Increased On-Target Rate and Risk of Concatemerization after CRISPR-Enhanced Targeting in ES Cells
Abstract
1. Introduction
2. Material and Methods
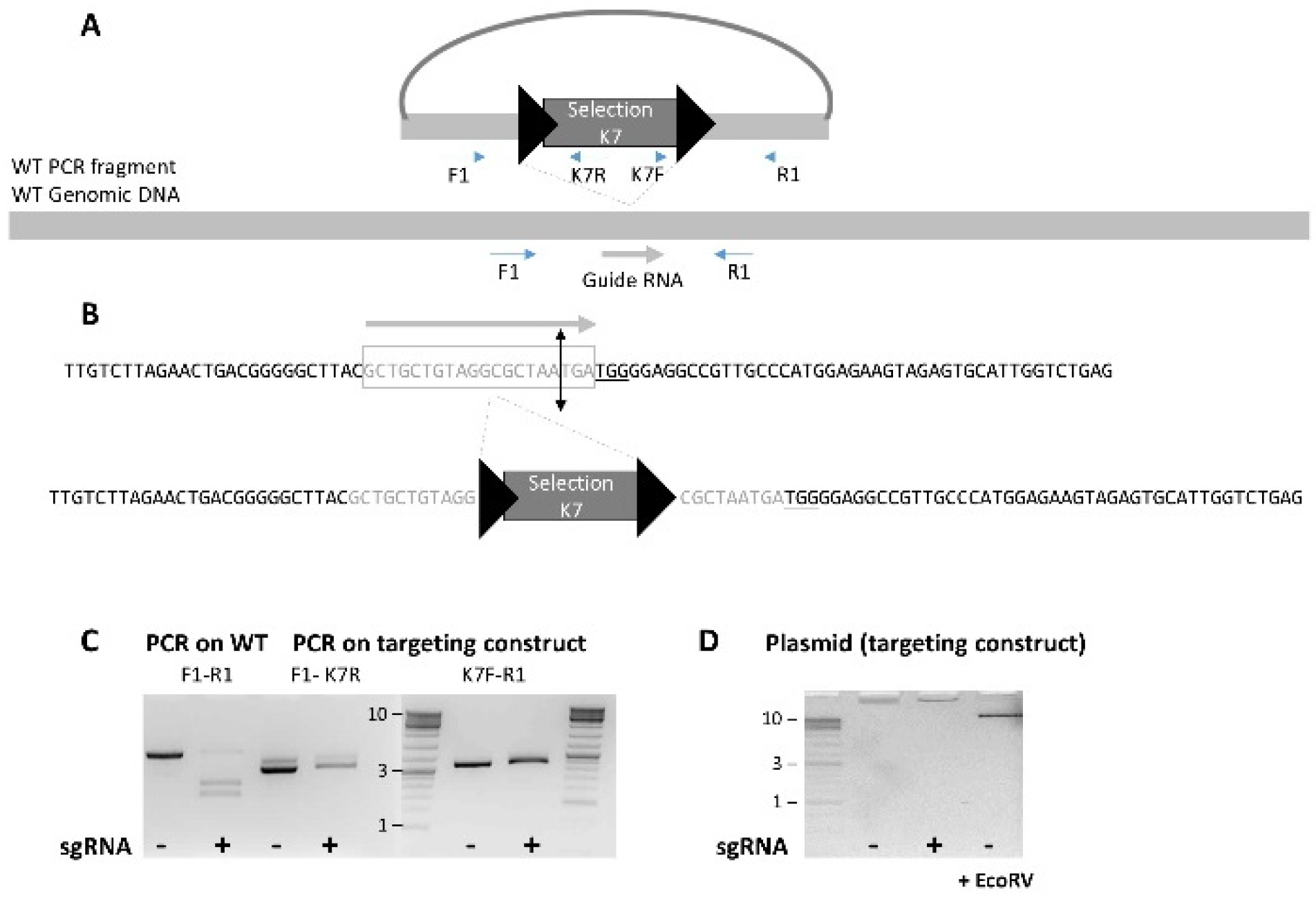
2.1. Selection of gRNAs and CRISPR/Cas9 Vector
2.2. Embryonic Stem Cell Techniques
2.2.1. Embryonic Stem Cell Culture
2.2.2. Electroporation and Selection
2.2.3. Cell Lysis
2.3. Embryonic Stem Cell Validation
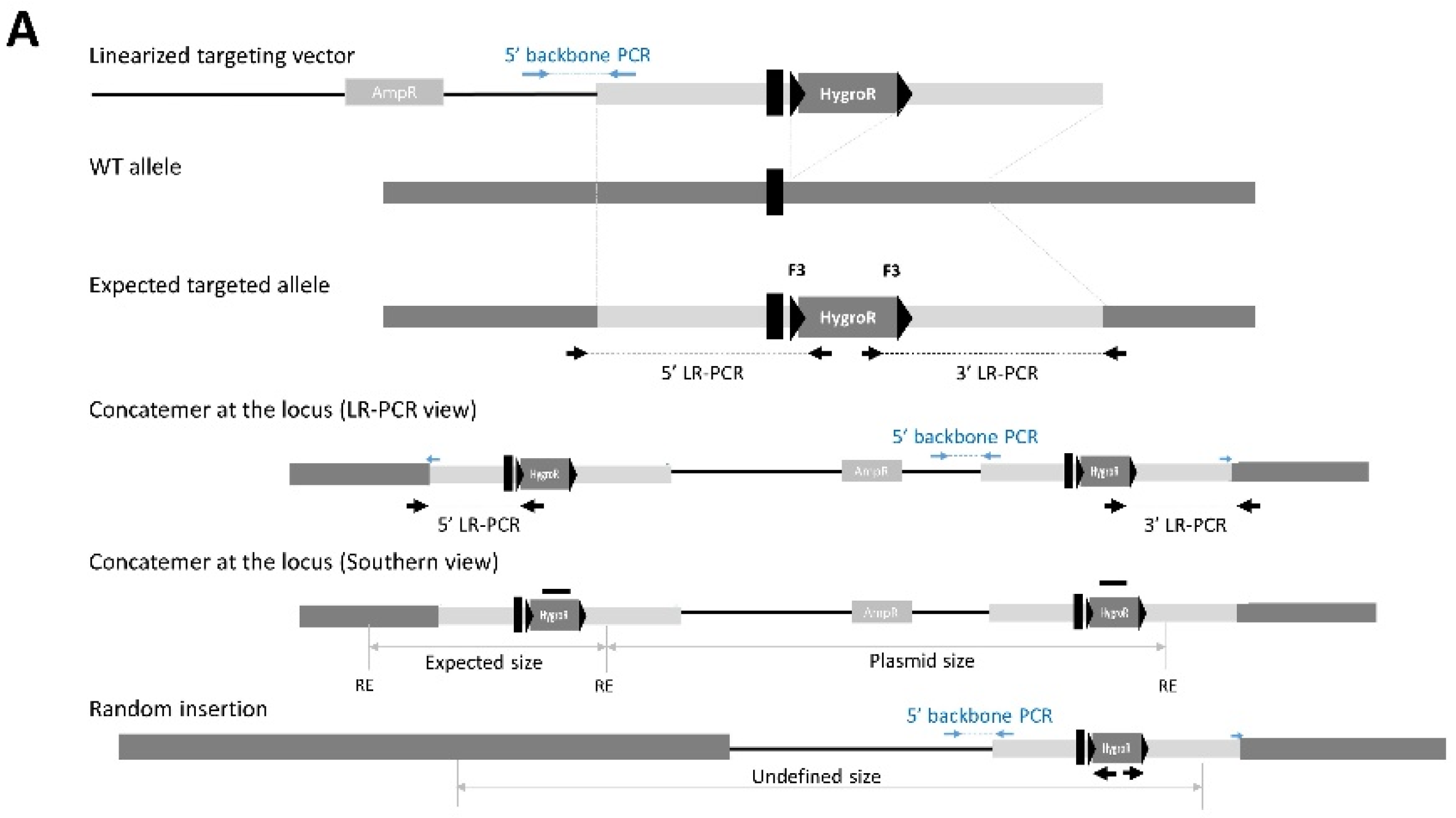
2.3.1. Long-Range PCR
Design of LR-PCR Genotyping Primers
Long-Range PCR Conditions
2.3.2. Southern Blotting
3. Results
3.1. Outcome of the Use of CRISPR/Cas9
3.1.1. Increased Homologous Recombination Frequency in the Presence of a Plasmid Expressing Cas9 and a Specific Guide RNA
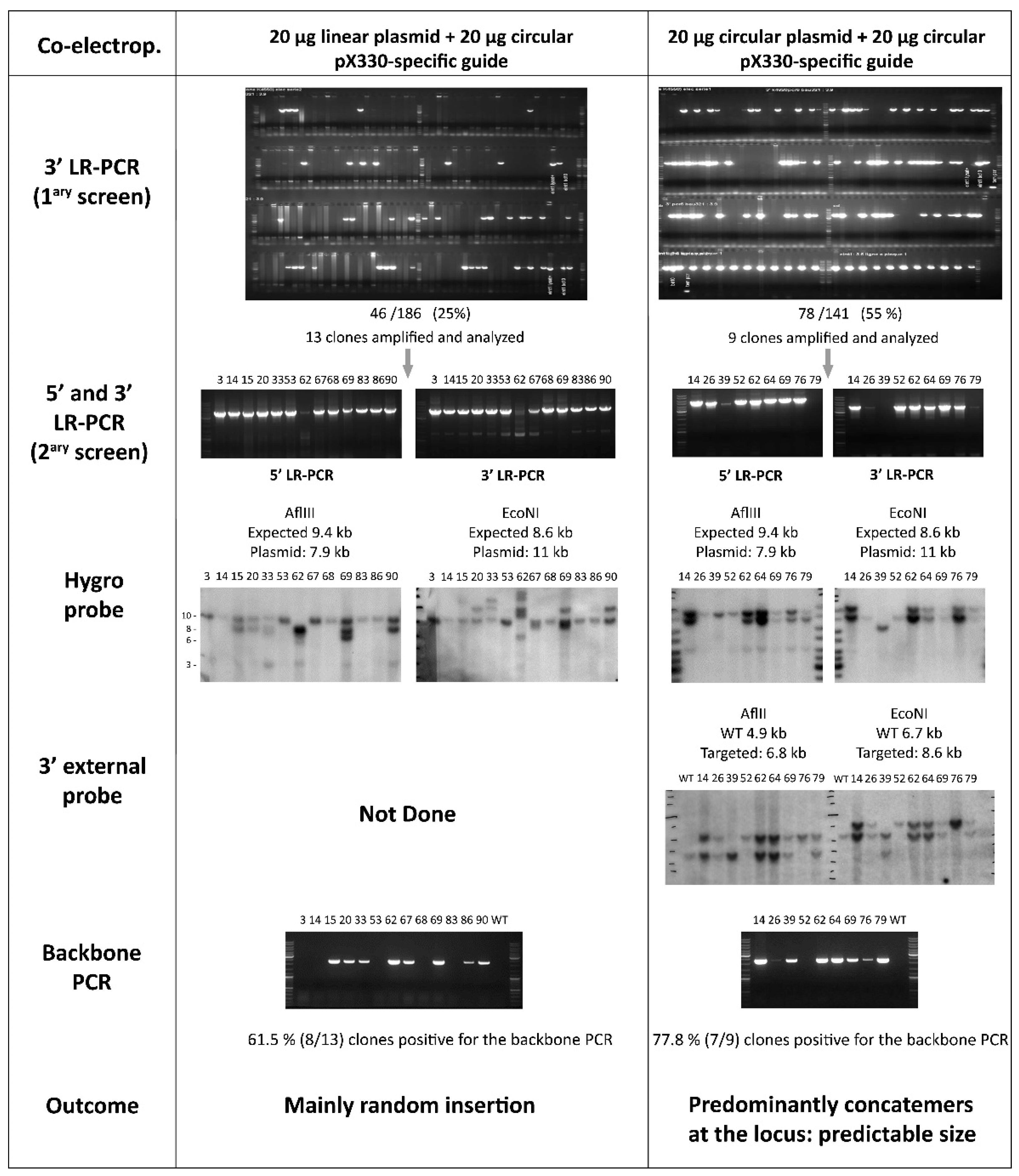
3.1.2. Linear Versus Circular Targeting Construct
3.1.3. An Unexpected and Specific Pattern
3.2. The Reduction of Homology Arm Size Does Not Affect Homologous Recombination Frequency
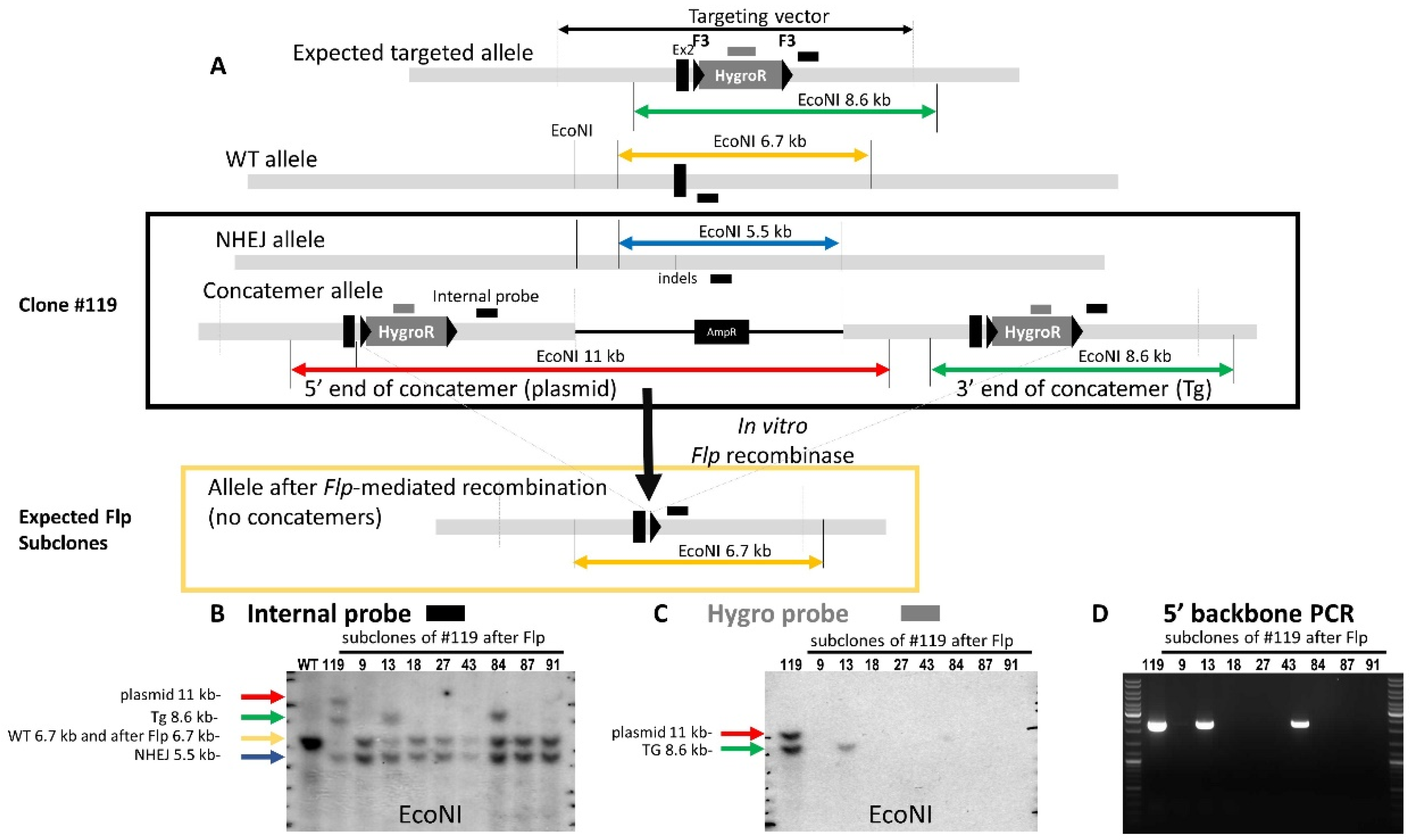
3.3. Confirmation of On-Target Concatemer(s)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurumurthy, C.B.; O’Brien, A.R.; Quadros, R.M.; Adams, J.; Alcaide, P.; Ayabe, S.; Ballard, J.; Batra, S.K.; Beauchamp, M.-C.; Becker, K.A.; et al. Reproducibility of CRISPR-Cas9 methods for generation of conditional mouse alleles: A multi-center evaluation. Genome Biol. 2019, 20, 171. [Google Scholar] [CrossRef]
