Gene Expression and Epigenetic Regulation in the Prefrontal Cortex of Schizophrenia
Abstract
1. Prefrontal Cortex Development and Function
2. Prefrontal Cortical Pathology in Schizophrenia
3. Genetic Background of Schizophrenia
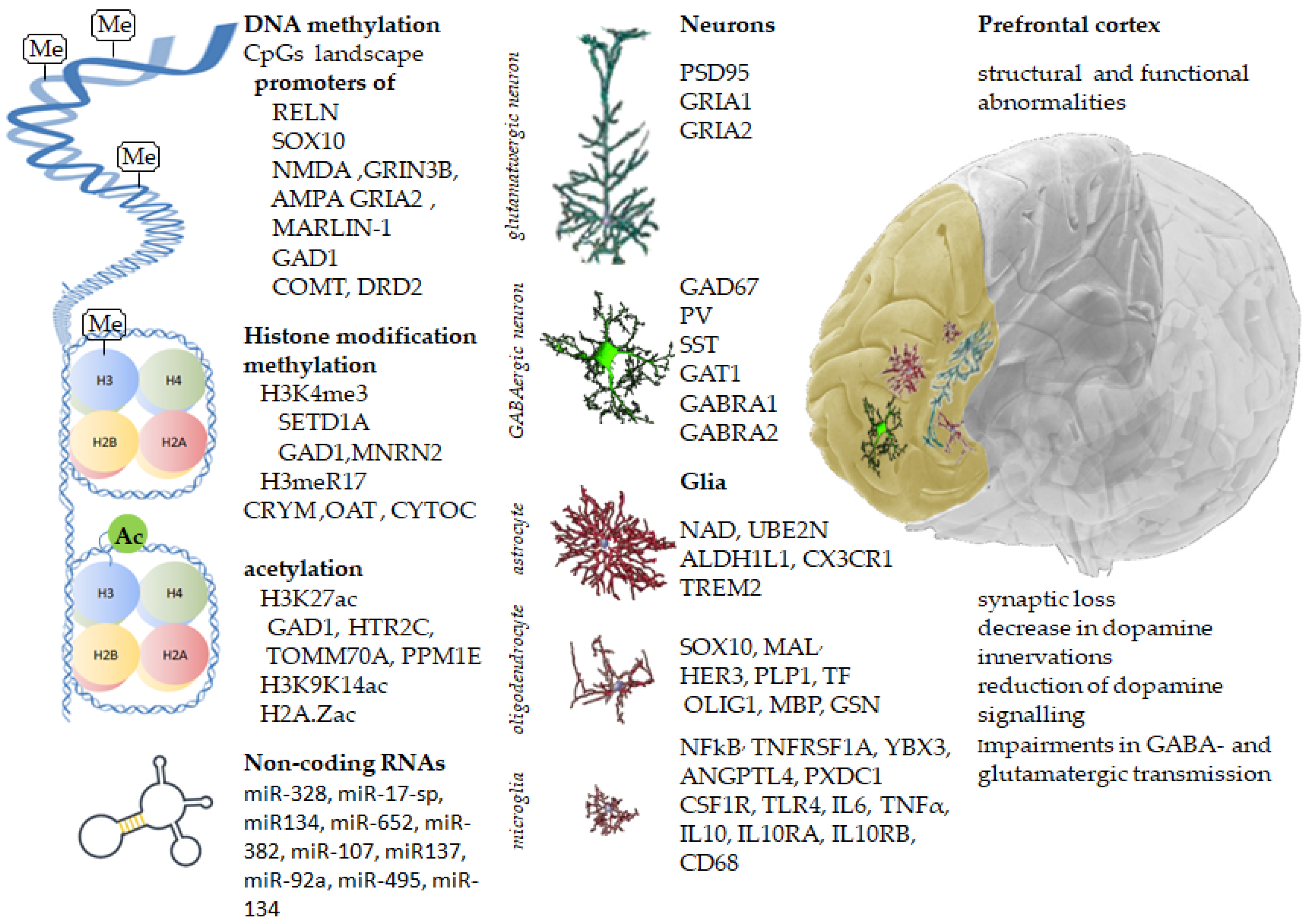
4. Abnormalities in Cortical Gene Expression in Schizophrenia
4.1. Glutamate-Related Genes
4.2. GABA-Related Genes
4.3. Dopamine-Related Genes
4.4. Plasticity-Related Genes
4.5. Myelination-Related Genes
4.6. Metabolic-Related Genes
4.7. Inflammation-Related Genes
5. Epigenetic Regulation
5.1. DNA Methylation
5.2. Histone Modifications
5.2.1. Histone Methylation
5.2.2. Histone Acetylation
5.3. Non-Coding RNAs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlen, M. What constitutes the prefrontal cortex? Science 2017, 358, 478–482. [Google Scholar] [CrossRef]
- Sakurai, T.; Gamo, N.J.; Hikida, T.; Kim, S.H.; Murai, T.; Tomoda, T.; Sawa, A. Converging models of schizophrenia--Network alterations of prefrontal cortex underlying cognitive impairments. Prog. Neurobiol 2015, 134, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Tsubomoto, M.; Kawabata, R.; Zhu, X.; Minabe, Y.; Chen, K.; Lewis, D.A.; Hashimoto, T. Expression of Transcripts Selective for GABA Neuron Subpopulations across the Cortical Visuospatial Working Memory Network in the Healthy State and Schizophrenia. Cereb. Cortex 2019, 29, 3540–3550. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.S.; Mack, N.R.; Shu, Y.; Gao, W.J. Prefrontal GABAergic Interneurons Gate Long-Range Afferents to Regulate Prefrontal Cortex-Associated Complex Behaviors. Front. Neural Circuits 2021, 15, 716408. [Google Scholar] [CrossRef] [PubMed]
- Dienel, S.J.; Lewis, D.A. Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiol. Dis. 2019, 131, 104208. [Google Scholar] [CrossRef]
- Ferguson, B.R.; Gao, W.-J. PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front. Neural Circuits 2018, 12, 37. [Google Scholar] [CrossRef]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic interneurons in the neocortex: From cellular properties to circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef]
- Selemon, L.D.; Zecevic, N. Schizophrenia: A tale of two critical periods for prefrontal cortical development. Transl. Psychiatry 2015, 5, e623. [Google Scholar] [CrossRef]
- Sakurai, T.; Gamo, N.J. Cognitive functions associated with developing prefrontal cortex during adolescence and developmental neuropsychiatric disorders. Neurobiol. Dis. 2018, 131, 104322. [Google Scholar] [CrossRef]
- Bennett, M.R. Synapse formation and regression in the cortex during adolescence and in schizophrenia. Med. J. Aust. 2009, 190, S14–S16. [Google Scholar] [CrossRef]
- Chini, M.; Hanganu-Opatz, I.L. Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends Neurosci. 2020, 44, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Sommer, I.E.; Bearden, C.E.; van Dellen, E.; Breetvelt, E.J.; Duijff, S.N.; Maijer, K.; van Amelsvoort, T.; de Haan, L.; Gur, R.E.; Arango, C.; et al. Early interventions in risk groups for schizophrenia: What are we waiting for? NPJ Schizophr. 2016, 2, 16003. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Nichenmetla, S.; Chhabra, H.; Sreeraj, V.S.; Rao, N.P.; Kesavan, M.; Varambally, S.; Venkatasubramanian, G.; Gangadhar, B.N. Prefrontal cortex activation during working memory task in schizophrenia: A fNIRS study. Asian J. Psychiatry 2020, 56, 102507. [Google Scholar] [CrossRef]
