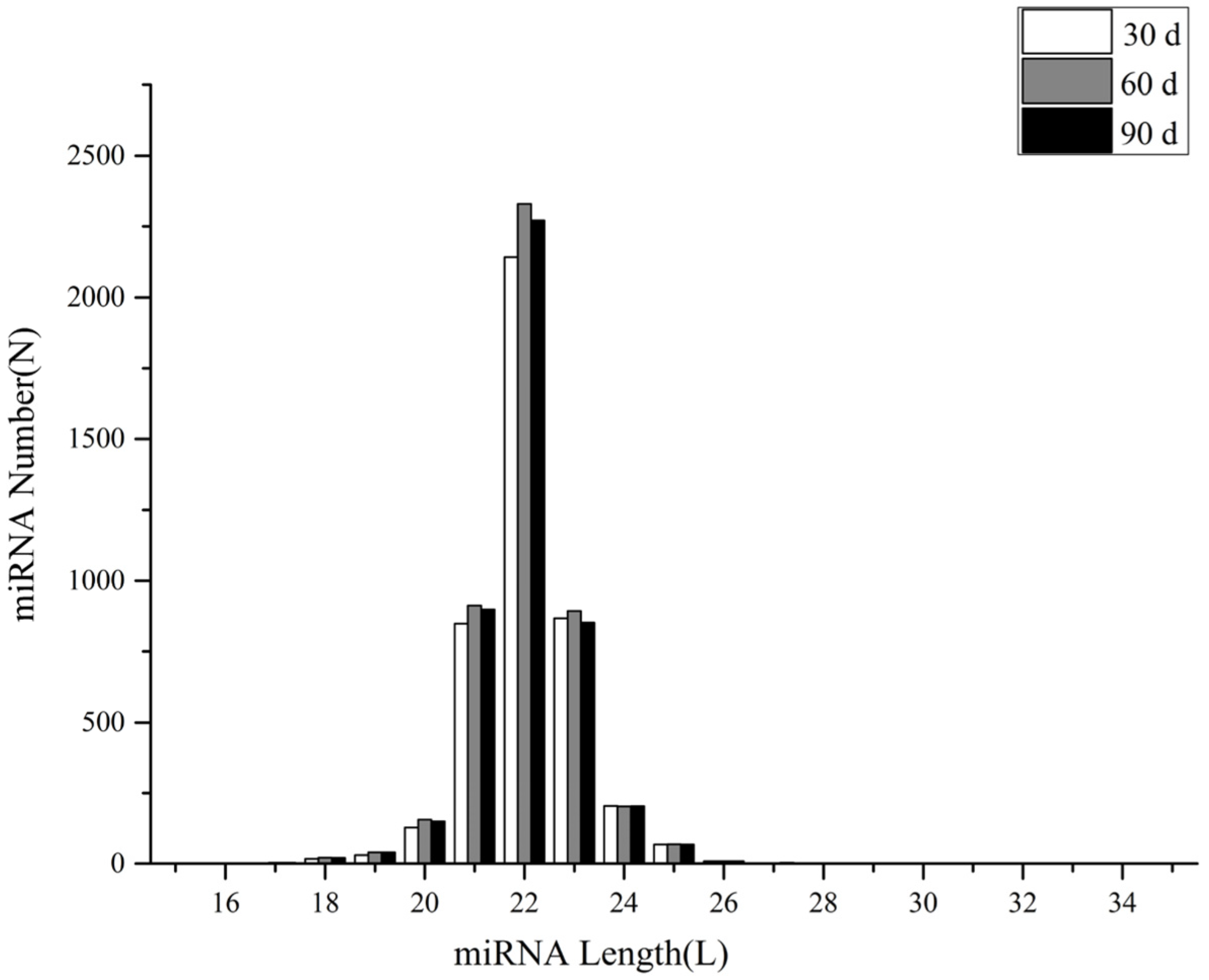
3.2. MiRNA Identification
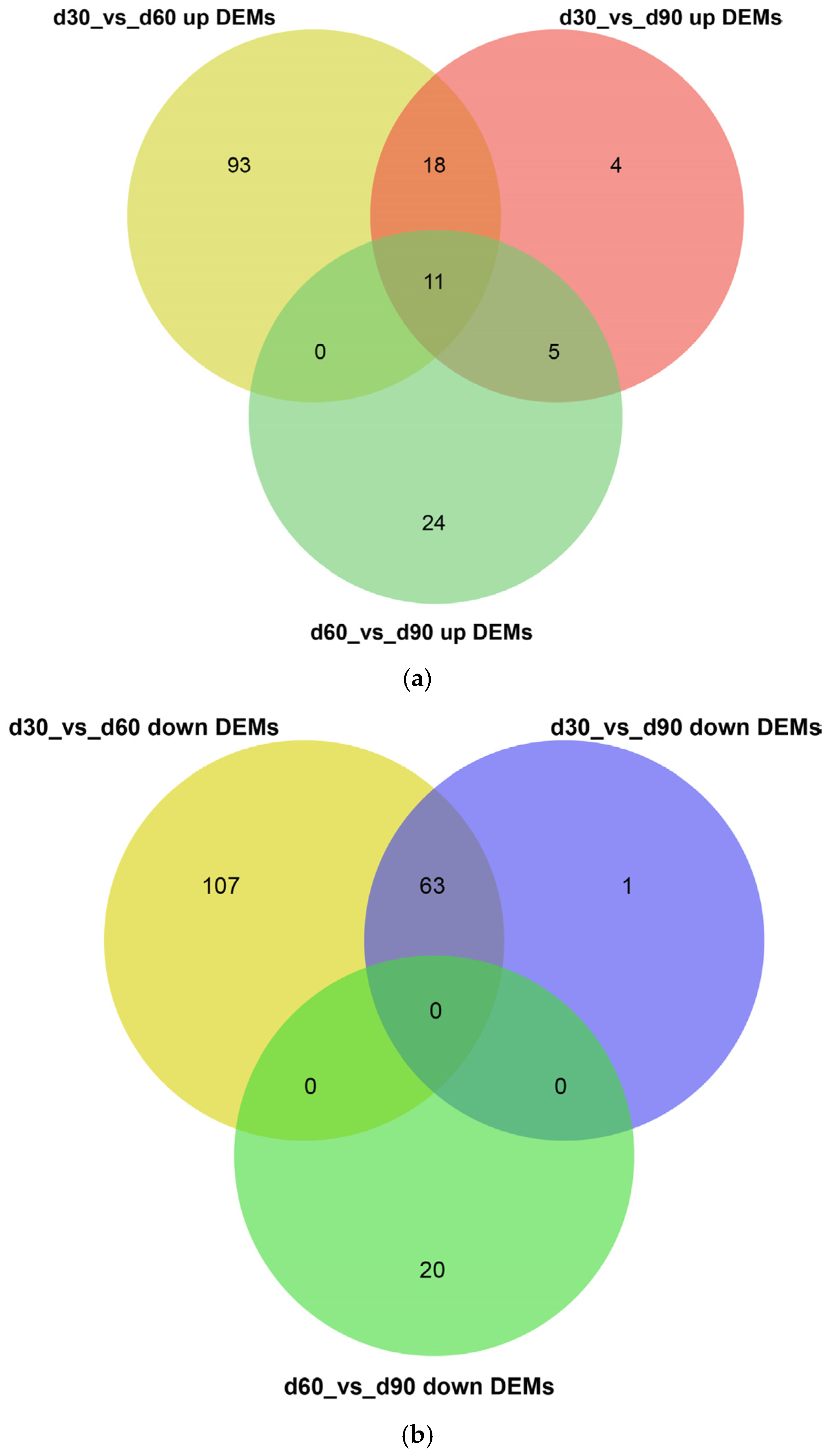
3.3. Screening for Differentially Expressed miRNAs
3.4. Target Genes’ Prediction of Differentially Expressed miRNAs
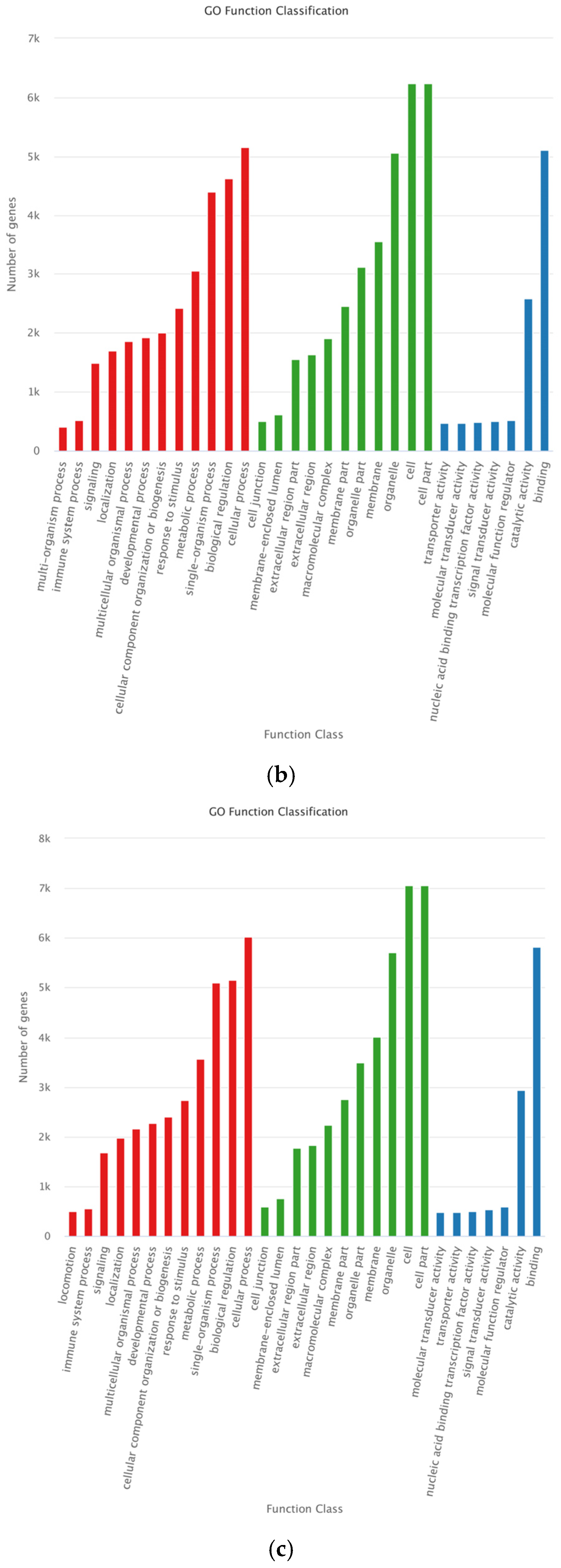
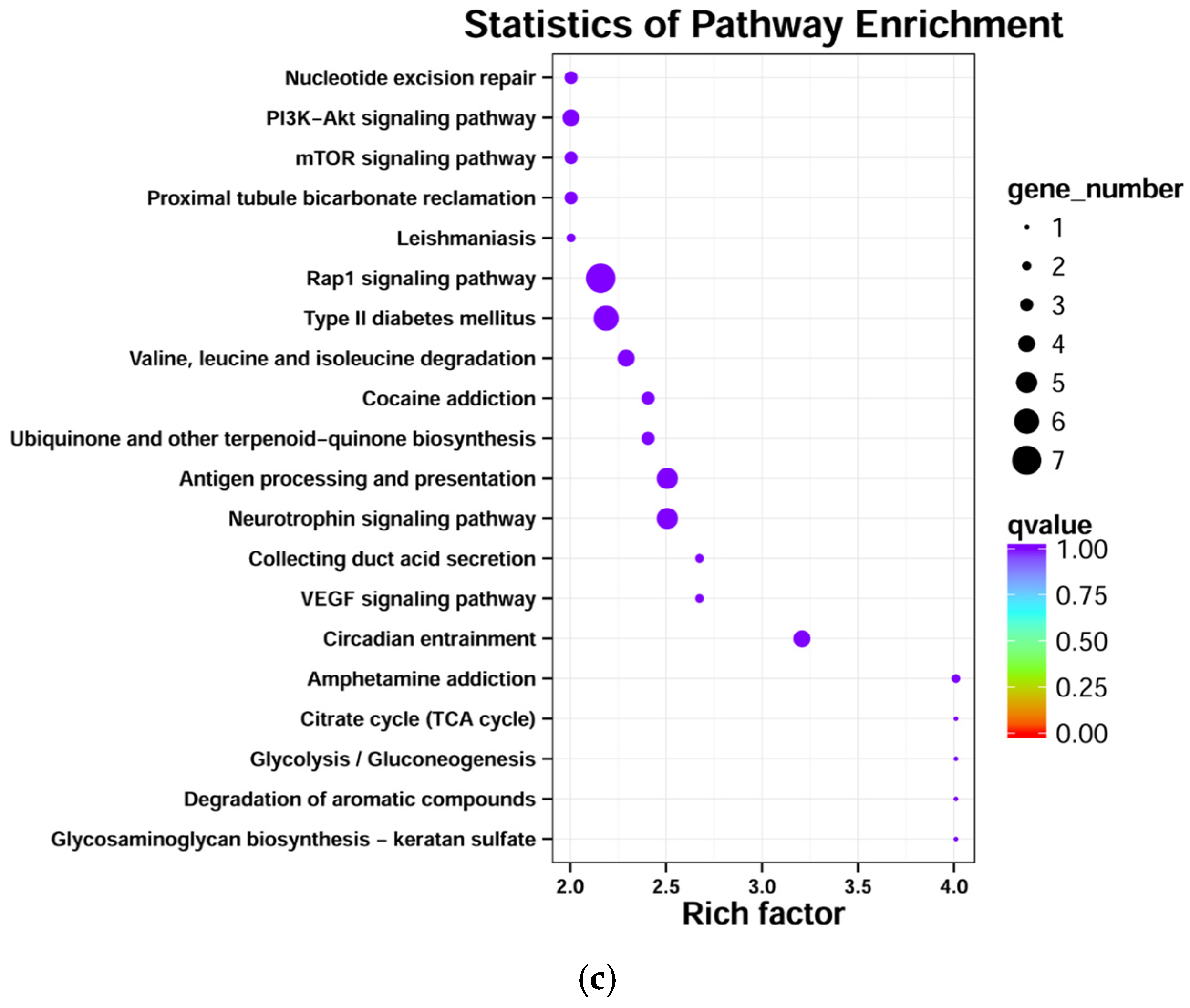
3.5. Target Genes’ Functional Analysis of Differentially Expressed miRNAs
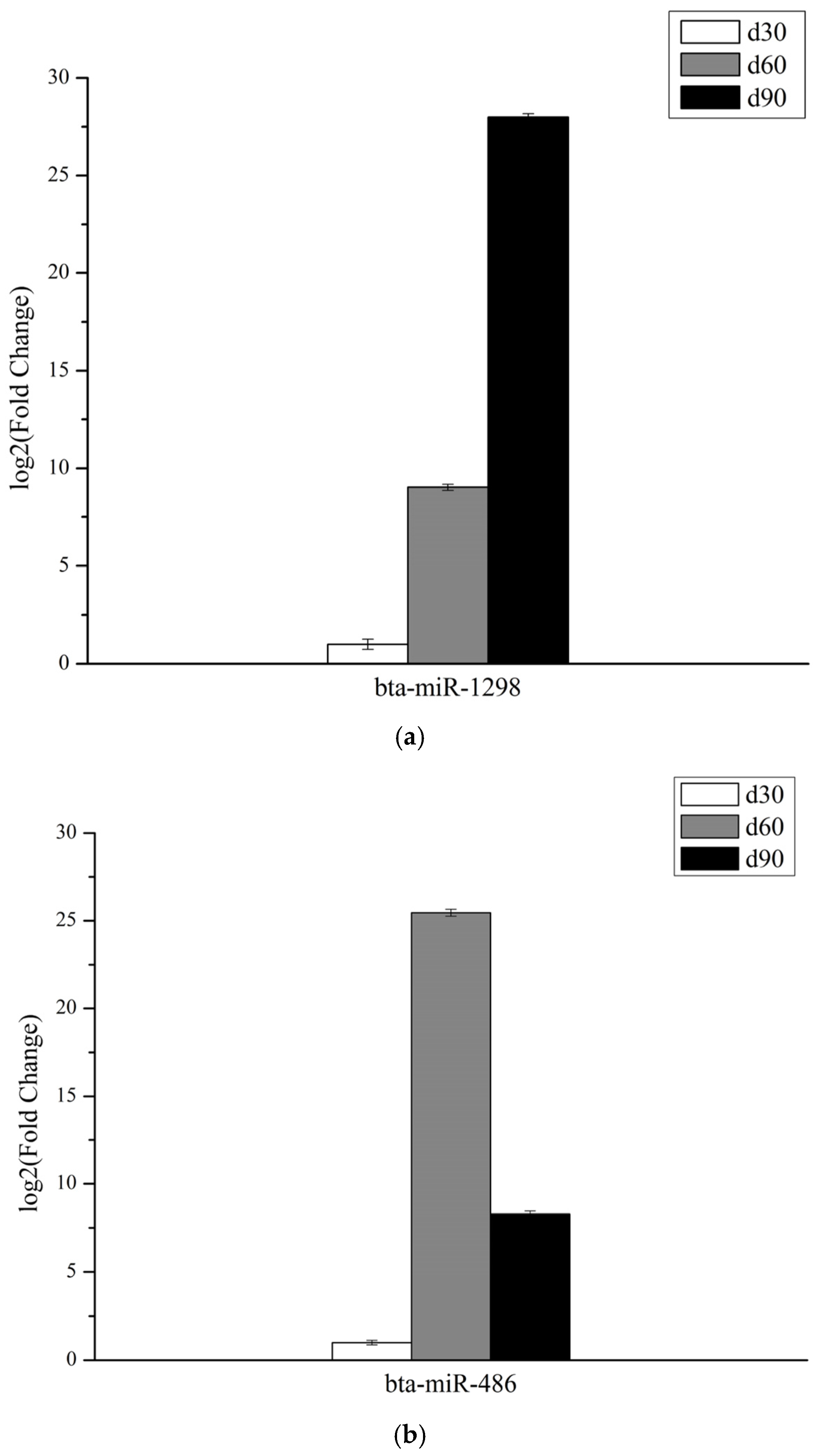
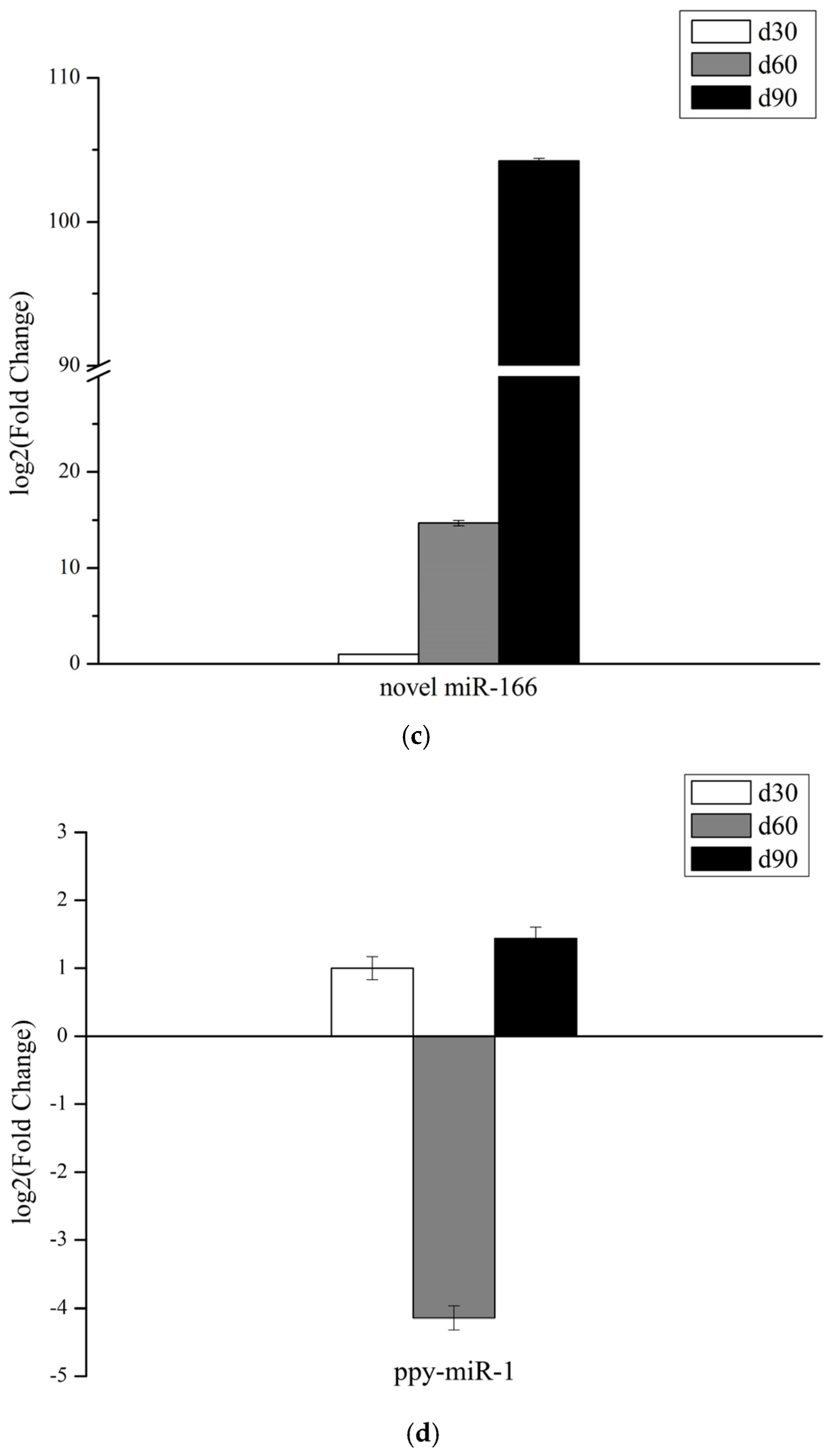
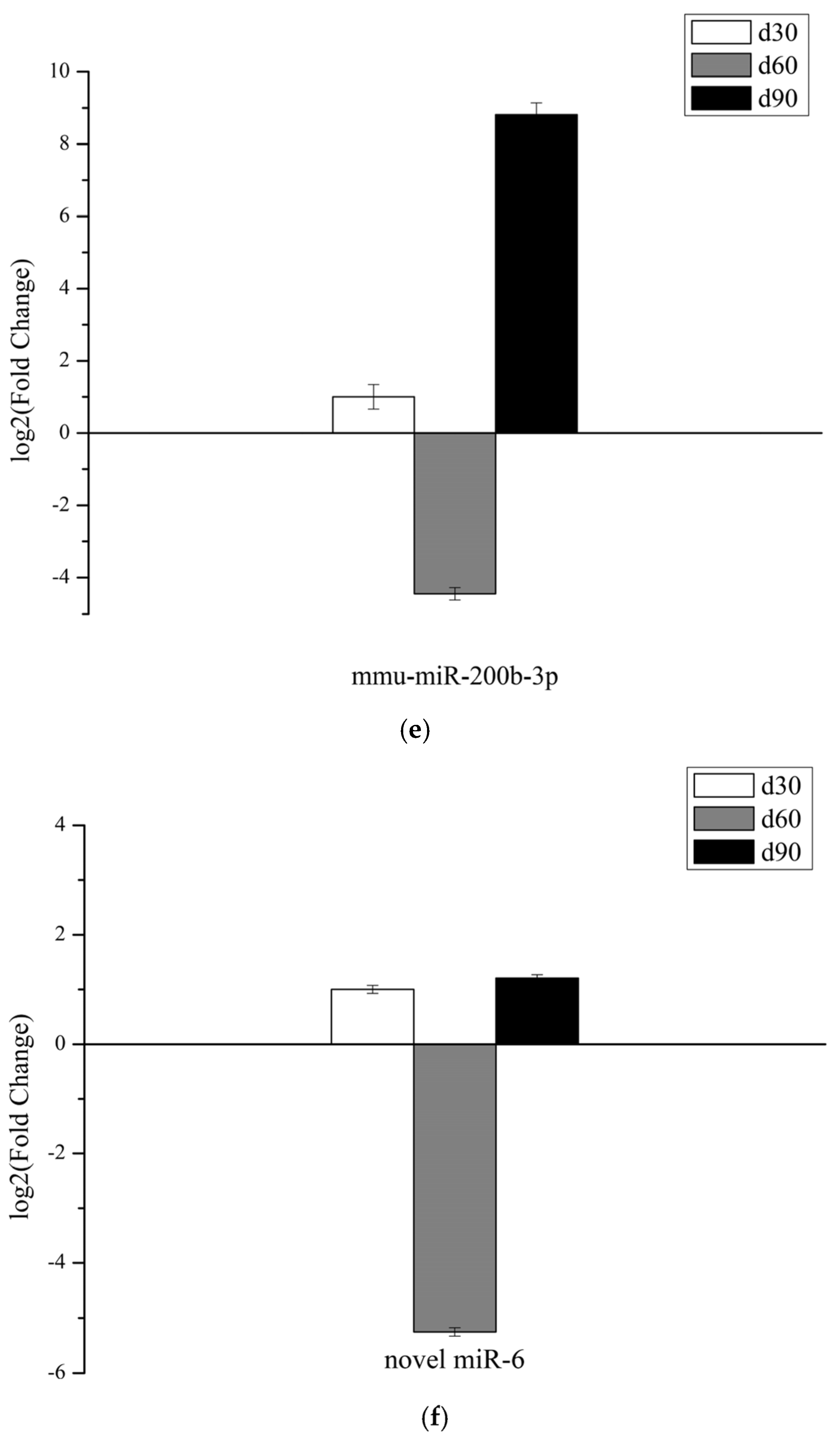
3.6. Validation of miRNAs Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, J.S.; Allen, S.; Faucheux, C.; Althnaian, T.; Mount, J.G. Deer antlers: A zoological curiosity or the key to understanding organ regeneration in mammals? J. Anat. 2005, 207, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.J. Future directions in antler research. Anat. Rec. 1995, 241, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, F.; Sheppard, A. Adult stem cells and mammalian epimorphic regeneration-insights from studying annual renewal of deer antlers. Curr. Stem Cell Res. Ther. 2009, 4, 237–251. [Google Scholar] [CrossRef]
- Dong, Z.; Coates, D. Bioactive Molecular Discovery Using Deer Antlers as a Model of Mammalian Regeneration. J. Proteome Res. 2021, 20, 2167–2181. [Google Scholar] [CrossRef]
- Li, C.; Suttie, J.M.; Clark, D.E. Morphological observation of antler regeneration in red deer (Cervus elaphus). J. Morphol. 2004, 262, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Fennessy, P.F. Deer Antlers: Regeneration, function and evolution. J. R. Soc. N. Z. 1984, 14, 290–291. [Google Scholar] [CrossRef]
- Gyurjan, I., Jr.; Molnar, A.; Borsy, A.; Steger, V.; Hackler, L., Jr.; Zomborszky, Z.; Papp, P.; Duda, E.; Deak, F.; Lakatos, P.; et al. Gene expression dynamics in deer antler: Mesenchymal differentiation toward chondrogenesis. Mol. Genet. Genom. 2007, 277, 221–235. [Google Scholar] [CrossRef]
- Waldo, C.M.; Wislocki, G.B.; Fawcett, D.W. Observations on the blood supply of growing antlers. Am. J. Anat. 1949, 84, 27–61. [Google Scholar] [CrossRef]
- Li, C.; Clark, D.E.; Lord, E.A.; Stanton, J.A.; Suttie, J.M. Sampling technique to discriminate the different tissue layers of growing antler tips for gene discovery. Anat. Rec. 2002, 268, 125–130. [Google Scholar] [CrossRef]
- Price, J.; Allen, S. Exploring the mechanisms regulating regeneration of deer antlers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 809–822. [Google Scholar] [CrossRef]
- Yao, B.; Zhao, Y.; Wang, Q.; Zhang, M.; Liu, M.; Liu, H.; Li, J. De novo characterization of the antler tip of Chinese Sika deer transcriptome and analysis of gene expression related to rapid growth. Mol. Cell. Biochem. 2012, 364, 93–100. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, B.; Zhang, M.; Wang, S.; Zhang, H.; Xiao, W. Comparative analysis of differentially expressed genes in Sika deer antler at different stages. Mol. Biol. Rep. 2013, 40, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Zhao, Y.; Zhang, H.; Zhang, M.; Liu, M.; Liu, H.; Li, J. Sequencing and de novo analysis of the Chinese Sika deer antler-tip transcriptome during the ossification stage using Illumina RNA-Seq technology. Biotechnol. Lett. 2012, 34, 813–822. [Google Scholar] [CrossRef]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Mohan, M. MicroRNAs. Vet. Pathol. 