Phenotypic Assessment of Pathogenic Variants in GNAO1 and Response to Caffeine in C. elegans Models of the Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Gene-Edited C. elegans Strains
2.2. Sensitivity to Aldicarb, Levamisole and PTZ
2.3. Behavioral Assays
2.4. Response to Caffeine and Istradefylline
2.5. Statistics
3. Results
3.1. Generation of Gene-Edited C. elegans Strains
3.2. Biallelic GNAO1 Mutations Result in Loss of Gαo Function in C. elegans
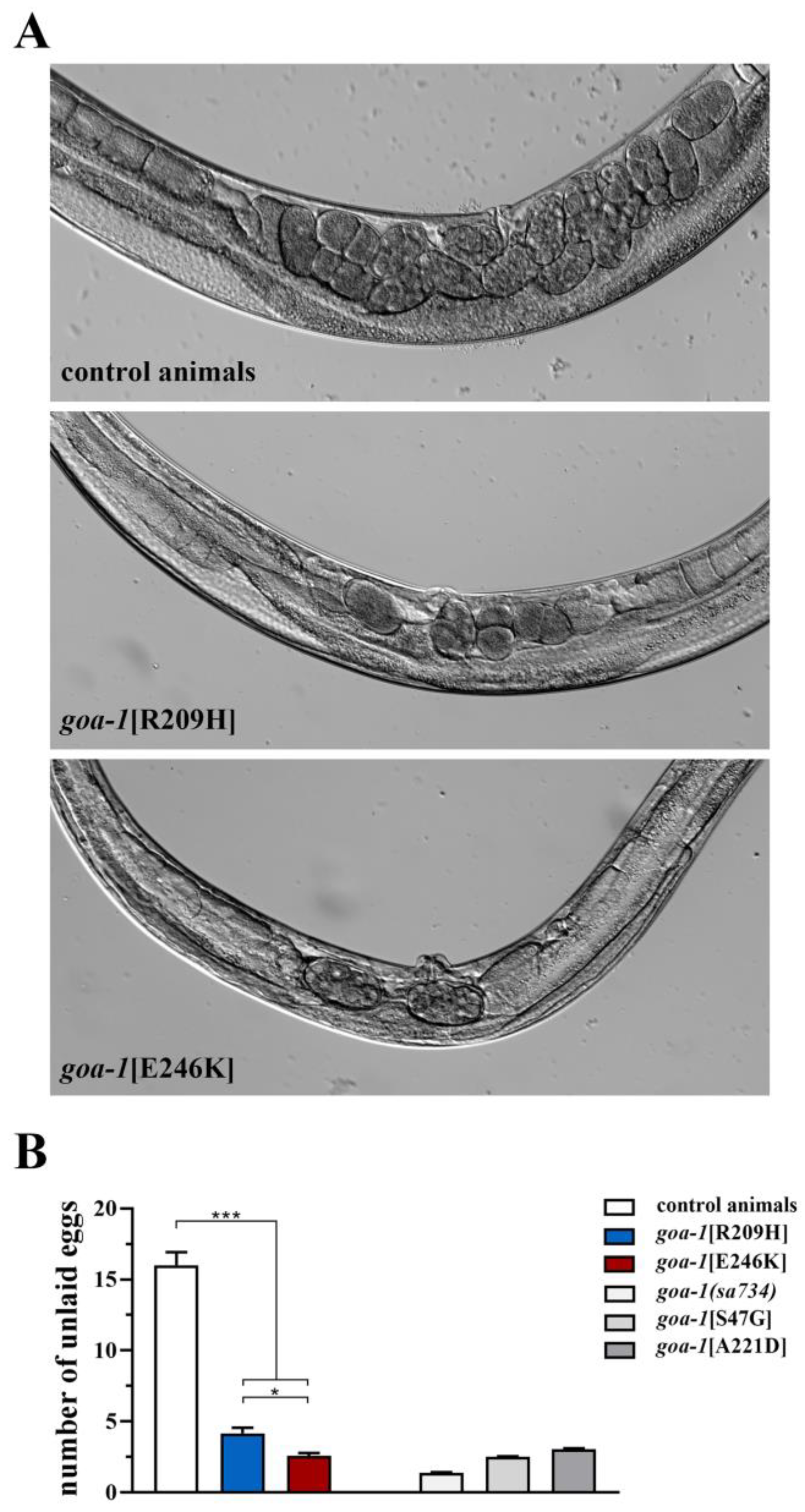
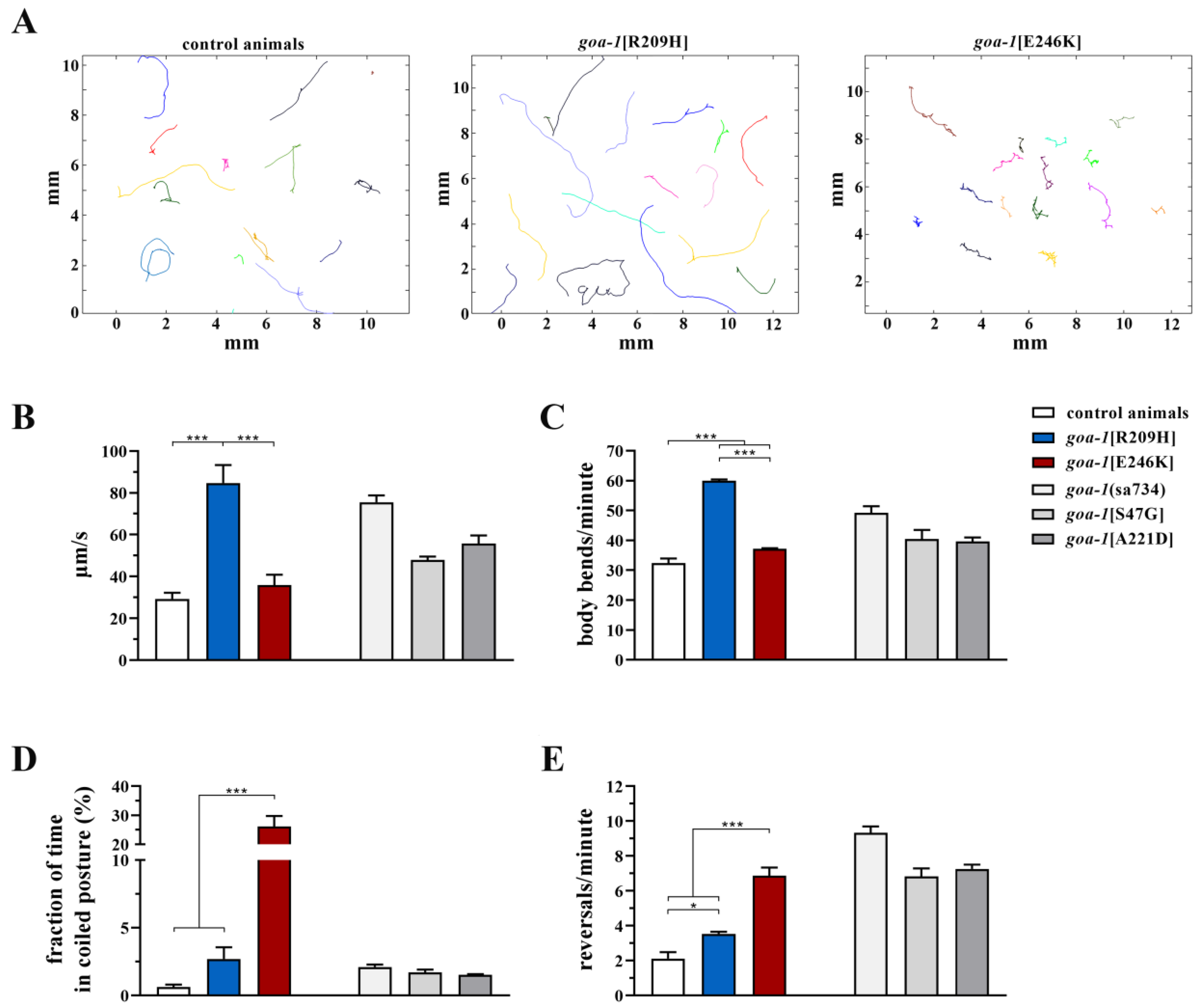
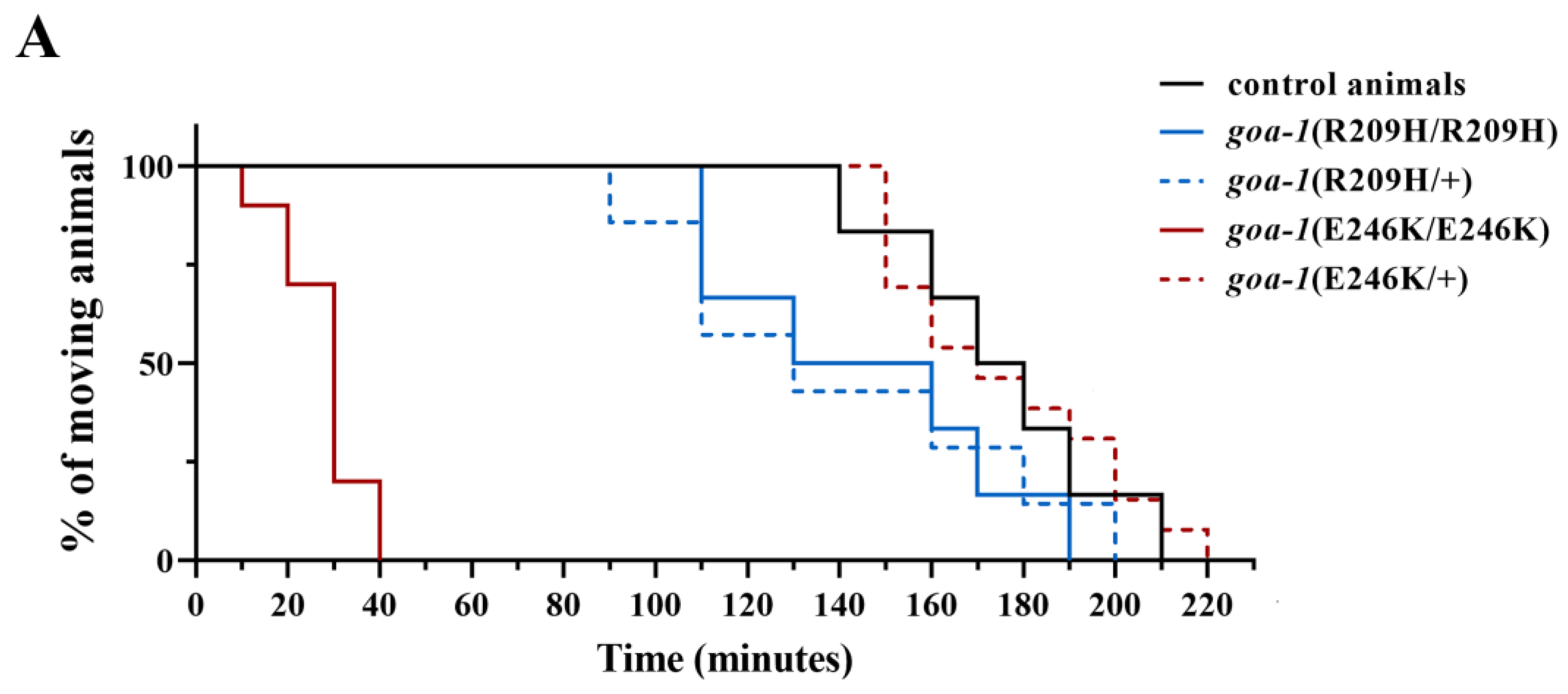
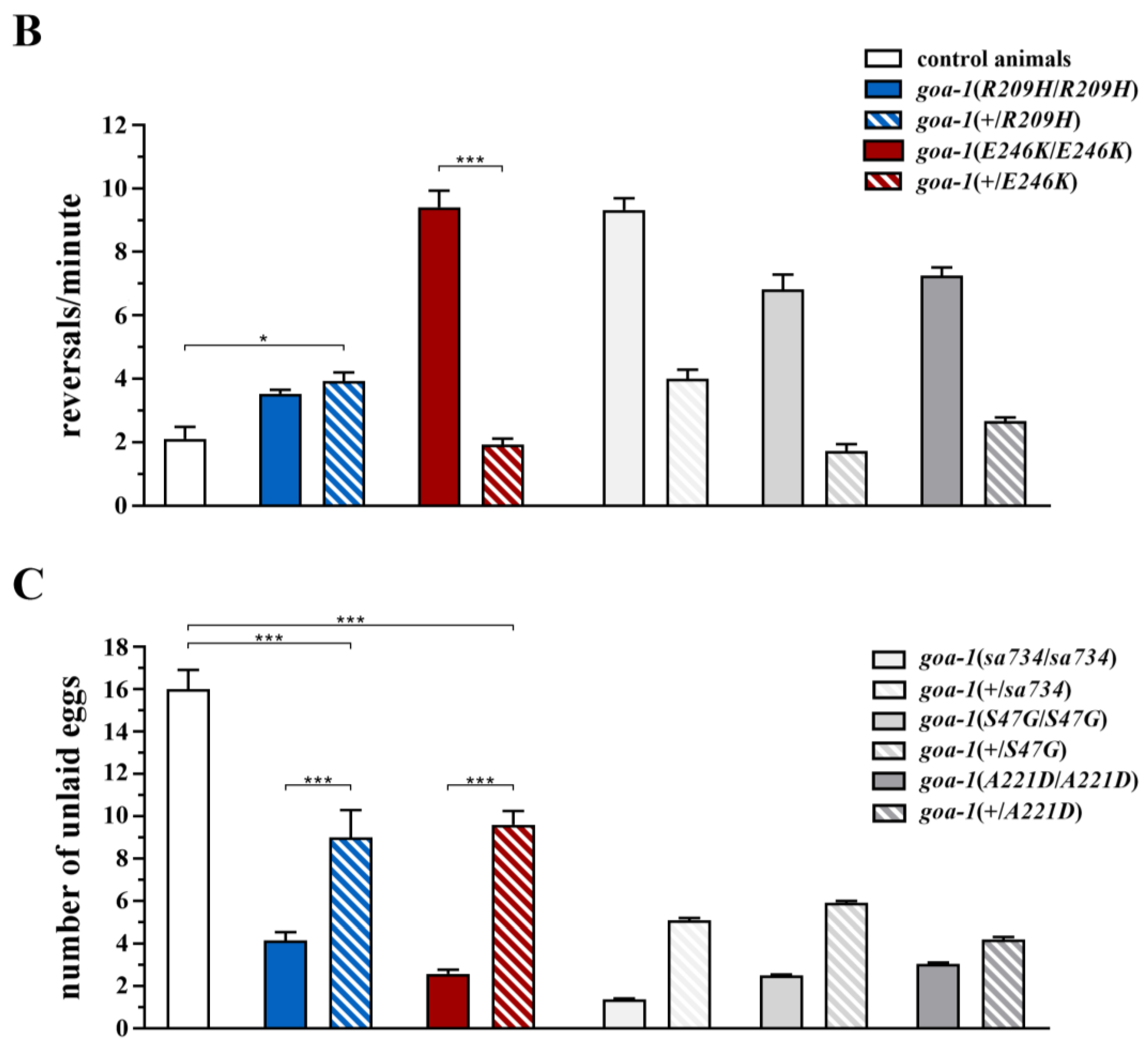
3.3. Heterozygous GNAO1 Mutations Display a Cell-Specific DN Behavior in C. elegans Neurons
3.4. Response to Caffeine and Istradefylline
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sternweis, P.C.; Robishaw, J.D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J. Biol. Chem. 1984, 259, 13806–13813. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, W.J.; Quan, F.; Goldsmith, P.; Unson, C.; Spiegel, A.; Forte, M. Immunolocalization of G protein alpha-subunits in the Drosophila CNS. J. Neurosci. 1990, 10, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Strittmatter, S.M.; Valenzuela, D.; Kennedy, T.E.; Neer, E.J.; Fishman, M.C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature 1990, 344, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Wettschureck, N.; Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Gold, M.S.; Boulay, G.; Spicher, K.; Peyton, M.; Brabet, P.; Srinivasan, Y.; Rudolph, U.; Ellison, G.; Birnbaumer, L. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc. Natl. Acad. Sci. USA 1998, 95, 3269–3274. [Google Scholar] [CrossRef]