- Yan, Z.; Rein, B. Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: Pathophysiological implications. Mol. Psychiatry 2021, 27, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Mirnics, K.; Middleton, F.A.; Marquez, A.; Lewis, D.A.; Levitt, P. Molecular Characterization of Schizophrenia Viewed by Microarray Analysis of Gene Expression in Prefrontal Cortex. Neuron 2000, 28, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Selemon, L.D.; Goldman-Rakic, P.S. The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol. Psychiatry 1999, 45, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Thune, J.J.; Uylings, H.B.; Pakkenberg, B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J. Psychiatr. Res. 2001, 35, 15–21. [Google Scholar] [CrossRef]
- Mauney, S.A.; Athanas, K.M.; Pantazopoulos, H.; Shaskan, N.; Passeri, E.; Berretta, S.; Woo, T.-U.W. Developmental Pattern of Perineuronal Nets in the Human Prefrontal Cortex and Their Deficit in Schizophrenia. Biol. Psychiatry 2013, 74, 427–435. [Google Scholar] [CrossRef]
- Kristiansen, L.; Huerta, I.; Beneyto, M.; Meador-Woodruff, J.H. NMDA receptors and schizophrenia. Curr. Opin. Pharmacol. 2007, 7, 48–55. [Google Scholar] [CrossRef]
- Smucny, J.; Carter, C.S.; Maddock, R.J. Medial Prefrontal Cortex Glutamate Is Reduced in Schizophrenia and Moderated by Measurement Quality: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. Biol. Psychiatry 2021, 90, 643–651. [Google Scholar] [CrossRef]
- Akil, M.; Pierri, J.N.; Whitehead, R.E.; Edgar, C.L.; Mohila, C.; Sampson, A.R.; Lewis, D.A. Lamina-Specific Alterations in the Dopamine Innervation of the Prefrontal Cortex in Schizophrenic Subjects. Am. J. Psychiatry 1999, 156, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Abi-Dargham, A.; Mawlawi, O.; Lombardo, I.; Gil, R.; Martinez, D.; Huang, Y.; Hwang, D.-R.; Keilp, J.; Kochan, L.; Van Heertum, R.; et al. Prefrontal Dopamine D1Receptors and Working Memory in Schizophrenia. J. Neurosci. 2002, 22, 3708–3719. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Weinberger, D.R. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol. Psychiatry 2004, 10, 40–68. [Google Scholar] [CrossRef]
- Cariaga-Martinez, A.; Saiz-Ruiz, J.; Alelú-Paz, R. From Linkage Studies to Epigenetics: What We Know and What We Need to Know in the Neurobiology of Schizophrenia. Front. Neurosci. 2016, 10, 202. [Google Scholar] [CrossRef]
- Madzarac, Z.; Tudor, L.; Sagud, M.; Erjavec, G.N.; Peles, A.M.; Pivac, N. The Associations between COMT and MAO-B Genetic Variants with Negative Symptoms in Patients with Schizophrenia. Curr. Issues Mol. Biol. 2021, 43, 618–636. [Google Scholar] [CrossRef]
- Williams, N.M.; Preece, A.; Morris, D.W.; Spurlock, G.; Bray, N.J.; Stephens, M.; Norton, N.; Williams, H.; Clement, M.; Dwyer, S.; et al. Identification in 2 Independent Samples of a Novel Schizophrenia RiskHaplotype of the Dystrobrevin Binding Protein Gene (DTNBP1). Arch. Gen. Psychiatry 2004, 61, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, G.; Frodl, T.; Morris, D.; Spoletini, I.; Cannon, D.; Cherubini, A.; Caltagirone, C.; Bossù, P.; McDonald, C.; Gill, M.; et al. Reduced Occipital and Prefrontal Brain Volumes in Dysbindin-Associated Schizophrenia. Neuropsychopharmacology 2009, 35, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Takahashi, A.; Kamatani, Y.; Momozawa, Y.; Saito, T.; Kondo, K.; Shimasaki, A.; Kawase, K.; Sakusabe, T.; Iwayama, Y.; et al. Genome-Wide Association Study Detected Novel Susceptibility Genes for Schizophrenia and Shared Trans-Populations/Diseases Genetic Effect. Schizophr. Bull. 2018, 45, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Nawalpuri, B.; Shah, D.; Sateesh, S.; Muddashetty, R.S.; Clement, J. Differential Regulation of Syngap1 Translation by FMRP Modulates eEF2 Mediated Response on NMDAR Activity. Front. Mol. Neurosci. 