2013, 51, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Wang, T.; Liu, H.; Xu, J.; Wang, L.; Zhao, P.; Xing, X. Full-length transcriptome and microRNA sequencing reveal the specific gene-regulation network of velvet antler in sika deer with extremely different velvet antler weight. Mol. Genet. Genom. 2019, 294, 431–443. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, M.; Liu, M.; Lu, B.; Leng, X.; Hu, Y.; Zhao, D.; Zhao, Y.U. Identification of the miRNA-mRNA regulatory network of antler growth centers. J. Biosci. 2019, 44, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, W. Progress of Noncoding RNA Regulating the Growth and Development of Antler Tissue Research. Biomed Res. Int. 2022, 2022, 3541577. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Zhang, J.; Chen, X.; Guo, Y.; Li, C. RNA sequencing-based identification of microRNAs in the antler cartilage of Gansu red deer (Cervus elaphus kansuensis). PeerJ 2022, 10, e13947. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Zhang, L.; Zhang, Y.; Ge, C.; Yang, F.; Du, R.; Ba, H. Integrated analysis of miRNA and mRNA transcriptomic reveals antler growth regulatory network. Mol. Genet. Genom. 2021, 296, 689–703. [Google Scholar] [CrossRef]
- Ba, H.; Wang, D.; Li, C. MicroRNA profiling of antler stem cells in potentiated and dormant states and their potential roles in antler regeneration. Mol. Genet. Genom. 2016, 291, 943–955. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Hofacker, I.L. Vienna RNA secondary structure server. Nucleic Acids Res. 2003, 31, 3429–3431. [Google Scholar] [CrossRef]
- Zhao, S.; Ye, Z.; Stanton, R. Misuse of RPKM or TPM normalization when comparing across samples and sequencing protocols. RNA 2020, 26, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Clark, D.E.; Li, C.; Wang, W.; Martin, S.K.; Suttie, J.M. Vascular localization and proliferation in the growing tip of the deer antler. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2006, 288, 973–981. [Google Scholar] [CrossRef]
- Rodriguez, R.E.; Schommer, C.; Palatnik, J.F. Control of cell proliferation by microRNAs in plants. Curr. Opin. Plant Biol. 2016, 34, 68–76. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model. Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef]
- Wu, N.; Gu, T.; Lu, L.; Cao, Z.; Song, Q.; Wang, Z.; Zhang, Y.; Chang, G.; Xu, Q.; Chen, G. Roles of miRNA-1 and miRNA-133 in the proliferation and differentiation of myoblasts in duck skeletal muscle. J. Cell. Physiol. 2019, 234, 3490–3499. [Google Scholar] [CrossRef]
- Wang, J.D.; Qiu, F.; Li, W. miRNA-1: A novel, potential therapeutic target in the treatment of acute myocardial infarction? Int. J. Cardiol. 2021, 333, 53. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Zhu, Y.; Gong, J. HOTAIR/miRNA-1/Cx43: A potential mechanism for treating myocardial ischemia-reperfusion injury. Int. J. Cardiol. 2020, 308, 11. [Google Scholar] [CrossRef]
- Jayawardena, E.; Medzikovic, L.; Ruffenach, G.; Eghbali, M. Role of miRNA-1 and miRNA-21 in Acute Myocardial Ischemia-Reperfusion Injury and Their Potential as Therapeutic Strategy. Int. J. Mol. Sci. 2022, 23, 1512. [Google Scholar] [CrossRef]