- Silachev, D.; Koval, A.; Savitsky, M.; Padmasola, G.; Quairiaux, C.; Thorel, F.; Katanaev, V.L. Mouse models characterize GNAO1 encephalopathy as a neurodevelopmental disorder leading to motor anomalies: From a severe G203R to a milder C215Y mutation. Acta Neuropathol. Commun. 2022, 10, 9. [Google Scholar] [CrossRef]
- Muntean, B.S.; Masuho, I.; Dao, M.; Sutton, L.P.; Zucca, S.; Iwamoto, H.; Patil, D.N.; Wang, D.; Birnbaumer, L.; Blakely, R.D.; et al. Gαo is a major determinant of cAMP signaling in the pathophysiology of movement disorders. Cell Rep. 2021, 34, 108718. [Google Scholar] [CrossRef]
- Nakamura, K.; Kodera, H.; Akita, T.; Shiina, M.; Kato, M.; Hoshino, H.; Terashima, H.; Osaka, H.; Nakamura, S.; Tohyama, J.; et al. De Novo mutations in GNAO1, encoding a Gαo subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am. J. Hum. Genet. 2013, 93, 496–505. [Google Scholar] [CrossRef]
- Saitsu, H.; Fukai, R.; Ben-Zeev, B.; Sakai, Y.; Mimaki, M.; Okamoto, N.; Suzuki, Y.; Monden, Y.; Saito, H.; Tziperman, B.; et al. Phenotypic spectrum of GNAO1 variants: Epileptic encephalopathy to involuntary movements with severe developmental delay. Eur. J. Hum. Genet. 2016, 24, 129–134. [Google Scholar] [CrossRef]
- Danti, F.R.; Galosi, S.; Romani, M.; Montomoli, M.; Carss, K.J.; Raymond, F.L.; Parrini, E.; Bianchini, C.; McShane, T.; Dale, R.C.; et al. GNAO1 encephalopathy: Broadening the phenotype and evaluating treatment and outcome. Neurol. Genet. 2017, 3, e143. [Google Scholar] [CrossRef]
- Feng, H.; Khalil, S.; Neubig, R.R.; Sidiropoulos, C. A mechanistic review on GNAO1-associated movement disorder. Neurobiol. Dis. 2018, 116, 131–141. [Google Scholar] [CrossRef]
