Abstract
Peanut (Arachis hypogaea) and its wild relatives are among the few species that naturally synthesize resveratrol, a well-known stilbenoid phytoalexin that plays a crucial role in plant defense against biotic and abiotic stresses. Resveratrol has received considerable attention due to its health benefits, such as preventing and treating various human diseases and disorders. Chalcone (CHS) and Stilbene (STS) Synthases are plant-specific type III Polyketide Synthases (PKSs) that share the same substrates and are key branch enzymes in the biosynthesis of flavonoids and stilbenoids, respectively. Although resveratrol accumulation in response to external stimulus has been described in peanut, there are no comprehensive studies of the CHS and STS gene families in the genus Arachis. In the present study, we identified and characterized 6 CHS and 46 STS genes in the tetraploid peanut and an average of 4 CHS and 22 STS genes in three diploid wild species (Arachis duranensis, Arachis ipaënsis and Arachis stenosperma). The CHS and STS gene and protein structures, chromosomal distributions, phylogenetic relationships, conserved amino acid domains, and cis-acting elements in the promoter regions were described for all Arachis species studied. Based on gene expression patterns of wild A. stenosperma STS genes in response to different biotic and abiotic stresses, we selected the candidate AsSTS4 gene, which is strongly induced by ultraviolet (UV) light exposure, for further functional investigation. The AsSTS4 overexpression in peanut hairy roots significantly reduced (47%) root-knot nematode infection, confirming that stilbene synthesis activation in transgenic plants can increase resistance to pathogens. These findings contribute to understanding the role of resveratrol in stress responses in Arachis species and provide the basis for genetic engineering for improved production of valuable secondary metabolites in plants.
1. Introduction
Chalcone (CHS) and Stilbene (STS) Synthases are enzymes belonging to the plant type III Polyketide Synthases (PKSs) superfamily that share the same substrates (one molecule of p-coumaroyl-CoA and three molecules of malonyl-CoA) to catalyze the first committed step of the flavonoid and stilbenoid biosynthesis pathways, respectively [1]. Resveratrol (trans-3,5,4′-trihydroxystilbene) is the best-known and the most studied stilbene compound due to its broad range of beneficial biological activities, such as antioxidant, antimicrobial, antiviral, anticancer, antidiabetic, anti-inflammatory, and cardio- and neuroprotective activities [2]. These health-promoting functional properties make resveratrol a promising natural agent for preventing and treating general health and various diseases and disorders. In addition, as a phytoalexin, resveratrol is elicited and accumulated in response to several biotic and abiotic stresses to protect plants against pathogen infection, herbivore attack, ultraviolet (UV) light irradiation, mechanical damage, heavy metals, treatments with chemicals, and others [3]. Due to its current relevance in the pharmaceutical, nutraceutical, food, veterinary, and cosmetic industries, large-scale resveratrol production is urgently required to meet the world’s market demand and new technologies should be developed to improve its extraction from plants [4,5].
CHS and STS share extensive similarities in their amino acid sequences, and evolutionary studies suggest that STS genes have evolved from CHS genes more than once in a lineage-specific manner through neofunctionalization [6,7,8]. However, unlike CHS, which is ubiquitously present in all higher plants, STS is restricted to a limited number of stilbene-producing plants, comprising 34 phylogenetically distant botanical families that include gymnosperms and angiosperms (monocots and dicots) [9].
Arachis species (Fabaceae) are among these few plants that naturally synthesize resveratrol, and despite the advances in exploring this molecule, only the cultivated species (Arachis hypogaea L.) has been considered for producing this compound [10]. In the last few years, however, the knowledge about resveratrol synthesis and metabolism in wild Arachis species belonging to distinct botanical sections has been expanded [11,12,13,14,15,16]. Our studies demonstrated that some wild species produced higher levels of resveratrol, associated with the induced expression of STS genes, than cultivated species upon UV exposure. In particular, A. lignosa L. (section Procumbentes), A. triseminata L. (section Triseminatae), A. duranensis L., A. stenosperma L., and A. ipaënsis L. (section Arachis) were identified as potential novel rich sources of resveratrol due to their high levels content, adding value to these overlooked genetic resources [11,12]. These findings open new opportunities to explore wild Arachis species directly as natural sources for resveratrol bioproduction or as donors of STS genes for resveratrol metabolic engineering in heterologous microorganisms or non-stilbene-producing plants [4,17].
Until recently, grapevine (Vitis spp.) and mulberry (Morus notabilis C.K. Schneid) were the only stilbene-producing plants for which a comprehensive characterization was conducted for the STS multigenic family consisting of closely related paralogs [18,19,20]. In this respect, the availability of the complete genome sequences of cultivated peanut (A. hypogaea) and their wild progenitors (A. duranensis and A. ipaënsis) [21,22,23,24,25] represents an excellent opportunity to advance in the knowledge of the organization, function, and evolution of the STS gene family in these stilbene-producing species. Furthermore, the recent release of the first draft genome sequence for the highly pathogen-resistant A. stenosperma (http://peanutbase.org) has greatly facilitated genome-wide studies in the genus.
In the present study, a genome-wide analysis was undertaken to characterize CHS and STS multigenic families in the genomes of four Arachis species. We identified 6 CHS and 46 STS genes in the tetraploid cultivated A. hypogaea and an average of 4 CHS and 22 STS genes in the diploid wild A. duranensis, A. ipaënsis, and A. stenosperma. Gene and protein structures, chromosomal distribution patterns, phylogenetic relationships, conserved amino acid domains, and cis-acting elements in the promoters were characterized in each species. Additionally, transcriptome analysis showed that A. stenosperma STS genes exhibited dynamic expression patterns in response to different biotic and abiotic stresses and revealed AsSTS4 as a promising candidate gene for genetic engineering towards enhanced resistance to pathogens. For in planta functional validation, AsSTS4 was cloned and successfully overexpressed in hairy roots of a peanut cultivar susceptible to the root-knot nematode (RKN) Meloidogyne arenaria, leading to a significant reduction of 47% in the number of nematode galls. To our knowledge, this is the first study describing the overexpression of a plant STS gene to enhance nematode resistance, pointing out AsSTS4 as a valuable candidate gene to be exploited in future genetic breeding programs.
2. Results
2.1. Identification and Characterization of CHS and STS Genes in Arachis spp.
Genome-wide searches were performed based on the presence of conserved CHS and STS domains on the genome of four Arachis species: A. duranensis (2n), A. ipaënsis (2n), A. stenosperma (2n), and A. hypogaea (4n). An average of 37 genes were identified as CHS- and STS-coding putative proteins in diploid species and 74 in the tetraploid, with 27–32% being pseudogenes, i.e., sequences containing partial CHS/STS domains, a premature stop codon, or frameshift mutations (Table 1). After manually removing redundancies, only proteins containing both the chalcone/stilbene synthases N- (PF00195) and C-terminal (PF02797) domains were kept. Proteins were then annotated as CHS or STS according to the presence of conserved residues around Met98 and Thr132 [26] as well as the nine residues recently predicted to be under positive selection in peanut by [6]. Each gene family was named based on the chromosome locations for each species.

Table 1.
Distribution of CHS and STS gene families in Arachis duranensis (2n), A. ipaënsis (2n), A. stenosperma (2n), and A. hypogaea (4n).