- Cai, Q.; Gao, M.L.; Huang, L.S.; Pan, L.H. lncRNA H19/miRNA-1: Another potential mechanism for treating myocardial ischemia-reperfusion injury. Int. J. Cardiol. 2021, 322, 57. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Yu, S.; Handler, A.M.; Tu, Z.; Saccone, G.; Xi, Z.; Zhang, H. miRNA-1-3p is an early embryonic male sex-determining factor in the Oriental fruit fly Bactrocera dorsalis. Nat. Commun. 2020, 11, 932. [Google Scholar] [CrossRef]
- Feng, B.; Cao, Y.; Chen, S.; Ruiz, M.; Chakrabarti, S. miRNA-1 regulates endothelin-1 in diabetes. Life Sci. 2014, 98, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wei, X.; Guan, Y.; Chen, Q.; Zhao, T.; Sun, C.; Wei, L. MicroRNA-1 regulates chondrocyte phenotype by repressing histone deacetylase 4 during growth plate development. FASEB J. 2014, 28, 3930–3941. [Google Scholar] [CrossRef]
- Chen, T.; Che, X.; Han, P.; Lu, J.; Wang, C.; Liang, B.; Hou, Z.; Wei, X.; Wei, L.; Li, P. MicroRNA-1 promotes cartilage matrix synthesis and regulates chondrocyte differentiation via post-transcriptional suppression of Ihh expression. Mol. Med. Rep. 2020, 22, 2404–2414. [Google Scholar] [CrossRef]
- Cong, L.; Jiang, P.; Wang, H.; Huang, L.; Wu, G.; Che, X.; Wang, C.; Li, P.; Duan, Q.; Guo, X.; et al. MiR-1 is a critical regulator of chondrocyte proliferation and hypertrophy by inhibiting Indian hedgehog pathway during postnatal endochondral ossification in miR-1 overexpression transgenic mice. Bone 2022, 165, 116566. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Chen, T.; Wei, L.; Gu, X.; Gao, Y.; Liang, S.; Li, P.; Shi, D.; Liang, B.; Wang, C.; et al. MicroRNA-1 regulates the development of osteoarthritis in a Col2a1-Cre-ERT2/GFPfl/fl-RFP-miR-1 mouse model of osteoarthritis through the downregulation of Indian hedgehog expression. Int. J. Mol. Med. 2020, 46, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Jia, H.; Li, B.; Xing, D.; Li, J.J. MicroRNA-1 Modulates Chondrocyte Phenotype by Regulating FZD7 of Wnt/β-Catenin Signaling Pathway. Cartilage 2021, 13, 1019S–1029S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Xing, X. Comparative antler proteome of sika deer from different developmental stages. Sci. Rep. 2021, 11, 10484. [Google Scholar] [CrossRef]
- Dong, Z.; Li, C.; Coates, D. PTN-PTPRZ signalling is involved in deer antler stem cell regulation during tissue regeneration. J. Cell. Physiol. 2021, 236, 3752–3769. [Google Scholar] [CrossRef]
- Hu, W.; Meng, X.; Lu, T.; Wu, L.; Li, T.; Li, M.; Tian, Y. MicroRNA1 inhibits the proliferation of Chinese sika deerderived cartilage cells by binding to the 3’-untranslated region of IGF1. Mol. Med. Rep. 2013, 8, 523–528. [Google Scholar] [CrossRef]
- Ren, S.; Liu, J.; Feng, Y.; Li, Z.; He, L.; Li, L.; Cao, X.; Wang, Z.; Zhang, Y. Knockdown of circDENND4C inhibits glycolysis, migration and invasion by up-regulating miR-200b/c in breast cancer under hypoxia. J. Exp. Clin. Cancer Res. 2019, 38, 388. [Google Scholar] [CrossRef]
- Guan, W.; Cui, H.; Huang, P.; Chun, W.J.; Lee, J.W.; Kim, H.; Zou, H. miR-200b/200a/429 Cluster Stimulates Ovarian Cancer Development by Targeting ING5. J. Oncol. 2020, 2020, 3404059. [Google Scholar] [CrossRef]