2019, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, M.; Tian, X.; Guo, Y.; Liu, F.; Li, Y.; Zhang, H.; Lu, X.; Xu, D.; Zhou, R.; et al. Transgenic overexpression of furin increases epileptic susceptibility. Cell Death Dis. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Yu, H.; Yan, H.; Li, J.; Li, Z.; Zhang, X.; Ma, Y.; Mei, L.; Liu, C.; Cai, L.; Wang, Q.; et al. Common variants on 2p16.1, 6p22.1 and 10q24.32 are associated with schizophrenia in Han Chinese population. Mol. Psychiatry 2016, 22, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Fromer, M.; Pocklington, A.J.; Kavanagh, D.H.; Williams, H.J.; Dwyer, S.; Gormley, P.; Georgieva, L.; Rees, E.; Palta, P.; Ruderfer, D.M.; et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 2014, 506, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kirov, G.; Pocklington, A.; Holmans, P.; Ivanov, D.; Ikeda, M.; Ruderfer, D.; Moran, J.; Chambert, K.; Toncheva, D.; Georgieva, L.; et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol. Psychiatry 2011, 17, 142–153. [Google Scholar] [CrossRef]
- Purcell, S.M.; Moran, J.L.; Fromer, M.; Ruderfer, D.; Solovieff, N.; Roussos, P.; O’Dushlaine, C.; Chambert, K.; Bergen, S.E.; Kähler, A.; et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature 2014, 506, 185–190. [Google Scholar] [CrossRef]
- The Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci 2015, 18, 199–209. [Google Scholar] [CrossRef]
- Liu, H.; Xu, L.; Fu, J.; Su, Q.; Liu, N.; Xu, J.; Tang, J.; Li, W.; Zhao, F.; Ding, H.; et al. Prefrontal Granule Cell-Related Genes and Schizophrenia. Cereb. Cortex 2020, 31, 2268–2277. [Google Scholar] [CrossRef]
- Stahl, S.M. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: Dopamine, serotonin, and glutamate. CNS Spectrums 2018, 23, 187–191. [Google Scholar] [CrossRef]
- Weickert, C.S.; Fung, S.J.; Catts, V.S.; Schofield, P.R.; Allen, K.M.; Moore, L.T.; Newell, K.A.; Pellen, D.; Huang, X.-F.; Catts, S.V.; et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol. Psychiatry 2013, 18, 1185–1192. [Google Scholar] [CrossRef]
- Rahman, T.; Purves-Tyson, T.; Geddes, A.E.; Huang, X.F.; Newell, K.A.; Weickert, C.S. N-Methyl-d-Aspartate receptor and inflammation in dorsolateral prefrontal cortex in schizophrenia. Schizophr. Res. 2022, 240, 61–70. [Google Scholar] [CrossRef]
- Beneyto, M.; Meador-Woodruff, J.H. Lamina-Specific Abnormalities of NMDA Receptor-Associated Postsynaptic Protein Transcripts in the Prefrontal Cortex in Schizophrenia and Bipolar Disorder. Neuropsychopharmacology 2007, 33, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.E.; The BrainSeq Consortium; Straub, R.E.; Shin, J.H.; Tao, R.; Gao, Y.; Collado-Torres, L.; Kam-Thong, T.; Xi, H.S.; Quan, J.; et al. Developmental and genetic regulation of the human cortex transcriptome illuminate schizophrenia pathogenesis. Nat. Neurosci. 2018, 21, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, L.V.; Beneyto, M.; Haroutunian, V.; Meador-Woodruff, J.H. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol. Psychiatry 2006, 11, 737–747. [Google Scholar] [CrossRef]
- Ohnuma, T.; Kato, H.; Arai, H.; Faull, R.L.M.; McKenna, P.J.; Emson, P.C. Gene expression of PSD95 in prefrontal cortex and hippocampus in schizophrenia. Neuroreport 2000, 11, 3133–3137. [Google Scholar] [CrossRef]