As a result, we identified 5 CHS and 22 STS in A. duranensis, 3 CHS and 23 STS in A. ipaënsis, 5 CHS and 20 STS in A. stenosperma and 6 CHS and 46 STS genes in A. hypogaea (Table 1). Similar to other plant PKS family genes [27,28,29], the 19 Arachis CHS exhibited either a 2-exon/1-intron (53%) or a 3-exon/2-intron (47%) architecture (Figure 1A). The great majority (90%) of the 111 STS genes was characterized by a very similar gene structure harboring a single intron and two exons in all Arachis species (Figure 1B–E). The intron and exon sizes of the STS family members are quite conserved within each species, with a few lacking annotated 5′UTR. Only five STS genes (AdSTS6 and 19; AiSTS6; AhSTS15 and 24) are the exception from this usual structure and exhibit a 3-exon/2-intron organization (Figure 1B,C,E). Some A. ipaënsis and A. hypogaea genes showed intron-exon structures that are uncharacteristic for STS genes (Figure 1C,E), which could be due to gene prediction problems, such as intronless (AiSTS9, 12, 13 and 14), multiple exons and introns (AiSTS5 and 7) or start codon introns (AhSTS3, 14 and 18). Detailed characteristics of CHS and STS genes in the four Arachis species are shown in Tables S1–S4.
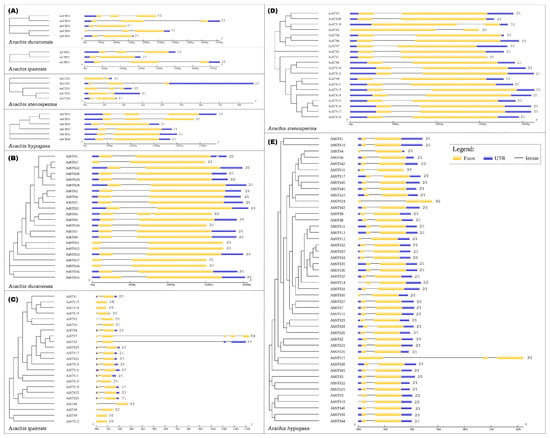
Figure 1.
Exon–intron gene structure of CHS (A) and STS in Arachis duranensis (B); A. ipaënsis (C); A. stenosperma (D); and A. hypogaea (E).
The predicted proteins encoded by Arachis CHS and STS genes ranged from 293 to 433 amino acids with an average of 383. The molecular weight (MW) varied from 31.80 to 48.14 kDa, while the theoretical isoelectric point (pI) values were between 5.17 and 7.18. Overall, other physicochemical properties, such as instability index, aliphatic index, and GRAVY, are similar among the Arachis CHS and STS proteins. The subcellular localization revealed that plastid (53%) and cytoplasm (42%) are the most frequent sites predicted for the 19 Arachis CHS proteins. In contrast, the great majority (82%) of the 111 Arachis STS proteins are predominantly localized in the plastid, with only a few found to be cytoplasm-localized (17%), indicating that the subcellular localization of the STS family proteins is highly conserved in the Arachis species studied. Interestingly, the plastid is considered a major compartment to produce defense-related signaling molecules. Apart from cytoplasm and plastid, members of CHS and STS were also found in relatively low distribution (5 and 1%, respectively) in the Golgi apparatus. Detailed information about the physicochemical properties and the subcellular localization of CHS and STS proteins in the four Arachis species are shown in Tables S1–S4.
2.2. Chromosomal Distribution and Organization of Arachis CHS and STS Genes
The genome localization and syntenic relationships of the CHS and STS genes were predicted for A. duranensis, A. ipaënsis, and A. hypogaea. A. stenosperma was not included in this analysis since the single whole genomic sequence available for this species does not yet have a gene structure annotation. The chromosomal location of the CHS genes showed that they were unevenly distributed on chromosomes 03, 04, 05, and 06 of the wild species and chromosomes 03, 05, 13, 14, and 15 of the cultivated species (Figure 2). Conversely, the great majority of the Arachis STS genes were grouped in gene clusters on the chromosome 04 of each diploid wild species (86%) and the collinear chromosomes 04 and 14 (85%) of the cultivated tetraploid species (Figure 2; Table 1). The few remaining STS genes were unevenly distributed on chromosomes 01 and 06 of the wild species and on chromosomes 01, 11, and 16 of the cultivated species. Moreover, most of the Arachis CHS and STS genes were located on the distal chromosomal regions, typical for the gene-rich characteristic of these recombination hotspots, as previously observed for other Arachis gene families [30,31,32]. No CHS and STS representatives were found for any species on chromosomes 02, 07 to 10, 12, and 17 to 20.
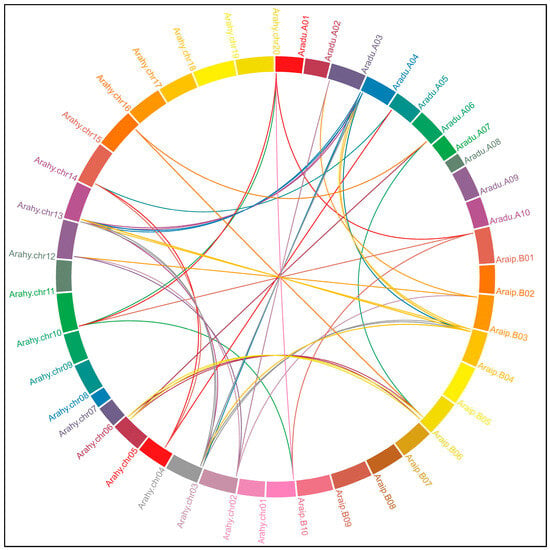
Figure 2.
Genomic distribution and syntenic relationships of the CHS and STS genes in Arachis spp. The ten chromosomes of A. duranensis is represented as Aradu (A01 to A10) and A. ipaënsis as Araip (B01 to B10), and the 20 chromosomes of A. hypogaea as Arahy (chr01 to chr20). The syntenic relationships between the genes are represented by lines.
For a more comprehensive investigation of the STS gene clusters in Arachis, we further analyzed their physical organization in chromosome 04 of diploid species and collinear chromosomes 04 and 14 of tetraploid one. The distribution and orientation of functional genes (STS, CHS, and others), pseudogenes, and transposable elements (TEs) were arranged in each cluster (Figure 3). In all three diploid wild species, STS genes were positioned in a single aligned gene cluster on each chromosome 04, harboring 17 to 21 complete STS genes occurring within less than 922 kb apart, with an average density of 2.4 genes per 100 kb (Table 1; Figure 3). Likewise, in the cultivated tetraploid species, 16 and 23 complete STS genes were identified on chromosomes 04 (622 kb) and 14 (943 kb), respectively, with an average density of 2.5 genes per 100 kb (Table 1; Figure 3). In all species studied, these complete STS genes are irregularly spaced within each cluster and often interrupted by STS pseudogenes and other functional genes encoding non-STS proteins (Figure 3). Moreover, Arachis STS gene clusters are also characterized by the presence of various TEs, with an average of 3.3 TEs per 100 kb (Figure 3), predominantly from retrotransposons classes LTR (42.6%) and non-LTR/LINE (15.9%) and DNA transposons superfamilies CACTA (15.3%) and MULE (12.4%), in accordance to [21].
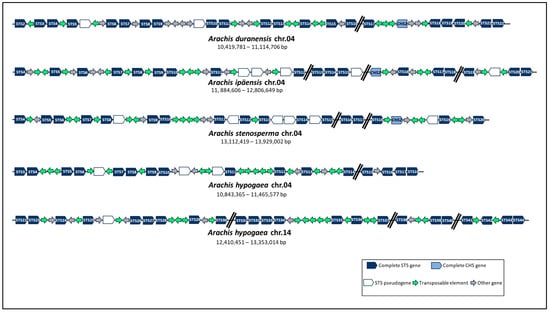
Figure 3.