- Chen, S.; Tu, Y.; Yuan, H.; Shi, Z.; Guo, Y.; Gong, W.; Tu, S. Regulatory functions of miR200b3p in tumor development (Review). Oncol. Rep. 2022, 47, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, T.; Chen, Y.; Xiao, J. Exosome-mediated miR-200b promotes colorectal cancer proliferation upon TGF-beta1 exposure. Biomed. Pharmacother. 2018, 106, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, N.; Du, J.; Zhang, H.; Zhang, C. MicroRNA-200b-3p promotes endothelial cell apoptosis by targeting HDAC4 in atherosclerosis. BMC Cardiovasc. Disord. 2021, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki-Takai, M.; Takai, H.; Iwai, Y.; Noda, K.; Mezawa, M.; Tsuruya, Y.; Yamaguchi, A.; Nakayama, Y.; Ogata, Y. MiR-200b suppresses TNF-α-induced AMTN production in human gingival epithelial cells. Odontology 2021, 109, 403–410. [Google Scholar] [CrossRef]
- Tamagawa, S.; Enomoto, K.; Gunduz, E.; Gunduz, M.; Sato, F.; Uchino, S.; Muragaki, Y.; Hotomi, M. MicroRNA 200b promotes mesenchymal-to-epithelial transition in anaplastic thyroid carcinoma. Oncol. Lett. 2020, 20, 3. [Google Scholar] [CrossRef]
- Sinha, M.; Ghatak, S.; Roy, S.; Sen, C.K. microRNA-200b as a Switch for Inducible Adult Angiogenesis. Antioxid. Redox Signal. 2015, 22, 1257–1272. [Google Scholar] [CrossRef]
- Moh-Moh-Aung, A.; Fujisawa, M.; Ito, S.; Katayama, H.; Ohara, T.; Ota, Y.; Yoshimura, T.; Matsukawa, A. Decreased miR-200b-3p in cancer cells leads to angiogenesis in HCC by enhancing endothelial ERG expression. Sci. Rep. 2020, 10, 10418. [Google Scholar] [CrossRef]
- Li, C. Deer antler regeneration: A stem cell-based epimorphic process. Birth Defects Res. C Embryo Today 2012, 96, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Stanton, J.A.; Robertson, T.M.; Suttie, J.M.; Sheard, P.W.; Harris, A.J.; Clark, D.E. Nerve growth factor mRNA expression in the regenerating antler tip of red deer (Cervus elaphus). PLoS ONE 2007, 2, e148. [Google Scholar] [CrossRef]
- Wei, G.; Qin, T.; Li, X.; Wang, Z.; Wang, Y.; Guan, Q.; Shi, W.; Xie, L.; Zhao, S.; Sun, H. Constructing the in vitro culture system of the sika deer (cervus nippon) antler periosteal cell to detect its function on antler regeneration. Front. Biosci. (Landmark Ed.) 2022, 27, 69. [Google Scholar] [CrossRef]
- Han, R.; Han, L.; Wang, S.; Li, H. Whole Transcriptome Analysis of Mesenchyme Tissue in Sika Deer Antler Revealed the CeRNAs Regulatory Network Associated With Antler Development. Front. Genet. 2019, 10, 1403. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Jin, W.; Li, Z.; Bao, C.; He, C.; Guo, Y.; Li, C. Integrative Analyses of Antler Cartilage Transcriptome and Proteome of Gansu Red Deer (Cervus elaphus kansuensis) at Different Growth Stages. Animals 2022, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.J. Tumor-like growth of antlers in castrated fallow deer: An electron microscopic study. Scanning Microsc. 1990, 4, 715–720; discussion 720–711. [Google Scholar]