- Vawter, M.P.; Crook, J.M.; Hyde, T.M.; Kleinman, J.E.; Weinberger, D.R.; Becker, K.G.; Freed, W.J. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: A preliminary study. Schizophr. Res. 2002, 58, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, K.; Tani, H.; Nakajima, S.; Nagai, N.; Koizumi, T.; Miyazaki, T.; Mimura, M.; Takahashi, T.; Uchida, H. AMPA receptors in schizophrenia: A systematic review of postmortem studies on receptor subunit expression and binding. Schizophr. Res. 2022, 243, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Volk, D.W.; Eggan, S.M.; Lewis, D.A. Alterations in Metabotropic Glutamate Receptor 1α and Regulator of G Protein Signaling 4 in the Prefrontal Cortex in Schizophrenia. Am. J. Psychiatry 2010, 167, 1489–1498. [Google Scholar] [CrossRef]
- Ghose, S.; Crook, J.M.; Bartus, C.L.; Sherman, T.G.; Herman, M.M.; Hyde, T.M.; Kleinman, J.E.; Akil, M. Metabotropic Glutamate Receptor 2 and 3 Gene Expression in The Human Prefrontal Cortex and Mesencephalon in Schizophrenia. Int. J. Neurosci. 2008, 118, 1609–1627. [Google Scholar] [CrossRef]
- Bauer, D.; Gupta, D.; Harotunian, V.; Meador-Woodruff, J.H.; McCullumsmith, R.E. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr. Res. 2008, 104, 108–120. [Google Scholar] [CrossRef]
- Parkin, G.M.; Gibbons, A.; Udawela, M.; Dean, B. Excitatory amino acid transporter (EAAT)1 and EAAT2 mRNA levels are altered in the prefrontal cortex of subjects with schizophrenia. J. Psychiatr. Res. 2020, 123, 151–158. [Google Scholar] [CrossRef]
- Scarr, E.; Udawela, M.; Dean, B. Changed frontal pole gene expression suggest altered interplay between neurotransmitter, developmental, and inflammatory pathways in schizophrenia. Schizophrenia 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Lauriat, T.; Dracheva, S.; Chin, B.; Schmeidler, J.; McInnes, L.; Haroutunian, V. Quantitative analysis of glutamate transporter mRNA expression in prefrontal and primary visual cortex in normal and schizophrenic brain. Neuroscience 2006, 137, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Hoftman, G.D.; Volk, D.W.; Bazmi, H.H.; Li, S.; Sampson, A.R.; Lewis, D.A. Altered Cortical Expression of GABA-Related Genes in Schizophrenia: Illness Progression vs Developmental Disturbance. Schizophr. Bull. 2013, 41, 180–191. [Google Scholar] [CrossRef]
- Torrey, E.F.; Barci, B.M.; Webster, M.J.; Bartko, J.J.; Meador-Woodruff, J.H.; Knable, M.B. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol. Psychiatry 2005, 57, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Volk, D.W.; Austin, M.C.; Pierri, J.N.; Sampson, A.R.; Lewis, D.A. GABA Transporter-1 mRNA in the Prefrontal Cortex in Schizophrenia: Decreased Expression in a Subset of Neurons. Am. J. Psychiatry 2001, 158, 256–265. [Google Scholar] [CrossRef]
- Beneyto, M.; Abbott, A.; Hashimoto, T.; Lewis, D.A. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb. Cortex 2011, 21, 999–1011. [Google Scholar] [CrossRef]
- Chen, J.; Lipska, B.K.; Halim, N.; Ma, Q.D.; Matsumoto, M.; Melhem, S.; Kolachana, B.S.; Hyde, T.M.; Herman, M.M.; Apud, J.; et al. Functional Analysis of Genetic Variation in Catechol-O-Methyltransferase (COMT): Effects on mRNA, Protein, and Enzyme Activity in Postmortem Human Brain. Am. J. Hum. Genet. 2004, 75, 807–821. [Google Scholar] [CrossRef]
- Matsumoto, M.; Weickert, C.S.; Beltaifa, S.; Kolachana, B.; Chen, J.; Hyde, T.M.; Herman, M.M.; Weinberger, D.R.; E Kleinman, J. Catechol O-Methyltransferase (COMT) mRNA Expression in the Dorsolateral Prefrontal Cortex of Patients with Schizophrenia. Neuropsychopharmacology 2003, 28, 1521–1530. [Google Scholar] [CrossRef]
- Kaalund, S.S.; Newburn, E.N.; Ye, T.; Tao, R.; Li, C.; A Deep-Soboslay, A.; Herman, M.M.; Hyde, T.M.; Weinberger, D.R.; Lipska, B.K.; et al. Contrasting changes in DRD1 and DRD2 splice variant expression in schizophrenia and affective disorders, and associations with SNPs in postmortem brain. Mol. Psychiatry 2013, 19, 1258–1266. [Google Scholar] [CrossRef]