Schematic representation of STS gene clusters organization on chromosomes 04 and 14 of Arachis spp. Right and left arrows indicate whether genes or transposable elements are located on the + or − strand, respectively.
2.3. Phylogenetic Analysis of CHS and STS Proteins
To explore the evolutionary relationships among the Arachis CHS and STS proteins, a phylogenetic tree was built based on the amino acid sequences encoded by 27 A. duranensis (5 CHS, 22 STS), 26 A. ipaënsis (3 CHS, 23 STS), 25 A. stenosperma (5 CHS, 20 STS), and 52 A. hypogaea (6 CHS, 46 STS) genes (Table 1). The phylogenic analysis showed that proteins belonging to the Arachis CHS and STS families formed two clearly separate major clades with a high bootstrap support (Figure 4).
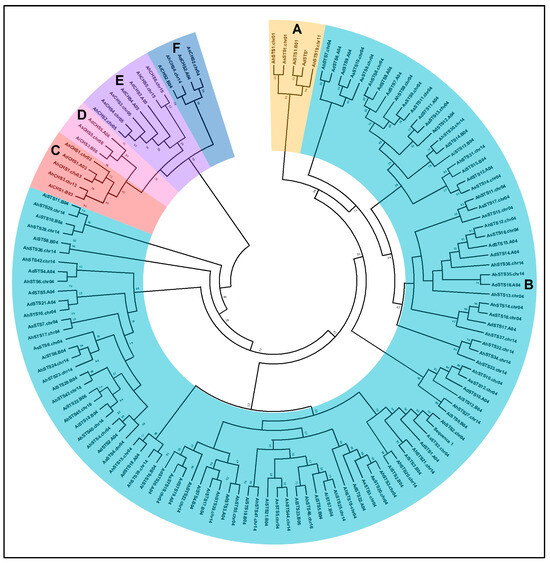
Figure 4.
Phylogenetic tree of STS (Groups A and B) and CHS (Groups C, D, E, and F) S amino acid sequences from Arachis duranensis; A. ipaënsis; A. stenosperma; and A. hypogaea.
The CHS sequences were distributed into four subclades, each formed by proteins encoded by genes with similar positions on chromosomes 03/13, 04/14, 05/15, and 06 of each diploid and tetraploid species (Figure 4). The STS sequences retrieved from the four Arachis species were grouped in several subclades, with sequences of each species spread into different clades having higher similarity to other species sequences than to those of the same species, as previously observed by our group [11]. While all STS proteins encoded by genes on chromosomes 01 and 11 were grouped together, those encoded by the 103 genes located on chromosomes 04 and 14 were more diverse and included the four STS genes from A. ipaënsis and A. hypogaea located, respectively, on chromosomes 06 and 16.
Overall, CHS and STS subclades comprise proteins from the four Arachis species located in similar positions on the chromosomes. For instance, some subclades are formed by proteins encoded by genes with sequential positions on chromosome 04 of the different species, like that comprising AhSTS7, AdSTS6, AdSTS9, AsSTS8, and AsSTS10. Likewise, subclades are formed by proteins whose genes have similar positions on chromosomes 04 and 14 but are located outside of the STS gene clusters (Figure 3), like the one comprised of AhSTS2, AsSTS2, AdSTS1, AiSTS2, and AiSTS3, (chromosome 04) and AhSTS20 and AhSTS21 (chromosome 14; Figure 4). Interestingly, the subclade formed entirely by STS proteins located at chromosomes 01 (AhSTS1, AsSTS1, and AiSTS1) and 11 (AhSTS19), also includes an STS pseudogene from A. duranensis (AdSTS*; Figure 4). Conversely, a unique subclade comprises sequences (AiSTS10, AiSTS11, AhSTS28, and AhSTS29) coding by genes exclusively located on the STS clusters in chromosomes 04 and 14. Vannozzi et al. [18] also observed that grapevine STS proteins encoded by genes located on different chromosomes formed separate clades. Here, only few clades are composed by Arachis proteins encoded by genes located on different chromosomes, such as AhSTS4 located at the STS cluster in chromosome 04 that was grouped with AhSTS40 and AhSTS43 located on the chromosome 14, and AhSTS45 on chromosome 16.
To better understand the evolution of CHS and STS families in legumes, we also performed a phylogenetic analysis based on the gene sequences of three Arachis species (A. duranensis, A. ipaënsis, and A. hypogaea) and eleven legume species for which the complete annotated genome is available at LIS (Legume Information System) database, using four non-legume species as outgroups (Figure S1). As observed with the phylogenetic tree based on Arachis amino acid sequences (Figure 4), the legume gene tree shows the generally expected phylogenetic relationships, with CHS and STS homologs falling in two distinct clades. The first large clade is composed entirely of STS syntenic genes from the three Arachis species, indicating that, among the major crop and model legume species examined here, Arachis is the only harboring STS genes. The second large clade is formed exclusively by CHS syntenic genes from all legume and non-legume species, including the three Arachis, mixed in distinct subclades, confirming that the CHS gene family is ubiquitous in higher plants. These results support that Arachis STS genes have independently evolved different mutations from the typical CHS genes in the ancestor of Fabaceae, as recently reported by [6].
2.4. Conserved Functional Domain and Motifs Analysis
The type III PKS active site residues of the enzymes and CHS/STS signature motif (‘WGVLFGFGPGLT’; [33]) were conserved among the Arachis CHS and STS proteins, which share an average of 90.7% sequence identity without significant insertions or deletions (Figure S2). In accordance with [26], the Arachis STS proteins contain 11 unvaried amino acid residues surrounding Met98 (‘EDMMIREVPRV’) and Thr132 (‘CTTSGVALPGV’) (Figure S2), as described for the peanut STS1 protein (ID AB027606). Likewise, at the same positions, Arachis CHS proteins show the consensus sequences ‘QDMVVVEVPRL’ and ‘CTTSGVDMPGA’ (Figure S2) that are highly conserved residues among the members of the CHS superfamily in distinct plant species such as alfalfa, pine, and sweet orange [26,34]. The nine positively selected sites of typical peanut STS, as recently predicted by [6], were also present in all Arachis species. Previous studies indicate that differences in a few amino acid residues in these conserved residues can lead to variations in the crystal structures and enzymatic activities or modifications in the loop regions among the type III PKS [1,6] and allow for the distinction of CHS and STS family members in the four Arachis species.
The distribution, position, and frequency of occurrence of PFAM domains showed a highly conserved protein structure across the CHS and STS families in Arachis spp. (Figure 5 and Figure 6), consisting of the chalcone and stilbene synthases N-terminal (PF00195; Motifs 1, 3, and 4) and C-terminal (PF02797; Motifs 2 and 5) domains, both used here to identify CHS and STS proteins. In addition, these proteins also share three common domains, FAE1_CUT1_RppA (PF08392), ACP_synthase_III (PF08545), and ACP_synthase_III_C (PF08541) (Figure 6), which are characteristic features of type III PKSs in plants and are involved with lipid metabolism [35].