- Li, C.; Waldrup, K.A.; Corson, I.D.; Littlejohn, R.P.; Suttie, J.M. Histogenesis of antlerogenic tissues cultivated in diffusion chambers in vivo in red deer (Cervus elaphus). J. Exp. Zool. 1995, 272, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Xu, M.; Zhang, X.; Meng, S.; Wang, Y.; Zhou, T.; Ma, Q.; Han, B.; Wei, Y.; Deng, X. Enhanced Osteogenic Behavior of ADSCs Produced by Deproteinized Antler Cancellous Bone and Evidence for Involvement of ERK Signaling Pathway. Tissue Eng. Part A 2015, 21, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, H.; Wang, D.; McMahon, C.; Li, C. Differential effects of the PI3K/AKT pathway on antler stem cells for generation and regeneration of antlers in vitro. Front. Biosci. (Landmark Ed.) 2018, 23, 1848–1863. [Google Scholar] [CrossRef]
- Dong, Z.; Ba, H.; Zhang, W.; Coates, D.; Li, C. iTRAQ-Based Quantitative Proteomic Analysis of the Potentiated and Dormant Antler Stem Cells. Int. J. Mol. Sci. 2016, 17, 1778. [Google Scholar] [CrossRef]
- Weng, R.; Lin, H.; Li, Z.; Chen, D.; Lin, X.; Zhang, Z.; Chen, Q.; Yao, Y.; Li, W. Mechanisms of Intervertebral Disc Degeneration Treatment with Deer Antlers Based on Network Pharmacology and Molecular Docking. Evid. Based Complement. Altern. Med. 2022, 2022, 8092848. [Google Scholar] [CrossRef]
- Chen, D.Y.; Yang, M.; Sun, Z.T.; Song, M.M.; Yao, H.B.; Long, G.H.; Hu, W. Notch4 affects the proliferation and differentiation of deer antler chondrocytes through the Smad3/lncRNA27785.1 axis. Cell. Signal. 2022, 98, 110429. [Google Scholar] [CrossRef]
- Ma, L.; Duan, C.C.; Yang, Z.Q.; Ding, J.L.; Liu, S.; Yue, Z.P.; Guo, B. Crosstalk between Activin A and Shh signaling contributes to the proliferation and differentiation of antler chondrocytes. Bone 2019, 123, 176–188. [Google Scholar] [CrossRef]
- Ma, L.; Yang, Z.Q.; Ding, J.L.; Liu, S.; Guo, B.; Yue, Z.P. Function and regulation of transforming growth factor beta1 signalling in antler chondrocyte proliferation and differentiation. Cell Prolif. 2019, 52, e12637. [Google Scholar] [CrossRef]
- Chen, D.Y.; Li, Y.J.; Jiang, R.F.; Li, Y.T.; Feng, J.; Hu, W. Effects and mechanism of lncRNA-27785.1 that regulates TGF-beta1 of Sika deer on antler cell proliferation. J. Cell. Physiol. 2021, 236, 5742–5756. [Google Scholar] [CrossRef]
- Zhang, H.L.; Yang, Z.Q.; Duan, C.C.; Geng, S.; Wang, K.; Yu, H.F.; Yue, Z.P.; Guo, B. WNT4 acts downstream of BMP2 to mediate the regulation of ATRA signaling on RUNX1 expression: Implications for terminal differentiation of antler chondrocytes. J. Cell. Physiol. 2018, 233, 1129–1145. [Google Scholar] [CrossRef]
- Ba, H.; Wang, D.; Yau, T.O.; Shang, Y.; Li, C. Transcriptomic analysis of different tissue layers in antler growth Center in Sika Deer (Cervus nippon). BMC Genom. 2019, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Mount, J.G.; Muzylak, M.; Allen, S.; Althnaian, T.; McGonnell, I.M.; Price, J.S. Evidence that the canonical Wnt signalling pathway regulates deer antler regeneration. Dev. Dyn. 2006, 235, 1390–1399. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Long, F. mTORC1 signaling controls mammalian skeletal growth through stimulation of protein synthesis. Development 2014, 141, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, R.; Li, D.; Qiao, T.; Guo, X. Fluoride regulates chondrocyte proliferation and autophagy via PI3K/AKT/mTOR signaling pathway. Chem. Biol. Interact. 2021, 349, 109659. [Google Scholar] [CrossRef]