- Stenkrona, P.; Matheson, G.J.; Halldin, C.; Cervenka, S.; Farde, L. D1-Dopamine Receptor Availability in First-Episode Neuroleptic Naive Psychosis Patients. Int. J. Neuropsychopharmacol. 2019, 22, 415–425. [Google Scholar] [CrossRef]
- Urigüen, L.; García-Fuster, M.J.; Callado, L.F.; Morentin, B.; La Harpe, R.; Casadó, V.; Lluis, C.; Franco, R.; García-Sevilla, J.A.; Meana, J.J. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: Effect of antipsychotic treatment. Psychopharmacology 2009, 206, 313–324. [Google Scholar] [CrossRef]
- Meador-Woodruff, J.H.; Haroutunian, V.; Powchik, P.; Davidson, M.; Davis, K.L.; Watson, S.J. Dopamine Receptor Transcript Expression in Striatum and Prefrontal and Occipital Cortex. Arch. Gen. Psychiatry 1997, 54, 1089–1095. [Google Scholar] [CrossRef]
- Bray, N.J.; Preece, A.; Williams, N.M.; Moskvina, V.; Buckland, P.R.; Owen, M.J.; O’Donovan, M.C. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Hum. Mol. Genet. 2005, 14, 1947–1954. [Google Scholar] [CrossRef]
- Baracskay, K.L.; Haroutunian, V.; Meador-Woodruff, J.H. Dopamine receptor signaling molecules are altered in elderly schizophrenic cortex. Synapse 2006, 60, 271–279. [Google Scholar] [CrossRef]
- Santarelli, D.M.; Carroll, A.P.; Cairns, H.M.; Tooney, P.A.; Cairns, M.J. Schizophrenia-associated MicroRNA-Gene Interactions in the Dorsolateral Prefrontal Cortex. Genom. Proteom. Bioinform. 2019, 17, 623–634. [Google Scholar] [CrossRef]
- Fung, S.J.; Sivagnanasundaram, S.; Weickert, C.S. Lack of Change in Markers of Presynaptic Terminal Abundance Alongside Subtle Reductions in Markers of Presynaptic Terminal Plasticity in Prefrontal Cortex of Schizophrenia Patients. Biol. Psychiatry 2011, 69, 71–79. [Google Scholar] [CrossRef]
- Hino, M.; Kunii, Y.; Matsumoto, J.; Wada, A.; Nagaoka, A.; Niwa, S.-I.; Takahashi, H.; Kakita, A.; Akatsu, H.; Hashizume, Y.; et al. Decreased VEGFR2 expression and increased phosphorylated Akt1 in the prefrontal cortex of individuals with schizophrenia. J. Psychiatr. Res. 2016, 82, 100–108. [Google Scholar] [CrossRef]
- Pillai, A. Decreased Expression of Sprouty2 in the Dorsolateral Prefrontal Cortex in Schizophrenia and Bipolar Disorder: A Correlation with BDNF Expression. PLoS ONE 2008, 3, e1784. [Google Scholar] [CrossRef]
- Iwamoto, K.; Bundo, M.; Yamada, K.; Takao, H.; Iwayama-Shigeno, Y.; Yoshikawa, T.; Kato, T. DNA Methylation Status of SOX10 Correlates with Its Downregulation and Oligodendrocyte Dysfunction in Schizophrenia. J. Neurosci. 2005, 25, 5376–5381. [Google Scholar] [CrossRef]
- Hakak, Y.; Walker, J.R.; Li, C.; Wong, W.H.; Davis, K.L.; Buxbaum, J.D.; Haroutunian, V.; Fienberg, A.A. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 4746–4751. [Google Scholar] [CrossRef]
- Santarelli, D.M.; Beveridge, N.J.; Tooney, P.A.; Cairns, M.J. Upregulation of Dicer and MicroRNA Expression in the Dorsolateral Prefrontal Cortex Brodmann Area 46 in Schizophrenia. Biol. Psychiatry 2011, 69, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Middleton, F.; Mirnics, K.; Pierri, J.N.; Lewis, D.; Levitt, P. Gene Expression Profiling Reveals Alterations of Specific Metabolic Pathways in Schizophrenia. J. Neurosci. 2002, 22, 2718–2729. [Google Scholar] [CrossRef] [PubMed]
- Arion, D.; Corradi, J.; Tang, S.; Datta, D.; Boothe, F.; He, A.; Cacace, A.M.; Zaczek, R.; Albright, C.F.; Tseng, G.; et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol. Psychiatry 2015, 20, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Verwer, R.W.; Lucassen, P.J.; Huitinga, I.; Swaab, D.F. Prefrontal cortex alterations in glia gene expression in schizophrenia with and without suicide. J. Psychiatr. Res. 2019, 121, 31–38. [Google Scholar] [CrossRef]