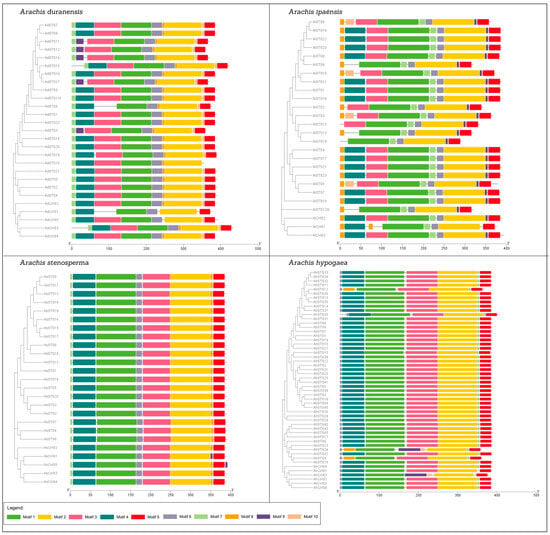
Figure 5.
Protein structure of CHS and STS in Arachis duranensis; A. ipaënsis; A. stenosperma; and A. hypogaea.
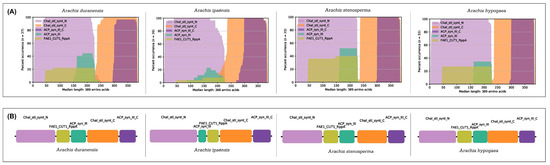
Figure 6.
Protein organization of CHS and STS in Arachis duranensis; A. ipaënsis; A. stenosperma; and A. hypogaea. (A) Percentage of occurrence and (B) organization of conserved protein domains in predicted proteins.
2.5. Cis-Acting Elements in CHS and STS Genes Promoter Regions
The 1500 bp sequences upstream of the first nucleotide of the 5’UTR could be extracted from all A. hypogaea (52) and A. stenosperma (25) CHS and STS genes. However, only 20 sequences were retrieved from the 27 A. duranensis genes and 11 out of 26 from A. ipaënsis due to the significant number of unspecified nucleotides in their respective promoter region sequences. A total of 93 cis-acting elements could be predicted in these 108 putative promoter sequences of CHS and STS genes from all four Arachis species, where the most common were the well-characterized TATA-box (49%) and CAAT-box (21%), which are considered core elements essential for eukaryotic promoter activity. Two other TATA elements (AT~TATA-box and TATAAAAT) were also frequently found (6.9 and 1.3%, respectively) in many promoters. The remaining cis-acting elements were clustered into four categories according to their putative roles and involvement in distinct pathways: 17 responsive to hormones (HRE), 28 to light (LRE), 18 to stresses (STRE), and 14 related to tissue specificity and development (TS&DEV). Although some promoters are responsive to more than one stimulus, here, they were classified based on only one response. The other 12 sequences identified as putative promoter elements (A-box, CCGTCC-motif, JERE, CTAG-motif, box S, OCT, DRE core, E2Fb, GC-motif, HD-Zip 3, AACA_motif and CARE) had no information about their functions in PlantCARE database and therefore were not further analyzed.
The total and mean number of putative cis-elements classified in the four categories (HRE, LRE, STRE, and TS&DEV) varied among the four Arachis species and the gene family (Table 2). In the tetraploid A. hypogaea, the total number of cis-elements found in the STS promoters was at least two times higher than the observed in the wild diploid species. Likewise, a larger number (>1.4 times) of elements was also observed in the CHS promoters of the tetraploid species compared to the diploids A. duranensis and A. stenosperma, whereas A. ipaënsis has only one CHS promoter identified due to errors in DNA sequences. When the mean number of cis-elements per promoter was taken into account for each species instead of the total number, the ratio tetraploid:diploid species was close to one for both CHS and STS genes (Table 2).

Table 2.
Total and mean number of cis-acting elements on STS and CHS gene promoters of Arachis duranensis, A. ipaënsis, A. stenosperma, and A. hypogaea.
Figure 7A,B shows the relationships between the STS and CHS genes, respectively, based on the observed numbers of cis-elements classified in the HRE, LRE, STRE, and TS&DEV categories. Regulatory elements belonging to the four categories were found in all putative promoter sequences analyzed, allowing the identification of promoter clusters based on the differences in the number of elements in each category. For instance, the first group (AiSTS16, AhSTS29, AhSTS35, and AhSTS42) showed the greatest number (>16) of elements related to hormone response (HRE), while a second group with eight sequences (AhSTS20, AhSTS22, AhSTS7, AhSTS21, AhSTS23, AsSTS16, AdSTS15, and AhSTS24) showed the highest number (>12) of light-responsive elements (LRE). Four genes (AhSTS12 and AsSTS3, AdSTS4, and AhSTS16) formed two groups with the largest number (>14) of elements responsive to stress (STRE). No clear STS promoter clustering was observed concerning the numbers of cis-elements classified as related to tissue specificity and development (TS&DEV). We also found significant clustering based on the number of cis-elements in the 15 Arachis CHS gene promoters for HRE, LRE, and STRE categories (Figure 7B). Overall, CHS and STS promoter sequences were not clustered together based on the species. For instance, A. hypogaea promoters were distributed in all clusters, including the sequences located at chromosomes 04 and 14 (Figure 7).
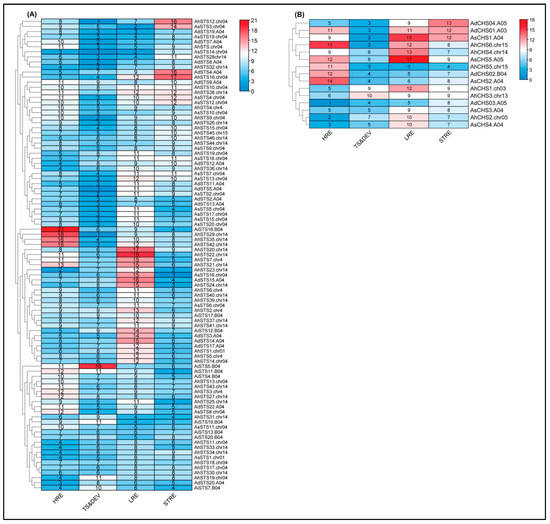
Figure 7.
Heatmap of cis-acting elements associated with responses to hormones (HRE), light (LRE), and stress (STRE) and related to tissue specificity and development (TS&DE) in the promoter regions of STS (A) and CHS (B) genes of A. duranensis (Ad), A. ipaënsis (Ai), A. stenosperma (As), and A. hypogaea (Ah). The heatmap colors range from red to blue scale, where darker colors indicate increasing and decreasing values in the numbers of cis-elements.
Among the 17 hormone responsive (HRE) cis-elements found in STS promoter genes, the most frequent in all species were ABRE and ERE, involved in ABA and ethylene responses, respectively, and CGTCA- and TGACG-motif, both involved in methyl jasmonate (MeJA) responsiveness (Figure 8A). Light-responsive (LRE) is the category that shows the greatest number of cis-elements (28) in STS promoter regions, with Box 4 being highly represented followed by G-Box. In the stress category, STRE, MYB, ARE, WUN-motif and W-box were the most abundant cis-elements and similarly distributed in the STS gene promoters. In the tissue specificity and development category, promoter regions of STS genes were enriched with TATA, MYC, and O2-site elements that are also related to general stress responses. For the four functional categories (Figure 8B), CHS promoter regions were less diverse than those found in the STS genes with a total of 71 cis-elements found in the 15 promoter regions analyzed.
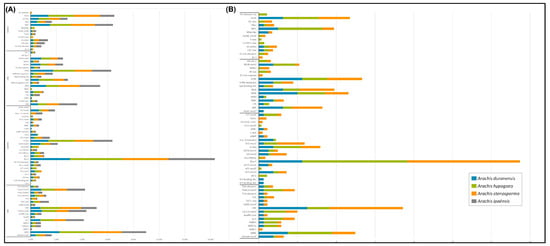
Figure 8.