- Kim, M.S.; Wu, K.Y.; Auyeung, V.; Chen, Q.; Gruppuso, P.A.; Phornphutkul, C. Leucine restriction inhibits chondrocyte proliferation and differentiation through mechanisms both dependent and independent of mTOR signaling. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1374. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.B.; Qiu, H.Y. Effects of Artesunate on chondrocyte proliferation, apoptosis and autophagy through the PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid arthritis. Biomed. Pharmacother. 2018, 102, 1209–1220. [Google Scholar] [CrossRef]
- Xu, K.; He, Y.; Moqbel, S.A.A.; Zhou, X.; Wu, L.; Bao, J. SIRT3 ameliorates osteoarthritis via regulating chondrocyte autophagy and apoptosis through the PI3K/Akt/mTOR pathway. Int. J. Biol. Macromol. 2021, 175, 351–360. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, F.; Lin, J.; Li, Y.; Johnson, N.; Zhang, J.; Gai, C.; Su, Z.; Cheng, H.; Wang, L.; et al. Erianin, the main active ingredient of Dendrobium chrysotoxum Lindl, inhibits precancerous lesions of gastric cancer (PLGC) through suppression of the HRAS-PI3K-AKT signaling pathway as revealed by network pharmacology and in vitro experimental verification. J. Ethnopharmacol. 2021, 279, 114399. [Google Scholar] [CrossRef]
- Hu, L.; Xie, X.; Xue, H.; Wang, T.; Panayi, A.C.; Lin, Z.; Xiong, Y.; Cao, F.; Yan, C.; Chen, L.; et al. MiR-1224-5p modulates osteogenesis by coordinating osteoblast/osteoclast differentiation via the Rap1 signaling target ADCY2. Exp. Mol. Med. 2022, 54, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Banerjee, R.; Rossa, C., Jr.; D’Silva, N.J. RAP1-RAC1 Signaling Has an Important Role in Adhesion and Migration in HNSCC. J. Dent. Res. 2020, 99, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, J.; Wang, J.; Han, J.; Li, S.; Huang, K.; Liu, C. Mex3a promotes oncogenesis through the RAP1/MAPK signaling pathway in colorectal cancer and is inhibited by hsa-miR-6887-3p. Cancer Commun. 2021, 41, 472–491. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Suttie, J. Morphogenetic aspects of deer antler development. Front. Biosci. (Elite Ed.) 2012, 4, 1836–1842. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef]




| Gene ID | miRNA Sequence | Primers | Sequence |
|---|---|---|---|
| U6 | RTP | AACGCTTCACGAATTTGCGT | |
| Forward primer | CTCGCTTCGGCAGCACA | ||
| Reverse primer | AACGCTTCACGAATTTGCG | ||
| bta-miR-1298 | UUCAUUCGGCUGUCCAGAUGUA | RTP | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG TACATCTG |
| Forward primer | ACACTCCAGCTGGGUUCAUUCGGCUGUCCA | ||
| Reverse primer | TGTCGTGGAGTCGGCAATTCAG | ||
| bta-miR-486 | UCCUGUACUGAGCUGCCCCGAG | RTP | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG CTCGGGGC |
| Forward primer | ACACTCCAGCTGGGUCCUGUACUGAGCUGC | ||
| Reverse primer | TGTCGTGGAGTCGGCAATTCAG | ||
| novel miR-166 | GUGGGCUUCCCUGGUGGCUCAGA | RTP | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG TCTGAGCC |
| Forward primer | ACACTCCAGCTGGGGUGGGCUUCCCUGGUGG | ||
| Reverse primer | TGTCGTGGAGTCGGCAATTCAG | ||
| ppy-miR-1 | UGGAAUGUAAAGAAGUAUGUAU | RTP | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG ATACATAC |