- Volk, D.W.; Moroco, A.E.; Roman, K.M.; Edelson, J.R.; Lewis, D.A. The Role of the Nuclear Factor-kappaB Transcriptional Complex in Cortical Immune Activation in Schizophrenia. Biol. Psychiatry 2019, 85, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, Y.; Webster, M.J.; Lee, D. Shared co-expression networks in frontal cortex of the normal aged brain and schizophrenia. Schizophr. Res. 2018, 204, 253–261. [Google Scholar] [CrossRef]
- López-González, I.; Pinacho, R.; Vila, .; Escanilla, A.; Ferrer, I.; Ramos, B. Neuroinflammation in the dorsolateral prefrontal cortex in elderly chronic schizophrenia. Eur. Neuropsychopharmacol. 2019, 29, 384–396. [Google Scholar] [CrossRef]
- Hilker, R.; Helenius, D.; Fagerlund, B.; Skytthe, A.; Christensen, K.; Werge, T.M.; Nordentoft, M.; Glenthøj, B. Heritability of Schizophrenia and Schizophrenia Spectrum Based on the Nationwide Danish Twin Register. Biol. Psychiatry 2017, 83, 492–498. [Google Scholar] [CrossRef]
- Imamura, A.; Morimoto, Y.; Ono, S.; Kurotaki, N.; Kanegae, S.; Yamamoto, N.; Kinoshita, H.; Tsujita, T.; Okazaki, Y.; Ozawa, H. Genetic and environmental factors of schizophrenia and autism spectrum disorder: Insights from twin studies. J. Neural Transm. 2020, 127, 1501–1515. [Google Scholar] [CrossRef]
- Rajarajan, P.; Jiang, Y.; Kassim, B.S.; Akbarian, S. Chromosomal Conformations and Epigenomic Regulation in Schizophrenia. Prog. Mol. Biol. Transl. Sci. 2018, 157, 21–40. [Google Scholar]
- Micale, V.; Di Bartolomeo, M.; Di Martino, S.; Stark, T.; Dell’Osso, B.; Drago, F.; D’Addario, C. Are the epigenetic changes predictive of therapeutic efficacy for psychiatric disorders? A translational approach towards novel drug targets. Pharmacol. Ther. 2023, 241, 108279. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.V.C.; Bourc’His, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Mendizabal, I.; Berto, S.; Chatterjee, P.; Layman, T.; Usui, N.; Toriumi, K.; Douglas, C.; Singh, D.; Huh, I.; et al. Evolution of DNA methylation in the human brain. Nat. Commun. 2021, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Bundo, M.; Ueda, J.; Oldham, M.C.; Ukai, W.; Hashimoto, E.; Saito, T.; Geschwind, D.H.; Kato, T. Neurons show distinctive DNA methylation profile and higher interindividual variations compared with non-neurons. Genome Res. 2011, 21, 688–696. [Google Scholar] [CrossRef]
- Kozlenkov, A.; Wang, M.; Roussos, P.; Rudchenko, S.; Barbu, M.; Bibikova, M.; Klotzle, B.; Dwork, A.J.; Zhang, B.; Hurd, Y.L.; et al. Substantial DNA methylation differences between two major neuronal subtypes in human brain. Nucleic Acids Res. 2015, 44, 2593–2612. [Google Scholar] [CrossRef]
- E Jaffe, A.; Gao, Y.; Deep-Soboslay, A.; Tao, R.; Hyde, T.M.; Weinberger, D.R.; E Kleinman, J. Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex. Nat. Neurosci. 2015, 19, 40–47. [Google Scholar] [CrossRef]
- Grayson, D.R.; Jia, X.; Chen, Y.; Sharma, R.P.; Mitchell, C.P.; Guidotti, A.; Costa, E. Reelin promoter hypermethylation in schizophrenia. Proc. Natl. Acad. Sci. USA 2005, 102, 9341–9346. [Google Scholar] [CrossRef]
- Mill, J.; Tang, T.; Kaminsky, Z.; Khare, T.; Yazdanpanah, S.; Bouchard, L.; Jia, P.; Assadzadeh, A.; Flanagan, J.; Schumacher, A.; et al. Epigenomic Profiling Reveals DNA-Methylation Changes Associated with Major Psychosis. Am. J. Hum. Genet. 2008, 82, 696–711. [Google Scholar] [CrossRef]