Distribution in the HRE, LRE, STRE, and TS&DEV categories of cis-acting elements found in the putative promoter sequences of STS (A) and CHS (B) genes from A. duranensis, A. ipaënsis, A. stenosperma and A. hypogaea.
2.6. Arachis Stenosperma CHS and STS Gene Expression Patterns under Biotic and Abiotic Stresses
The expression patterns of the 20 genes encoding for STS and five for CHS in A. stenosperma were analyzed using our previous transcriptome surveys to investigate their transcriptional dynamics in response to biotic and abiotic stresses. The wild A. stenosperma was chosen for this expression analysis as it is a high-resveratrol producer, is adapted to marginal habitats, harbors stress-resilience traits, and is highly responsive to multiple environmental stimuli [36]. Transcriptome data were obtained from A. stenosperma plants submitted to different types of stresses: UV exposure; drought treatments in moderate (dry-down) and severe (dehydration) conditions; nematode infection (at 3, 6, 7, and 9 days after infection; DAI); and combined drought imposition and nematode infection (cross-stress).
Expression profiling showed that all A. stenosperma STS genes were upregulated in response to all isolated or combined stresses analyzed, with variations in their expression levels according to the treatment (Figure 9). The exceptions were the AsSTS13 and AsSTS7 genes that were downregulated in response to only one stress each: cross-stress and nematode infection at 9 DAI, respectively. Remarkably, a general strong induction by UV exposure (Log2 FC > 9.25) was observed for all A. stenosperma STS genes, exhibiting differences in expression at least eight times more than the other abiotic and biotic stresses (Figure 9). Across the 20 A. stenosperma genes, AsSTS4 was the one that exhibited the most significant induction (Log2 FC = 14.7) upon UV stress and among those that displayed the highest levels of expression in response to all other applied stresses. (Figure 9). Although it is well-known that controlled UV radiation can lead to increased plant resistance to pathogens by inducing specialized metabolites and hormones, antioxidative activities, and defensive responses [37], the molecular mechanisms behind the general induction of wild Arachis STS genes following UV exposure or in response to different types of stresses are still not completely understood.
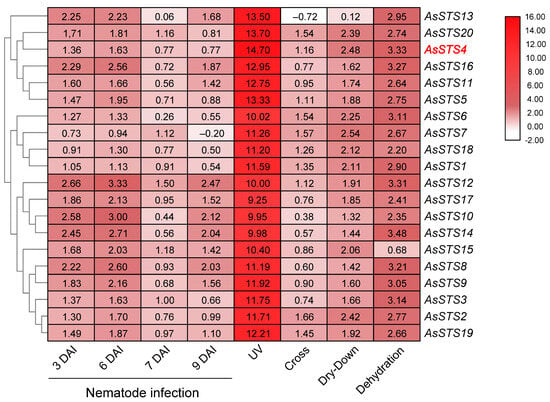
Figure 9.
Heatmap of the in silico expression patterns of 20 Arachis stenosperma STS genes in response to different types of stresses: nematode infection (at 3, 6, 7, and 9 days after infection; DAI); ultraviolet (UV) exposure; drought treatments (dry-down and dehydration); and combined drought imposition and nematode infection (cross). The color key represents differential gene expression magnitude in Log2 fold change (FC) values.
On the other hand, the transcript levels of the five A. stenosperma CHS genes were slightly affected by abiotic and biotic stresses analyzed, showing general magnitudes of expression much lower than those observed for STS genes (Figure S3). AsCHS5 was the only gene significantly induced by dehydration and repressed upon UV stress or during the earlier stages (3 and 6 DAI) of nematode infection.
Further analysis showed that the number of transcriptional regulatory elements in the promoter region varied among the 20 A. stenosperma STS genes, ranging from 15 to 37 (Figure S4). We observed that the six genes forming the group with the highest expression abundance across all stresses (AsSTS4, 5, 11, 13, 16, and 20; Figure 9) have different types and numbers of cis-elements in their promoters (Figure S4). The exception was AsSTS4, which, besides exhibiting the most significant induction upon UV stress, also showed the highest number of cis-elements (33 in total) distributed in the three categories (LRE, HRE, and STRE) involved in plant stress responses, such as MYB, ABRE, STRE, MYC, and Box4 (Figure S4). Therefore, to better understand the role of wild Arachis STS genes in the molecular response underlying their general induction by different biotic and abiotic stresses, we selected the AsSTS4 gene for further in planta functional studies.
2.7. Functional Characterization of AsSTS4 by Overexpression in Peanut Hairy Roots
Roots emerging from the petiole-wounding site of peanut detached leaves 20 days after A. rhizogenes transformation were analyzed for GFP fluorescence. Most (90%) detached leaves transformed with pPZP-empty and pPZP-AsSTS4 vectors produced eGFP-positive hairy roots, with an average of six hairy roots per leaf. Only roots that displayed GFP fluorescence and typical hairy root phenotype were inoculated with nematodes (M. arenaria) approximately 30 days after A. rhizogenes transformation (Figure 10A,B).
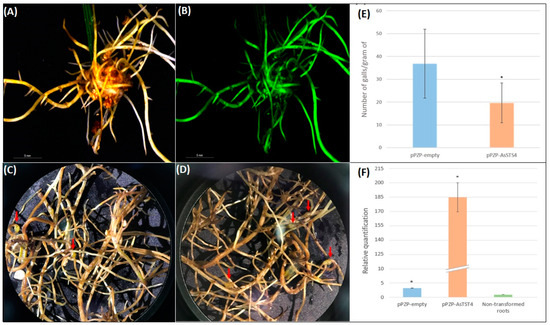
Figure 10.
Peanut hairy roots transformed with A. rhizogenes harboring pPZP-AsSTS4 vector and observed under a stereomicroscope using bright field (A) and epifluorescence (B) 30 days after A. rhizogenes transformation. Transgenic peanut hairy roots transformed with pPZP- AsSTS4 (C) and pPZP-empty vectors (D), showing gall formation 60 days after M. arenaria inoculation. Mean number of galls per gram of transgenic hairy roots (E) and relative quantification of AsSTS4 gene in transgenic peanut hairy roots transformed with pPZP-empty, pPZP-ASSTS4, and in non-transformed control roots (F). Arrows indicate nematode galls. Error bars mean the standard error of samples, and asterisks mean significant differences between samples (p < 0.05; Student’s t-test).
The effect of AsSTS4 overexpression in nematode infection was evaluated in the hairy roots at 60 days after inoculation by analyzing the root biomass and the number of galls. Hairy roots transformed with pPZP-AsSTS4 showed an average biomass of 0.29 ± 0.09 g per detached leaf that did not differ significantly (p < 0.05, t-test) from that observed in roots transformed with pPZP-empty vector (0.25 ± 0.16 g) (Figure 10C,D), suggesting that the induction and development of hairy roots were not affected by AsSTS4 overexpression. Nematode infection was further confirmed in GFP-positive hairy roots derived from pPZP-empty vector transformation by gall development (average of 36.87 galls per root gram) (Figure 10C,E), corroborating previous studies demonstrate that M. arenaria could penetrate and develop inside RNK-susceptible peanut hairy roots [38,39,40]. In contrast, the overexpression of AsSTS4 reduced the ability of M. arenaria to complete its life cycle in transgenic hairy roots (average of 19.65 galls per root gram) (Figure 10D,E), promoting a substantial and significant (p < 0.05, t-test) reduction of 46.71% in nematode infection in comparison to the control roots that did not express the transgene.