- Gennequin, B.; Otte, D.-M.; Zimmer, A. CRISPR/Cas-induced double-strand breaks boost the frequency of gene replacements for humanizing the mouse Cnr2 gene. Biochem. Biophys. Res. Commun. 2013, 441, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, Y.; Propson, N.E.; Howden, S.E.; Chu, L.-F.; Sontheimer, E.J.; Thomson, J.A. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 2013, 110, 15644–15649. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Staahl, B.T.; Alla, R.K.; Doudna, J.A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife 2014, 3, e04766. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, J.; Xiong, M.; Petersen, A.J.; Dong, Y.; Tao, Y.; Huang, C.T.-L.; Du, Z.; Zhang, S.-C. Engineering Human Stem Cell Lines with Inducible Gene Knockout using CRISPR/Cas9. Cell Stem Cell 2015, 17, 233–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Vanoli, F.; LaRocque, J.R.; Krawczyk, P.M.; Jasin, M. Biallelic targeting of expressed genes in mouse embryonic stem cells using the Cas9 system. Methods 2014, 69, 171–178. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Shivalila, C.S.; Dawlaty, M.M.; Cheng, A.W.; Zhang, F.; Jaenisch, R. One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas-Mediated Genome Engineering. Cell 2013, 153, 910–918. [Google Scholar] [CrossRef]
- Schick, J.A.; Seisenberger, C.; Beig, J.; Bürger, A.; Iyer, V.; Maier, V.; Perera, S.; Rosen, B.; Skarnes, W.C.; Wurst, W. CRISPR-Cas9 enables conditional mutagenesis of challenging loci. Sci. Rep. 2016, 6, 32326. [Google Scholar] [CrossRef]
- Lindner, L.; Cayrou, P.; Rosahl, T.W.; Zhou, H.H.; Birling, M.-C.; Herault, Y.; Pavlovic, G. Droplet digital PCR or quantitative PCR for in-depth genomic and functional validation of genetically altered rodents. Methods 2021, 191, 107–119. [Google Scholar] [CrossRef]