- Schirinzi, T.; Garone, G.; Travaglini, L.; Vasco, G.; Galosi, S.; Rios, L.; Castiglioni, C.; Barassi, C.; Battaglia, D.; Gambardella, M.L.; et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: Results from an analytical review. Parkinsonism Relat. Disord. 2019, 61, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Galosi, S.; Pollini, L.; Novelli, M.; Bernardi, K.; Di Rocco, M.; Martinelli, S.; Leuzzi, V. Motor, epileptic, and developmental phenotypes in genetic disorders affecting G protein coupled receptors-cAMP signaling. Front. Neurol. 2022, 13, 886751. [Google Scholar] [CrossRef]
- Abela, L.; Kurian, M.A. Postsynaptic movement disorders: Clinical phenotypes, genotypes, and disease mechanisms. J. Inherit. Metab. Dis. 2018, 41, 1077–1091. [Google Scholar] [CrossRef]
- Wirth, T.; Garone, G.; Kurian, M.A.; Piton, A.; Millan, F.; Telegrafi, A.; Drouot, N.; Rudolf, G.; Chelly, J.; Marks, W.; et al. Highlighting the Dystonic Phenotype Related to GNAO1. Mov. Disord. 2022, 37, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Krenn, M.; Sommer, R.; Sycha, T.; Zech, M. GNAO1 Haploinsufficiency Associated with a Mild Delayed-Onset Dystonia Phenotype. Mov. Disord. 2022, 37, 2464–2466. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dao, M.; Muntean, B.S.; Giles, A.C.; Martemyanov, K.A.; Grill, B. Genetic modeling of GNAO1 disorder delineates mechanisms of Gαo dysfunction. Hum. Mol. Genet. 2022, 31, 510–522. [Google Scholar] [CrossRef]
- Di Rocco, M.; Galosi, S.; Lanza, E.; Tosato, F.; Caprini, D.; Folli, V.; Friedman, J.; Bocchinfuso, G.; Martire, A.; Di Schiavi, E.; et al. Caenorhabditis elegans provides an efficient drug screening platform for GNAO1-related disorders and highlights the potential role of caffeine in controlling dyskinesia. Hum. Mol. Genet. 2022, 31, 929–941. [Google Scholar] [CrossRef]
- Larasati, Y.A.; Savitsky, M.; Koval, A.; Solis, G.P.; Valnohova, J.; Katanaev, V.L. Restoration of the GTPase activity and cellular interactions of Gαo mutants by Zn2+ in GNAO1 encephalopathy models. Sci. Adv. 2022, 8, eabn9350. [Google Scholar] [CrossRef]