In addition, qRT-PCR analysis showed that AsSTS4 overexpression in transgenic roots was 185 times higher than in the non-transformed controls (Figure 10F), confirming the transgenic status of the leaf-derived peanut hairy roots. We also observed the expression of an endogenous AsSTS4 ortholog gene (3.2 X) in the hairy roots transformed with the pPZP-empty (Figure 10F), probably due to the intrinsic ability of peanut hairy roots to enhance resveratrol production, even under non-elicited conditions [41].
3. Discussion
Wild Arachis species evolved in their native South America several adaptive traits associated with defense and survival under stressful environmental conditions, among which the production and accumulation of phenolic-like compounds or phytoalexins seem to play a primary role [12,13,42]. Resveratrol is a major phytoalexin involved in constitutive and inducible defense reactions of Arachis species against bioaggressors, including fungi, bacteria, nematodes, and herbivores, and in response to several abiotic stressors, such as UV irradiation, wounding, drought, or extreme temperatures [10,42]. Arachis is one of the few plant genera that naturally produce resveratrol, and our recent findings demonstrate that this particularity is ubiquitously present in wild and cultivated species belonging to the different sections of the genus [12,13]. Thus, resveratrol and derivatives are important components of the defense mechanisms evolved by Arachis to cope with environmental constraints. Moreover, besides resveratrol, interest has been increasing in novel bioactive prenylated stilbenoids produced by both wild and cultivated Arachis, such as arachidins, and their potential medical benefits have been reported [16,41,42]. Here, the comprehensive characterization of CHS and STS genes in four Arachis species expands the molecular and evolutionary understanding of gene families involved in developing defensive secondary metabolites found during the environmental adaptation of wild species.
Previous studies have identified and characterized members of CHS and STS gene families in peanut (A. hypogaea), whereas there have been no reports on these families on wild Arachis species [43,44,45,46]. The allopolyploid domesticated peanut, with an AABB genome, arose from natural hybridization between the diploid ancestors A. duranensis (genome A) and A. ipaënsis (genome B), followed by spontaneous chromosome duplication [23]. Arachis genome evolution studies suggest that wild progenitors experienced one round of whole genome duplication, whereas the cultivated peanut experienced two rounds, with few changes in A- and B-subgenomes since polyploidization [24]. In the present study, the comprehensive characterization of CHS and STS gene families identified 52 members in A. hypogaea, whereas 27 and 26 members in A. duranensis and A. ipaënsis, respectively. As expected, the number of CHS (6) and STS (46) genes in the tetraploid A. hypogaea is almost equivalent to the combined value in its diploid wild progenitors, i.e., 8 CHS and 45 STS. Similar results have been reported for genome-wide analysis of the Phospholipase D gene family in Arachis [47] and for other gene families in Brassica and Gossypium allotetraploid species when compared with their corresponding diploid progenitors [48,49,50].
Analysis of the intron-exon organization of the 111 Arachis STS genes revealed a well-conserved intra- and interspecies structure in terms of both number and length of introns, which is consistent with the previously described structure for STS genes in plants [19,20]. The exception from the general 2-exon/1-intron organization of Arachis STS genes is A. ipaënsis, which showed dissimilar structures for some genes, such as intron absence or too big introns, which can be due to sequencing errors or genome annotation problems in this species. This highly conserved exon–intron architecture in both tetraploid and diploid genomes suggests that A. hypogaea STS genes did not undergo any mutation impact, either intron loss or intron gain during the polyploidization.
In addition to sharing very similar exon–intron arrangements, almost all Arachis STS genes (88%) are physically located in single aligned clusters in a single chromosome of each diploid species or the collinear chromosomes of the tetraploid species. This clustering within a limited region in a specific chromosome seems to be a particular feature of plant STS genes and was previously reported for grapevine and mulberry, two other resveratrol-producing plants for which a comprehensive analysis of the STS family was conducted [7,18,19,20]. In accordance with these studies, we found that the Arachis STS gene clusters are characterized by the presence of STS pseudogenes and other non-STS functional genes along with functional STS genes and enriched for TE copies, including retrotransposons and DNA transposons. TEs play a predominant role in cluster formation by chromosomal rearrangements, and their occurrence throughout the STS clusters can be associated with the frequency of recombination events in these regions. TE-rich regions are also often associated with genes involved in immunity or secondary metabolism in plants and are considered relevant compartments for phenotypic plasticity and adaptation to stress [51]. In addition, TEs are dynamic components of wild Arachis genomes, contributing very substantially as a primary driving force for the divergence of the A and B genomes [21,22,23,52]. In plants, genes involved in the biosynthesis of specialized metabolites are commonly clustered together with a coordinated expression to ensure sufficient production of metabolites and prevent the accumulation of toxic metabolic intermediates [53]. The presence of TEs facilitates the formation of these metabolic gene clusters. Moreover, as observed for Arachis STS, plant metabolic gene clusters comprise primarily the genes responsible for determining a class of metabolites with sizes ranging from 35 kb to several hundred kb [53]. However, to date, the phenomenon of plant metabolic gene clustering has not been associated with stilbenoids, with only two other gene clusters involved with the phenylpropanoid biosynthetic pathways so far being described [54].
Here, the clustered Arachis STS genes are also characterized by the presence of several common cis-acting elements in their promoter regions. As observed for the number of STS genes, ploidy level is the leading cause of cis-acting element number variation among Arachis species. In the category related to hormone response, cis-elements involved in ABA, ethylene, and MeJA signaling and biosynthesis (ABRE, ERE, and CGCTCA-, and TGACG-motif) were found with the highest frequency (72%) in the STS promoters of all Arachis species. In the category related to stress response, the most common (88%) cis-elements are those involved in plant responsiveness to drought, cold, salt, wounding, heat, and anaerobic conditions, such as STRE, MYB, ARE, W-box, and WUN-motif. Likewise, two ubiquitous regulatory elements associated with light-controlled transcriptional activities in plants (Box 4 and G-Box) are highly represented (58%) in the light-responsive category. The occurrence pattern of these well-recognized cis-elements involved in plant responsiveness to different environmental elicitors might reflect the importance of transcriptional regulation of STS genes and the resulting resveratrol accumulation in the adaptation and appropriate development of wild Arachis under stressful environmental conditions [55,56]. In particular, the promoter regions of the majority of STS genes contain the MBS (MYB-binding site) cis-elements that can be recognized by MYB transcription factors, which have been characterized as regulators of stilbene synthesis in grapevine [57]. Likewise, W-box elements (WRKY-binding sites) are also found in the promoter regions of some primary and specialized metabolism genes, including CHS and PAL [56]. Some of the most frequent elements found in this study (Box 4, ABRE, G-Box, ARE, and CGTCA- and TGACG-motif) were also identified in the promoter regions of four A. hypogaea STS genes, suggesting that they may be responsible for the regulation of STS expression during biotic and abiotic stress responses through MeJA and SA signaling [43].
We also observed that the clustered A. stenosperma STS genes displayed very similar gene expression behavior in response to each of the eight distinct biotic and abiotic stresses, both in terms of behavior and magnitude. In particular, they are strongly induced by UV exposure, which agrees with our previous studies showing that UV radiation drastically alters the expression of STS genes in various Arachis species [11,12,13,14]. This marked activation of STS gene expression in wild and cultivated Arachis is associated with increased resveratrol content after UV exposure. In particular, A. stenosperma is well-known to harbor high resistance levels against the RKN M. arenaria and multiple fungal diseases [58,59]. Therefore, as a critical phytoalexin selectively accumulated in response to biotic stress, enhanced accumulation of resveratrol could play a direct role in the broad-spectrum defense mechanisms developed by this wild species to withstand pathogen pressure.