- Numata, S.; Ye, T.; Herman, M.; Lipska, B.K. DNA methylation changes in the postmortem dorsolateral prefrontal cortex of patients with schizophrenia. Front. Genet. 2014, 5, 280. [Google Scholar] [CrossRef]
- Tao, R.; Davis, K.N.; Li, C.; Shin, J.H.; Gao, Y.; Jaffe, A.; Gondré-Lewis, M.C.; Weinberger, D.R.; Kleinman, J.E.; Hyde, T.M. GAD1 alternative transcripts and DNA methylation in human prefrontal cortex and hippocampus in brain development, schizophrenia. Mol. Psychiatry 2017, 23, 1496–1505. [Google Scholar] [CrossRef]
- A Fachim, H.; Srisawat, U.; Dalton, C.F.; Reynolds, G.P. Parvalbumin promoter hypermethylation in postmortem brain in schizophrenia. Epigenomics 2018, 10, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Li, C.; Jaffe, A.E.; Shin, J.H.; Deep-Soboslay, A.; Yamin, R.; Weinberger, D.R.; Hyde, T.M.; Kleinman, J.E. Cannabinoid receptor CNR1 expression and DNA methylation in human prefrontal cortex, hippocampus and caudate in brain development and schizophrenia. Transl. Psychiatry 2020, 10, 158. [Google Scholar] [CrossRef]
- Wockner, L.; Noble, E.P.; Lawford, B.R.; Young, R.; Morris, P.; Whitehall, V.L.J.; Voisey, J. Genome-wide DNA methylation analysis of human brain tissue from schizophrenia patients. Transl. Psychiatry 2014, 4, e339. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Chen, J.; Duan, K.; Perrone-Bizzozero, N.; Sui, J.; Calhoun, V.; Liu, J. Network modules linking expression and methylation in prefrontal cortex of schizophrenia. Epigenetics 2021, 16, 876–893. [Google Scholar] [CrossRef] [PubMed]
- Alelú-Paz, R.; Carmona, F.J.; Sanchez-Mut, J.V.; Cariaga-Martínez, A.; González-Corpas, A.; Ashour, N.; Orea, M.J.; Escanilla, A.; Monje, A.; Márquez, C.G.; et al. Epigenetics in Schizophrenia: A Pilot Study of Global DNA Methylation in Different Brain Regions Associated with Higher Cognitive Functions. Front. Psychol. 2016, 7, 1496. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, K.; Kim, K.; Yi, S.-J. The role of histone modifications: From neurodevelopment to neurodiseases. Signal Transduct. Target. Ther. 2022, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Girdhar, K.; Hoffman, G.E.; Jiang, Y.; Brown, L.; Kundakovic, M.; Hauberg, M.E.; Francoeur, N.J.; Wang, Y.-C.; Shah, H.; Kavanagh, D.H.; et al. Cell-specific histone modification maps in the human frontal lobe link schizophrenia risk to the neuronal epigenome. Nat. Neurosci. 2018, 21, 1126–1136. [Google Scholar] [CrossRef]
- Huang, H.-S.; Matevossian, A.; Whittle, C.; Kim, S.Y.; Schumacher, A.; Baker, S.P.; Akbarian, S. Prefrontal Dysfunction in Schizophrenia Involves Mixed-Lineage Leukemia 1-Regulated Histone Methylation at GABAergic Gene Promoters. J. Neurosci. 2007, 27, 11254–11262. [Google Scholar] [CrossRef]
- Gusev, F.E.; Reshetov, D.A.; Mitchell, A.C.; Andreeva, T.V.; Dincer, A.; Grigorenko, A.P.; Fedonin, G.; Halene, T.; Aliseychik, M.; Filippova, E.; et al. Chromatin profiling of cortical neurons identifies individual epigenetic signatures in schizophrenia. Transl. Psychiatry 2019, 9, 256. [Google Scholar] [CrossRef]
- Girdhar, K.; Hoffman, G.E.; Bendl, J.; Rahman, S.; Dong, P.; Liao, W.; Hauberg, M.E.; Sloofman, L.; Brown, L.; Devillers, O.; et al. Chromatin domain alterations linked to 3D genome organization in a large cohort of schizophrenia and bipolar disorder brains. Nat. Neurosci. 2022, 25, 474–483. [Google Scholar] [CrossRef]