- Sulston, J.E.; Hodgkin, J. Methods. In The Nematode Caenorhabditis Elegans; The Community of C. elegans Researchers, Wood, W.B., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1988; pp. 587–606. [Google Scholar]
- Mello, C.C.; Kramer, J.M.; Stinchcomb, D.; Ambros, V. Efficient gene transfer in C. elegans: Extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991, 10, 3959–3970. [Google Scholar] [CrossRef]
- Yang, B.; Schwartz, M.; McJunkin, K. In vivo CRISPR screening for phenotypic targets of the mir-35-42 family in C. elegans. Genes. Dev. 2020, 34, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Paix, A.; Folkmann, A.; Rasoloson, D.; Seydoux, G. High Efficiency, Homology-Directed Genome Editing in Caenorhabditis elegans Using CRISPR-Cas9 Ribonucleoprotein Complexes. Genetics 2015, 201, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, T.R.; Luo, S.; Nonet, M.L. Analysis of Synaptic Transmission in Caenorhabditis elegans Using an Aldicarb-Sensitivity Assay. Nat. Protoc. 2006, 1, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Thapliyal, S.; Babu, K. Pentylenetetrazole (PTZ)-induced Convulsion Assay to Determine GABAergic Defects in Caenorhabditis elegans. Bio. Protoc. 2018, 8, e2989. [Google Scholar] [CrossRef]
- Davis, A.N.; Tanis, J.E. Measuring Caenorhabditis elegans Sensitivity to the Acetylcholine Receptor Agonist Levamisole. J Vis. Exp. 2022, 184. [Google Scholar] [CrossRef]
- Hart, A.C. Behavior. In WormBook: The Online Review of C. elegans Biology. 2006. Available online: http://www.wormbook.org/ (accessed on 10 December 2022).
- Miller, K.G.; Rand, J.B. A role for RIC-8 (Synembryn) and GOA-1 (G(o)alpha) in regulating a subset of centrosome movements during early embryogenesis in Caenorhabditis elegans. Genetics 2000, 156, 1649–1660. [Google Scholar] [CrossRef]