- Sailer, S.; Coassin, S.; Lackner, K.; Fischer, C.; McNeill, E.; Streiter, G.; Kremser, C.; Maglione, M.; Green, C.M.; Moralli, D.; et al. When the genome bluffs: A tandem duplication event during generation of a novel Agmo knockout mouse model fools routine genotyping. Cell Biosci. 2021, 11, 54. [Google Scholar] [CrossRef]
- de Vree, P.J.; de Wit, E.; Yilmaz, M.; van de Heijning, M.; Klous, P.; Verstegen, M.J.A.M.; Wan, Y.; Teunissen, H.; Krijger, P.H.L.; Geevan, G.; et al. Targeted sequencing by proximity ligation for comprehensive variant detection and local haplotyping. Nat. Biotechnol. 2014, 32, 1019–1025. [Google Scholar] [CrossRef]
- Haeussler, M.; Schönig, K.; Eckert, H.; Eschstruth, A.; Mianné, J.; Renaud, J.-B.; Schneider-Maunoury, S.; Shkumatava, A.; Teboul, L.; Kent, J.; et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Wang, Y.; Dausman, J.; Jaenisch, R. A transgenic mouse strain expressing four drug-selectable marker genes. Nucleic Acids Res. 1997, 25, 3745–3746. [Google Scholar] [CrossRef] [PubMed]
- Codner, G.F.; Erbs, V.; Loeffler, J.; Chessum, L.; Caulder, A.; Jullien, N.; Wells, S.; Birling, M.-C.; Teboul, L. Universal Southern blot protocol with cold or radioactive probes for the validation of alleles obtained by homologous recombination. Methods 2021, 191, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Scekic-Zahirovic, J.; Sendscheid, O.; El Oussini, H.; Jambeau, M.; Sun, Y.; Mersmann, S.; Wagner, M.; Dieterlé, S.; Sinniger, J.; Dirrig-Grosch, S.; et al. Toxic gain of function from mutant FUS protein is crucial to trigger cell autonomous motor neuron loss. EMBO J. 2016, 35, 1077–1097. [Google Scholar] [CrossRef]