| Forward primer | ACACTCCAGCTGGG UGGAAUGUAAAGAAGU | ||
| Reverse primer | TGTCGTGGAGTCGGCAATTCAG | ||
| mmu-miR-200b-3p | UAAUACUGCCUGGUAAUGAUGA | RTP | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG TCATCATT |
| Forward primer | ACACTCCAGCTGGGUAAUACUGCCUGGUAA | ||
| Reverse primer | TGTCGTGGAGTCGGCAATTCAG | ||
| Novel miR-6 | CAAAUUCGUGAAGCGUUCCAUAUUU | RTP | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG AAATATGG |
| Forward primer | ACACTCCAGCTGGGCAAAUUCGUGAAGCGUUCC | ||
| Reverse primer | TGTCGTGGAGTCGGCAATTCAG | ||
| Novel miR-94 | GAAUUAUAGGAAUUGAACC | RTP | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG GGTTCAAT |
| Forward primer | ACACTCCAGCTGGGGAAUUAUAGGAAU | ||
| Reverse primer | TGTCGTGGAGTCGGCAATTCAG |
| d30 | d60 | d90 | ||||||
|---|---|---|---|---|---|---|---|---|
| miRNA ID | Count | TPM | miRNA ID | Count | TPM | miRNA ID | Count | TPM |
| xla-miR-148a-3p | 141,308 | 22,996.78 | xla-miR-148a-3p | 154,290 | 17,939.06 | xla-miR-148a-3p | 143,637 | 21,242.91 |
| chi-miR-148a-3p | 131,464 | 21,394.74 | chi-miR-148a-3p | 144,249 | 16,771.61 | chi-miR-148a-3p | 131,088 | 19,387 |
| mmu-miR-148a-3p | 131,464 | 21,394.74 | mmu-miR-148a-3p | 144,249 | 16,771.61 | mmu-miR-148a-3p | 131,088 | 19,387 |
| ggo-miR-148a | 131,461 | 21,394.25 | ggo-miR-148a | 144,248 | 16,771.5 | ggo-miR-148a | 131,087 | 19,386.85 |
| rno-miR-148a-3p | 131,461 | 21,394.25 | rno-miR-148a-3p | 144,248 | 16,771.5 | rno-miR-148a-3p | 131,087 | 19,386.85 |
| oan-miR-148-3p | 131,423 | 21,388.07 | oan-miR-148-3p | 144,240 | 16,770.57 | oan-miR-148-3p | 131,062 | 19,383.16 |
| bta-miR-148a | 131,378 | 21,380.75 | bta-miR-148a | 144,213 | 16,767.43 | bta-miR-148a | 131,046 | 19,380.79 |
| cgr-miR-148a | 131,378 | 21,380.75 | cgr-miR-148a | 144,213 | 16,767.43 | cgr-miR-148a | 131,046 | 19,380.79 |
| eca-miR-148a | 131,378 | 21,380.75 | eca-miR-148a | 144,213 | 16,767.43 | eca-miR-148a | 131,046 | 19,380.79 |
| hsa-miR-148a-3p | 131,378 | 21,380.75 | hsa-miR-148a-3p | 144,213 | 16,767.43 | hsa-miR-148a-3p | 131,046 | 19,380.79 |
| mdo-miR-148-3p | 131,378 | 21,380.75 | mdo-miR-148-3p | 144,213 | 16,767.43 | mdo-miR-148-3p | 131,046 | 19,380.79 |
| mml-miR-148a-3p | 131,378 | 21,380.75 | mml-miR-148a-3p | 144,213 | 16,767.43 | mml-miR-148a-3p | 131,046 | 19,380.79 |
| oar-miR-148a | 131,378 | 21,380.75 | oar-miR-148a | 144,213 | 16,767.43 | oar-miR-148a | 131,046 | 19,380.79 |
| ppy-miR-148a | 131,378 | 21,380.75 | ppy-miR-148a | 144,213 | 16,767.43 | ppy-miR-148a | 131,046 | 19,380.79 |
| ssc-miR-148a-3p | 131,378 | 21,380.75 | ssc-miR-148a-3p | 144,213 | 16,767.43 | ssc-miR-148a-3p | 131,046 | 19,380.79 |
| ptr-miR-148a | 131,336 | 21,373.91 | ptr-miR-148a | 144,189 | 16,764.64 | ptr-miR-148a | 131,017 | 19,376.5 |
| ppa-miR-148a | 130,305 | 21,206.12 | ppa-miR-148a | 143,467 | 16,680.69 | ppa-miR-148a | 130,182 | 19,253.01 |
| cfa-miR-148a | 128,867 | 20,972.1 | cfa-miR-148a | 142,161 | 16,528.84 | cfa-miR-148a | 128,908 | 19,064.59 |
| ocu-miR-148a-3p | 128,867 | 20,972.1 | ocu-miR-148a-3p | 142,161 | 16,528.84 | ocu-miR-148a-3p | 128,908 | 19,064.59 |
| tch-miR-148a-3p | 128,867 | 20,972.1 | tch-miR-148a-3p | 142,161 | 16,528.84 | tch-miR-148a-3p | 128,908 | 19,064.59 |