- Gotta, M.; Ahringer, J. Distinct roles for Galpha and Gbetagamma in regulating spindle position and orientation in Caenorhabditis elegans embryos. Nat. Cell Biol. 2001, 3, 297–300. [Google Scholar] [CrossRef]
- Govindan, J.A.; Cheng, H.; Harris, J.E.; Greenstein, D. Gαo/i andGαs signaling function in parallel with the MSP/Eph receptor to control meiotic diapause in C.elegans. Curr. Biol. 2006, 16, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Grill, B.; (University of Washington School of Medicine, Seattle, WA, USA). Personal communication, 2022.
- Robatzek, M.; Thomas, J.H. Calcium/calmodulin-dependent protein kinase II regulates Caenorhabditis elegans locomotion in concert with a Go/Gq signaling network. Genetics 2000, 156, 1069–1082. [Google Scholar] [CrossRef]
- Koelle, M.R. Neurotransmitter signaling through heterotrimeric G proteins: Insights from studies in C. elegans. In The C. elegans Research Community; Worm Book: Tel Aviv, Israel, 2018; pp. 1–52. [Google Scholar]
- Mendel, J.E.; Korswagen, H.C.; Liu, K.S.; Hajdu-Cronin, Y.M.; Simon, M.I.; Plasterk, R.H.; Sternberg, P.W. Participation of the protein Go in multiple aspects of behavior in C. elegans. Science 1995, 267, 1652–1655. [Google Scholar] [CrossRef]
- Ségalat, L.; Elkes, D.A.; Kaplan, J.M. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science 1995, 267, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Corson, F.; Keil, W.; Singhal, A.; Bae, A.; Lu, Y.; Liang, Y.; Shaham, S. Glutamate spillover in C. elegans triggers repetitive behavior through presynaptic activation of MGL-2/mGluR5. Nat. Commun. 2019, 10, 1882. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.G.; Emerson, M.D.; Rand, J.B. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron 1999, 24, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Zhen, M.; Samuel, A.D. C. elegans locomotion: Small circuits, complex functions. Curr. Opin. Neurobiol. 2015, 33, 117–126. [Google Scholar] [CrossRef]