- Bailly, J.; Del Rossi, N.; Runtz, L.; Li, J.-J.; Park, D.; Scherrer, G.; Tanti, A.; Birling, M.-C.; Darcq, E.; Kieffer, B.L. Targeting Morphine-Responsive Neurons: Generation of a Knock-In Mouse Line Expressing Cre Recombinase from the Mu-Opioid Receptor Gene Locus. Eneuro 2020, 7, ENEURO.0433-19.2020. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; German, B.; Chraa, D.; Braud, A.; Hugel, C.; Meyer, P.; Davidson, G.; Laurette, P.; Mengus, G.; Flatter, E.; et al. Keratinocyte-derived cytokine TSLP promotes growth and metastasis of melanoma by regulating the tumor-associated immune microenvironment. JCI Insight 2022, 7, e161438. [Google Scholar] [CrossRef]
- Bodakuntla, S.; Yuan, X.; Genova, M.; Gadadhar, S.; Leboucher, S.; Birling, M.-C.; Klein, D.; Martini, R.; Janke, C.; Magiera, M.M. Distinct roles of alpha- and beta-tubulin polyglutamylation in controlling axonal transport and in neurodegeneration. EMBO J. 2022, 41, e111373. [Google Scholar] [CrossRef]
- Hasty, P.; Rivera-Pérez, J.; Bradley, A. The role and fate of DNA ends for homologous recombination in embryonic stem cells. Mol. Cell. Biol. 1992, 12, 2464–2474. [Google Scholar]
- Codner, G.F.; Lindner, L.; Caulder, A.; Wattenhofer-Donzé, M.; Radage, A.; Mertz, A.; Eisenmann, B.; Mianné, J.; Evans, E.P.; Beechey, C.V.; et al. Aneuploidy screening of embryonic stem cell clones by metaphase karyotyping and droplet digital polymerase chain reaction. BMC Cell Biol. 2016, 17, 30. [Google Scholar] [CrossRef]