| d30 | d60 | d90 | ||||||
|---|---|---|---|---|---|---|---|---|
| miRNA ID | Count | TPM | miRNA ID | Count | TPM | miRNA ID | Count | TPM |
| novel miR-249 | 683 | 111.1529 | novel miR-249 | 1171 | 136.1504 | novel miR-249 | 1008 | 149.0762 |
| novel miR-77 | 654 | 106.4334 | novel miR-77 | 782 | 90.92195 | novel miR-77 | 684 | 101.1588 |
| novel miR-93 | 274 | 44.59137 | novel miR-93 | 521 | 60.57588 | novel miR-166 | 457 | 67.58711 |
| novel miR-15 | 187 | 30.4328 | novel miR-15 | 311 | 36.1595 | novel miR-267 | 456 | 67.43922 |
| novel miR-114 | 177 | 28.80537 | novel miR-114 | 207 | 24.06758 | novel miR-5 | 456 | 67.43922 |
| novel miR-6 | 138 | 22.45843 | novel miR-57 | 164 | 19.06803 | novel miR-86 | 456 | 67.43922 |
| novel miR-150 | 121 | 19.69181 | novel miR-21 | 118 | 13.71968 | novel miR-93 | 413 | 61.07982 |
| novel miR-57 | 102 | 16.59971 | novel miR-150 | 115 | 13.37088 | novel miR-52 | 290 | 42.88898 |
| novel miR-91 | 102 | 16.59971 | novel miR-44 | 115 | 13.37088 | novel miR-15 | 226 | 33.42382 |
| novel miR-48 | 101 | 16.43696 | novel miR-10 | 112 | 13.02207 | novel miR-114 | 183 | 27.06442 |
| novel miR-84 | 94 | 15.29777 | novel miR-91 | 108 | 12.557 | novel miR-57 | 142 | 21.00081 |
| novel miR-83 | 91 | 14.80954 | novel miR-48 | 102 | 11.85939 | novel miR-94 | 118 | 17.45138 |
| novel miR-72 | 64 | 10.4155 | novel miR-265 | 98 | 11.39431 | novel miR-48 | 114 | 16.85981 |
| novel miR-193 | 62 | 10.09002 | novel miR-84 | 98 | 11.39431 | novel miR-6 | 114 | 16.85981 |
| novel miR-21 | 61 | 9.927275 | novel miR-247 | 82 | 9.534016 | novel miR-150 | 109 | 16.12034 |
| novel miR-94 | 60 | 9.764533 | novel miR-41 | 77 | 8.952673 | novel miR-265 | 100 | 14.7893 |
| novel miR-71 | 57 | 9.276307 | novel miR-107 | 76 | 8.836405 | novel miR-91 | 93 | 13.75405 |
| novel miR-107 | 55 | 8.950822 | novel miR-83 | 76 | 8.836405 | novel miR-81 | 82 | 12.12723 |
| novel miR-41 | 55 | 8.950822 | novel miR-71 | 68 | 7.906257 | novel miR-44 | 72 | 10.6483 |
| novel miR-90 | 49 | 7.974369 | novel miR-6 | 64 | 7.441183 | novel miR-72 | 72 | 10.6483 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; He, C.; Bao, C.; Li, Z.; Jin, W.; Li, C.; Chen, Y. MiRNA Profiling and Its Potential Roles in Rapid Growth of Velvet Antler in Gansu Red Deer (Cervus elaphus kansuensis). Genes 2023, 14, 424. https://doi.org/10.3390/genes14020424
Zhang Z, He C, Bao C, Li Z, Jin W, Li C, Chen Y. MiRNA Profiling and Its Potential Roles in Rapid Growth of Velvet Antler in Gansu Red Deer (Cervus elaphus kansuensis). Genes. 2023; 14(2):424. https://doi.org/10.3390/genes14020424
Chicago/Turabian StyleZhang, Zhenxiang, Caixia He, Changhong Bao, Zhaonan Li, Wenjie Jin, Changzhong Li, and Yanxia Chen. 2023. "MiRNA Profiling and Its Potential Roles in Rapid Growth of Velvet Antler in Gansu Red Deer (Cervus elaphus kansuensis)" Genes 14, no. 2: 424. https://doi.org/10.3390/genes14020424
APA StyleZhang, Z., He, C., Bao, C., Li, Z., Jin, W., Li, C., & Chen, Y. (2023). MiRNA Profiling and Its Potential Roles in Rapid Growth of Velvet Antler in Gansu Red Deer (Cervus elaphus kansuensis). Genes, 14(2), 424. https://doi.org/10.3390/genes14020424