- Chen, J.F.; Cunha, R.A. The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signal 2020, 16, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Savitsky, M.; Solis, G.P.; Kryuchkov, M.; Katanaev, V.L. Humanization of Drosophila Gαo to Model GNAO1 Paediatric Encephalopathies. Biomedicines 2020, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Padgett, W.L.; Daly, J.W. Caffeine analogs: Effects on ryanodine-sensitive calcium-release channels and GABAA receptors. Cell. Mole. Neurobiol. 2003, 23, 331–347. [Google Scholar] [CrossRef]
- Boswell-Smith, V.; Spina, D.; Page, C.P. Phosphodiesterase inhibitors. Brit. J. Pharmacol. 2006, 147, S252–S257. [Google Scholar] [CrossRef]
- Saki, M.; Yamada, K.; Koshimura, E.; Sasaki, K.; Kanda, T. In vitro pharmacological profile of the A2A receptor antagonist istradefylline. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Hong, J.H.; Choi, I.Y.; Che, Y.; Lee, J.K.; Yang, S.D.; Song, C.W.; Kang, H.S.; Lee, J.H.; Noh, J.S.; et al. Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase. J. Neurosci. 2002, 22, 7931–7940. [Google Scholar] [CrossRef]
- Feng, H.; Sjögren, B.; Karaj, B.; Shaw, V.; Gezer, A.; Neubig, R.R. Movement disorder in GNAO1 encephalopathy associated with gain-of-function mutations. Neurology. 2017, 89, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Méneret, A.; Gras, D.; McGovern, E.; Roze, E. Caffeine and the Dyskinesia Related to Mutations in the ADCY5 Gene. Ann. Intern. Med. 2019, 171, 439. [Google Scholar] [CrossRef] [PubMed]