- Collias, D.; Beisel, C.L. CRISPR technologies and the search for the PAM-free nuclease. Nat. Commun. 2021, 12, 555. [Google Scholar] [CrossRef]
- Auer, T.O.; Duroure, K.; De Cian, A.; Concordet, J.-P.; Del Bene, F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 2013, 24, 142–153. [Google Scholar] [CrossRef]
- Skryabin, B.V.; Kummerfeld, D.-M.; Gubar, L.; Seeger, B.; Kaiser, H.; Stegemann, A.; Roth, J.; Meuth, S.G.; Pavenstädt, H.; Sherwood, J.; et al. Pervasive head-to-tail insertions of DNA templates mask desired CRISPR-Cas9–mediated genome editing events. Sci. Adv. 2020, 6, eaax2941. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Battulin, N. Concatenation of Transgenic DNA: Random or Orchestrated? Genes 2021, 12, 1969. [Google Scholar] [CrossRef]
- Rohan, R.M.; King, D.; Frels, W.I. Direct sequencing of PCR-amplified junction fragments from tandemly repeated transgenes. Nucleic Acids Res. 1990, 18, 6089–6095. [Google Scholar] [CrossRef]
- Pesenti, E.; Liskovykh, M.; Okazaki, K.; Mallozzi, A.; Reid, C.; Abad, M.A.; Jeyaprakash, A.A.; Kouprina, N.; Larionov, V.; Masumoto, H.; et al. Analysis of Complex DNA Rearrangements during Early Stages of HAC Formation. ACS Synth. Biol. 2020, 9, 3267–3287. [Google Scholar] [CrossRef]
- Brinster, R.L.; Chen, H.Y.; E Trumbauer, M.; Yagle, M.K.; Palmiter, R.D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc. Natl. Acad. Sci. USA 1985, 82, 4438–4442. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Sasaki, H.; Seki, R.; Sakaki, Y. Mechanism of chromosomal integration of transgenes in microinjected mouse eggs: Sequence analysis of genome-transgene and transgene-transgene junctions at two loci. Gene 1993, 128, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Folger, K.R.; Wong, E.A.; Wahl, G.; Capecchi, M.R. Patterns of integration of DNA microinjected into cultured mammalian cells: Evidence for homologous recombination between injected plasmid DNA molecules. Mol. Cell. Biol. 1982, 2, 1372–1387. [Google Scholar] [PubMed]
- Folger, K.R.; Thomas, K.; Capecchi, M.R. Nonreciprocal exchanges of information between DNA duplexes coinjected into mammalian cell nuclei. Mol. Cell Biol. 1985, 5, 59–69. [Google Scholar] [PubMed]
- Suzuki, K.; Tsunekawa, Y.; Hernandez-Benitez, R.; Wu, J.; Zhu, J.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Li, Z.; et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 2016, 540, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.D.; Usher, C.L.; McCarroll, S.A. Analyzing Copy Number Variation with Droplet Digital PCR. Methods Mol. Biol. 2018, 1768, 143–160. [Google Scholar] [PubMed]
- Mazaika, E.; Homsy, J. Digital Droplet PCR: CNV Analysis and Other Applications. Curr. Protoc. Hum. Genet. 2014, 82, 7.24.1–7.24.13. [Google Scholar] [CrossRef] [PubMed]

| Selection Cassette | Universal Forward Primer for 3′ LR-PCR | Universal Reverse Primer for 5′ LR-PCR |
|---|---|---|
| Neomycin resistance (NeoR) | AGGGGCTCGCGCCAGCCGAACTGTT | GCGGCCGGAGAACCTGCGTGCAATC |
| Hygromycin resistance (HygroR) | CCGTCTGGACCGATGGCTGTGTAG | CTGCATCAGGTCGGAGACGCTGTCG |