In resveratrol-producing plants, CHS and STS share the same substrate to produce naringenin chalcone and resveratrol, respectively. Previous studies suggest that under stress conditions, these plants divert the common substrate to the resveratrol synthesis pathway over the naringenin chalcone synthesis in a competitive or inhibitory relationship [18,60,61]. Here, we also found that UV exposure strongly induced the expression of all 20 A. stenosperma STS genes, which are directly involved in resveratrol biosynthesis. In contrast, the five CHS genes are slightly responsive to UV. Likewise, STSs were upregulated by all the other biotic and abiotic stresses tested, whilst they did not significantly affect the expression of CHS genes. Notably, the number of STS members identified in the four Arachis species is four to eight times higher when compared to CHS members, regardless of their ploidy levels. This larger size of the STS family compared to CHS was also reported in other resveratrol-producing plants, with grapevine and mulberry showing 3.5 and 1.6 more STS than CHS complete genes, respectively [19,20]. Conversely, in non-producing resveratrol eudicots plants, CHS form large gene families, such as in soybean and mango (both with 21 members) and cotton (20 members), whilst no STS genes have been identified [27,28,29]. In accordance with [43], our phylogenetic analysis confirms the independent evolution of STS genes in Arachis species from 11 non-producing resveratrol legumes. Therefore, as proposed for grapevine and Polygonum [18,60,61], our findings reinforce the existence of antagonism between flavonol and stilbene biosynthesis, which prioritizes resveratrol accumulation under stress conditions and is likely to play an important role in the adaptation of wild Arachis to stressful environments [6].
Based on the highly conserved gene structure, the presence of common regulatory elements, and the very similar gene expression behavior in response to distinct types of stress, functional conservation of Arachis STS corresponding proteins might be predicted among the different Arachis species. Indeed, all Arachis STS share extensive amino acid sequence identity, with the conserved motifs 1, 3, and 4 representing the Chal_sti_synt_N domain and motifs 2 and 5 comprising the Chal_sti_synt_C domain present in all CHS and STS proteins, suggesting conserved evolution. Moreover, they show absolute conservation of the internal active site, including the Cys-His-Asn catalytic triad inherited from the KAS III ancestor, which is considered a very important feature for the catalytic function of CHS and STS enzymes in plants [1]. The phylogenetic relationships of Arachis proteins also showed that the CHS clustered separately, as outgroups, from all STS. Both CHS and STS clades formed subclades according to the chromosome position of their corresponding genes. The existence of a unique clade composed exclusively of STS suggests a conservation of the biological function amongst all Arachis STS proteins.
STS are stress-inducible downstream genes, and the consequent accumulation of resveratrol seems to be an important component of Arachis mechanisms underlying defense responses against biotic and abiotic stresses. In order to shed light on the role of wild Arachis STS genes in stress responses, we selected the A. stenosperma AsSTS4 gene as the candidate for a further functional assignment. Besides being highly responsive to different types of stresses, AsSTS4 presented the highest number of cis-regulatory elements involved in sensing environmental signals. The in planta validation of biological functions of candidate genes is a critical step to reveal its usefulness in the future for genetic breeding programs towards resistance to environmental stresses. Therefore, AsSTS4 was overexpressed in transgenic hairy roots of an RKN-susceptible peanut genotype, and its effect on RKN infection was evaluated. Our results showed that AsSTS4 significantly improved resistance against M. arenaria, one of the most damaging phytoparasites to peanut yield worldwide, causing significant crop loss and expensive control measures [62].
Previous studies suggested that resveratrol, besides its classical role as antimicrobial phytoalexin, constitutes an important regulator for the initiation of a hypersensitive response (HR) cell death, as described for A. stenosperma against M. arenaria [58,63,64]. Overexpression of STS genes isolated from cultivated peanut has already been reported in transgenic sweet potato and rice, both aiming to produce resveratrol in heterologous systems [17,45,46]. To our knowledge, the present study is the first report that applies the heterologous overexpression of an STS gene to increase nematode resistance, providing new functional insights into its role in biotic stress responses. As wild Arachis is one of the few plant genera that naturally synthesize resveratrol, our findings offer new perspectives for the biotechnological exploitation of these wild STS genes to increase plant resistance to biotic and abiotic stresses along with their pharmaceutical, cosmetic, and industrial purposes.
4. Conclusions
In the present study, peanut and its wild relatives join grapevine and mulberry as resveratrol-producing plants for which a comprehensive analysis of the CHS and STS gene families has been conducted. A total of 52 members were identified in allopolyploid domesticated peanut, while half were found in its wild diploid relatives A. duranensis, A. stenosperma, and A. ipaënsis (27, 25, and 26 members, respectively). This comprehensive analysis revealed strong conservation of these Arachis genes regarding gene and protein structures and the presence of common regulatory elements involved in stress signaling responses. In particular, the clustering of Arachis STS genes in one chromosome and their coordinated response to stress suggest a functional role for this physical organization, such as the plant metabolic gene clustering phenomenon, which has not yet been described for plant metabolic genes involved with the phenylpropanoid biosynthetic pathways. The general induction of Arachis STS genes in response to biotic and abiotic stress conditions, not observed for the CHS genes, corroborates the existence of an antagonism between these competitive biosynthesis pathways that prioritizes induction of STS genes under stressful conditions. Moreover, the overexpression of the wild AsSTS4 gene enhanced the resistance of peanut roots to RKN, which might be further exploited for biotechnological purposes. Overall, these findings support the idea that Arachis STS family members and resveratrol accumulation play an important role in adapting wild Arachis to stressful environments, increasing our understanding of the constitutive and inducible defense reactions mediated by this relevant secondary metabolite.
5. Materials and Methods
5.1. Identification of CHS and STS Gene Families in Four Arachis Species
Two steps were used to identify CHS and STS gene family members in Arachis spp. First, a gene family search in the PeanutBase database (http://peanutbase.org/; accessed on 15 May 2023) was performed using the Prosite entry PS00441 equivalent to CHS/STS active site (https://prosite.expasy.org/, accessed on 5 May 2023), and also the InterPro entry IPR011141, corresponding to type III PKSs family domains (https://www.ebi.ac.uk/interpro/, accessed on 15 May 2023). The search was conducted in the annotated genomes of cultivated peanut (A. hypogaea Tifrunner v. 1.0) and two wild Arachis species (A. duranensis V14167 v.1.0 and A. ipaënsis K30076 v. 1.0). Next, each putative CHS and STS genemodel sequence was used as a query for nucleotide BLAST (BLASTn) search against the genomic sequences of A. hypogaea, A. duranensis, A. ipaënsis, and A. stenosperma from both NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 10 June 2023) and PeanutBase (http://peanutbase.org/, accessed on 10 June 2023). Non-redundant CHS and STS genes were selected. The molecular weight and other physicochemical properties were predicted for inferred CHS and STS protein sequences using the web-based tool ExPASy (https://web.expasy.org/, accessed on 21 July 2023). The CHS and STS protein sequences were used as input for the Plant-mSubP webserver (http://bioinfo.usu.edu/Plant-mSubP/, accessed on 30 July 2023) to predict their subcellular localization. All databases and tools were used with default settings.
5.2. Gene Localization and Synteny Analysis of Arachis STS/CHS Genes
The MCScanX toolkit [65] was used to search for duplication and synteny in a cross-species manner for three species (A. duranensis, A. ipaënsis, and A. hypogaea), using the collinear relations to plot the synteny graph. The output from McScanX was formatted using an in-house script (https://github.com/lbi-cenargen, accessed on 10 August 2023) to retrieve all syntenic genes from CHS and STS gene families and produce an input file for the circa visualization software v1.0 (OMGenomics; http://omgenomics.com/circa/, accessed on 20 August 2023).