- Méneret, A.; Mohammad, S.S.; Cif, L.; Doummar, D.; DeGusmao, C.; Anheim, M.; Barth, M.; Damier, P.; Demonceau, N.; Friedman, J.; et al. Efficacy of Caffeine in ADCY5-Related Dyskinesia: A Retrospective Study. Mov. Disord. 2022, 37, 1294–1298. [Google Scholar] [CrossRef] [PubMed]


| Genotype | Embryonic Lethality (%) | Larval Arrest (%) | Brood Size (% of Controls) | Pvl Phenotype (%) |
|---|---|---|---|---|
| goa-1[WT] | 0.5 1 | 0 | - | 1 |
| goa-1[R209H] | 5 (p < 0.01) 2 | 5 (p < 0.01) | 80 (p < 0.01) | 10 (p < 0.01) |
| goa-1[E246K] | 21 (p < 0.001) | 40 (p < 0.001) | 18 (p < 0.001) | 50 (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Rocco, M.; Galosi, S.; Follo, F.C.; Lanza, E.; Folli, V.; Martire, A.; Leuzzi, V.; Martinelli, S. Phenotypic Assessment of Pathogenic Variants in GNAO1 and Response to Caffeine in C. elegans Models of the Disease. Genes 2023, 14, 319. https://doi.org/10.3390/genes14020319
Di Rocco M, Galosi S, Follo FC, Lanza E, Folli V, Martire A, Leuzzi V, Martinelli S. Phenotypic Assessment of Pathogenic Variants in GNAO1 and Response to Caffeine in C. elegans Models of the Disease. Genes. 2023; 14(2):319. https://doi.org/10.3390/genes14020319
Chicago/Turabian StyleDi Rocco, Martina, Serena Galosi, Francesca C. Follo, Enrico Lanza, Viola Folli, Alberto Martire, Vincenzo Leuzzi, and Simone Martinelli. 2023. "Phenotypic Assessment of Pathogenic Variants in GNAO1 and Response to Caffeine in C. elegans Models of the Disease" Genes 14, no. 2: 319. https://doi.org/10.3390/genes14020319
APA StyleDi Rocco, M., Galosi, S., Follo, F. C., Lanza, E., Folli, V., Martire, A., Leuzzi, V., & Martinelli, S. (2023). Phenotypic Assessment of Pathogenic Variants in GNAO1 and Response to Caffeine in C. elegans Models of the Disease. Genes, 14(2), 319. https://doi.org/10.3390/genes14020319