- Nguyen, P.; Bar-Sela, G.; Sun, L.; Bisht, K.S.; Cui, H.; Kohn, E.; Feinberg, A.P.; Gius, D. BAT3 and SET1A Form a Complex with CTCFL/BORIS To Modulate H3K4 Histone Dimethylation and Gene Expression. Mol. Cell. Biol. 2008, 28, 6720–6729. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; I Kurki, M.; Curtis, D.; Purcell, S.M.; Crooks, L.; McRae, J.; Suvisaari, J.; Chheda, H.; Blackwood, D.; Breen, G.; et al. Rare loss-of-function variants in SETD1A are associated with schizophrenia and developmental disorders. Nat. Neurosci. 2016, 19, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, S.; Ruehl, M.G.; Bliven, E.; Luiz, L.A.; Peranelli, A.C.; Baker, S.P.; Roberts, R.C.; Bunney, W.E.; Conley, R.C.; Jones, E.G.; et al. Chromatin Alterations Associated With Down-regulated Metabolic Gene Expression in the Prefrontal Cortex of Subjects With Schizophrenia. Arch. Gen. Psychiatry 2005, 62, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Dean, B.; A Thomas, E. Disease- and age-related changes in histone acetylation at gene promoters in psychiatric disorders. Transl. Psychiatry 2011, 1, e64. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, L.A.; Zheng, S.; Schrode, N.; Topol, A.; Bhanu, N.V.; Bastle, R.M.; Ramakrishnan, A.; Chan, J.C.; Cetin, B.; Flaherty, E.; et al. Chromatin profiling in human neurons reveals aberrant roles for histone acetylation and BET family proteins in schizophrenia. Nat. Commun. 2022, 13, 2195. [Google Scholar] [CrossRef]
- Sharma, R.P.; Grayson, D.R.; Gavin, D.P. Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: Analysis of the National Brain Databank microarray collection. Schizophr. Res. 2008, 98, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, F.A.; Gilbert, T.M.; Feng, N.; Taillon, B.D.; Volkow, N.D.; Innis, R.B.; Hooker, J.M.; Lipska, B.K. Expression of HDAC2 but Not HDAC1 Transcript Is Reduced in Dorsolateral Prefrontal Cortex of Patients with Schizophrenia. ACS Chem. Neurosci. 2016, 8, 662–668. [Google Scholar] [CrossRef]
- Gilbert, T.; Zürcher, N.R.; Wu, C.J.; Bhanot, A.; Hightower, B.G.; Kim, M.; Albrecht, D.S.; Wey, H.-Y.; Schroeder, F.A.; Rodriguez-Thompson, A.; et al. PET neuroimaging reveals histone deacetylase dysregulation in schizophrenia. J. Clin. Investig. 2018, 129, 364–372. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A Brief Review on the Mechanisms of miRNA Regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef]
- Morgunova, A.; Flores, C. MicroRNA regulation of prefrontal cortex development and psychiatric risk in adolescence. Semin. Cell Dev. Biol. 2021, 118, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, A.; Liu, Y.; Li, J.; Wang, M.; Sun, Y.; Qin, W.; Yu, C.; Jiang, T.; Liu, B. MIR137 polygenic risk is associated with schizophrenia and affects functional connectivity of the dorsolateral prefrontal cortex. Psychol. Med. 2019, 50, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, N.J.; Gardiner, E.; Carroll, A.P.; Tooney, P.; Cairns, M.J. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry 2009, 15, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilecki, W.; Maćkowiak, M. Gene Expression and Epigenetic Regulation in the Prefrontal Cortex of Schizophrenia. Genes 2023, 14, 243. https://doi.org/10.3390/genes14020243
Bilecki W, Maćkowiak M. Gene Expression and Epigenetic Regulation in the Prefrontal Cortex of Schizophrenia. Genes. 2023; 14(2):243. https://doi.org/10.3390/genes14020243
Chicago/Turabian StyleBilecki, Wiktor, and Marzena Maćkowiak. 2023. "Gene Expression and Epigenetic Regulation in the Prefrontal Cortex of Schizophrenia" Genes 14, no. 2: 243. https://doi.org/10.3390/genes14020243
APA StyleBilecki, W., & Maćkowiak, M. (2023). Gene Expression and Epigenetic Regulation in the Prefrontal Cortex of Schizophrenia. Genes, 14(2), 243. https://doi.org/10.3390/genes14020243