| 10 µg + 10 µg | 5 µg + 5 µg | 2.5 µg + 2.5 µg | 1 µg + 1 µg | |
| Project 1: Positive clones by long-range PCR (%) | 67/93 (72%) | 79/93 (84.9%) | 41/48 (85.4%) | 35/40 (87.5%) |
| Project 2: Positive clones by long-range PCR (%) | 18/93 (19.4%) | ND | ND | 5/93 (5.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erbs, V.; Lorentz, R.; Eisenman, B.; Schaeffer, L.; Luppi, L.; Lindner, L.; Hérault, Y.; Pavlovic, G.; Wattenhofer-Donzé, M.; Birling, M.-C. Increased On-Target Rate and Risk of Concatemerization after CRISPR-Enhanced Targeting in ES Cells. Genes 2023, 14, 401. https://doi.org/10.3390/genes14020401
Erbs V, Lorentz R, Eisenman B, Schaeffer L, Luppi L, Lindner L, Hérault Y, Pavlovic G, Wattenhofer-Donzé M, Birling M-C. Increased On-Target Rate and Risk of Concatemerization after CRISPR-Enhanced Targeting in ES Cells. Genes. 2023; 14(2):401. https://doi.org/10.3390/genes14020401
Chicago/Turabian StyleErbs, Valérie, Romain Lorentz, Benjamin Eisenman, Laurence Schaeffer, Laurence Luppi, Loic Lindner, Yann Hérault, Guillaume Pavlovic, Marie Wattenhofer-Donzé, and Marie-Christine Birling. 2023. "Increased On-Target Rate and Risk of Concatemerization after CRISPR-Enhanced Targeting in ES Cells" Genes 14, no. 2: 401. https://doi.org/10.3390/genes14020401
APA StyleErbs, V., Lorentz, R., Eisenman, B., Schaeffer, L., Luppi, L., Lindner, L., Hérault, Y., Pavlovic, G., Wattenhofer-Donzé, M., & Birling, M.-C. (2023). Increased On-Target Rate and Risk of Concatemerization after CRISPR-Enhanced Targeting in ES Cells. Genes, 14(2), 401. https://doi.org/10.3390/genes14020401