The physical organization of the STS clusters on chromosomes 04 and 14 of Arachis spp. was predicted using the GBrowse tool from PeanutBase (http://peanutbase.org/, accessed on 10 July 2023), accessed on 21 July 2023. To search putative transposable elements (TEs), the mixed repeats sequences from A. ipaënsis, A. duranensis, and A. hypogaea identified by [21] were used as a query for BLASTn search against the genome sequence of each STS cluster. The distribution and orientation of STS, CHS, TEs, STS pseudogenes, and other functional genes within each cluster were then arranged using SnapGene® software v5.0.8 (https://www.snapgene.com, accessed on 25 July 2023).
5.3. Arachis CHS and STS Gene and Protein Structures
The exon–intron organization of Arachis CHS and STS genes retrieved from PeanutBase (http://peanutbase.org/, accessed on 10 June 2023) was represented using GSDS software v2.0 (http://gsds.gao-lab.org, accessed on 21 June 2023). Protein sequences were submitted to the web-server based tool DomainViz (https://uhrigprotools.biology.ualberta.ca/, accessed on 15 June 2023) to visualize the distribution and organization of conserved PFAM domains. The online tool MEME Suite (https://meme-suite.org/meme/, accessed on 25 June 2023) was used to predict conserved motifs in Arachis CHS and STS protein sequences, with the motif length ranging from 6 to 100 residues and maximum number of motifs to 10. A phylogenetic tree was constructed using MEGA 11 software v11.0.13 [66] and then visualized with protein motifs via TBtools-II software v2.0 [67].
5.4. Phylogenetic Analysis of CHS and STS Members in Arachis spp.
The amino acid sequences predicted from the STS and CHS gene sequences of A. duranensis, A. ipaënsis, A. stenosperma, and A. hypogaea were aligned and used to obtain a phylogenetic tree using MEGA 11 software [66]. The sequence alignment, obtained using Clustal W, was used to infer evolutionary history using the Maximum Parsimony method that yielded a 1000 replicates bootstrap consensus tree.
5.5. Analysis of Cis-Elements of CHS and STS Upstream Sequences
For the analysis of cis-acting regulatory elements, the sequence of 1500 nucleotides upstream of the translation initiation codon of each CHS and STS gene from A. duranensis, A. ipaënsis, A. stenosperma, and A. hypogaea was retrieved from Phytozome (https://phytozome-next.jgi.doe.gov/, accessed on 30 August 2023) and PeanutBase (https://peanutbase.org/home, accessed on 30 August 2023) databases.
The extracted sequences of promoter regions were submitted to the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 17 September 2023 [68] for the prediction of cis-acting elements. All results were manually mined, and resultant hits of different cis-regulatory elements in each Arachis CHS and STS gene were visualized using TBtools-II [67].
5.6. Expression Analysis of CHS and STS Genes in Response to Biotic and Abiotic Stresses
The expression profiles of A. stenosperma STS genes were subjected to heatmap construction by TBtools-II software 2.0 [67] using our previously produced transcriptome RNA-Seq data from plants submitted to different types of stresses: M. arenaria infection (at 3, 6, 7 and 9 DAI); UV exposure; moderate (dry-down) and severe (dehydration) drought treatments; and combined drought and nematode stresses (cross-stress) [36,63,69,70].
5.7. Overexpression of AsSTS4 Gene in Peanut Hairy Roots
The predicted coding region (1173 bp; Table S3) of AsSTS4 from A. stenosperma was synthesized and cloned, under the control of the Arabidopsis thaliana actin 2 promoter, into the unique XhoI restriction site of the binary vector pPZP-BAR [70] by Epoch Life Science (Missouri City, TX, USA). The obtained vector pPZP-AsSTS4 and the corresponding empty pPZP-BAR vector, hereafter called pPZP-empty, were then introduced into the wild cucumopine-type Agrobacterium rhizogenes strain ‘K599’ and grown on selective Luria–Bertani (LB) medium to produce a fresh bacterial paste inoculum, essentially as described by [39].
Hairy roots of the RKN-susceptible peanut ‘Runner IAC-866’ were produced by the ex vitro detached leaf method previously established by our group [38,39], using quadrifoliate leaves harvested from one-month-old peanut plants grown under growth chamber conditions (25 ± 2 °C; 12 h photoperiod; 120 µmols m−2 s−1 light intensity). Detached leaves containing only eGFP-positive hairy roots were selected approximately 20 days after A. rhizogenes transformation and covered with medium-grained (5 to 8 mm) vermiculite for nematode infection.
5.8. Hairy Roots Inoculation with Meloidogyne arenaria
M. arenaria were multiplied for three months on greenhouse-grown tomato (Solanum lycopersicum ‘Santa Clara’) plants and second-stage juveniles (J2) collected essentially as described by [40]. The identity of M. arenaria inoculum was confirmed by PCR analysis using a specific SCAR marker [71], as previously established [72]. GFP-positive hairy roots transformed with pPZP-AsSTS4 and pPZP-empty vectors were then challenged with approximately 1000 J2 of M. arenaria according to [38] and maintained in growth chamber conditions.
At 60 DAI, hairy roots were carefully removed from the vermiculite, washed in running water, and weighted. The nematode infection in hairy roots transformed with both pPZP-AsAsSTS4 and pPZP-empty vectors was assessed by counting the number of galls under a stereomicroscope (Stemi 508, Zeiss, Oberkochen, Germany) and statistically analyzed using Student’s t-test (p < 0.05).
5.9. Transgene Expression Profiling
qRT-PCR analysis of AsSTS4 expression in peanut hairy roots was evaluated using specific primer pairs (5′-3′) STS1 (AAGGCCATCAAGGAATGGGG/ATGGGTCGAGGCCTAAGAGT) [36]. Total RNA was extracted from hairy roots in biological triplicates using the RNeasy Plant Mini kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. As a negative control, total RNA was extracted from non-transformed roots of two-week-old peanut plantlets grown under growth chamber conditions. RNA purification, assessment of the RNA integrity, and cDNA synthesis were performed according to [73]. qRT-PCR reactions were conducted on a StepOne Plus Real-Time PCR System (Applied Biosystems, Foster City, USA), as described by [72]. Primer efficiency was determined by the online real-time PCR Miner tool [74], and the quantification of AsSTS4 expression was estimated using the SATqPCR website [75]. Arachis GAPDH and 60S reference genes were used to normalize AsSTS4 expression in accordance with [76].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14122181/s1, Table S1: Members of CHS and STS families in Arachis duranensis genome. Table S2: Members of CHS and STS families in Arachis ipaënsis genome. Table S3: Members of CHS and STS families in Arachis stenosperma genome. Table S4. Members of CHS and STS families in Arachis hypogaea genome. Figure S1: Gene tree of Arachis CHS and STS syntenic genes of three Arachis species (A. duranensis, A. ipaënsis, and A. hypogaea), 11 legumes and four non-legume species. Figure S2: Alignment of CHS and STS amino acid sequences of (A) Arachis duranensis; (B) A. ipaënsis; (C) A. stenosperma; and (D) A. hypogaea. (1) = STS and CHS specific residues around Met98; (2) = STS and CHS specific residues around Thr132; (3) = CHS/STS active site (Prosite entry PS00441); (4) = CHS/STS signature motif. Figure S3: Heatmap of the in silico expression patterns of five Arachis stenosperma CHS genes in response to different types of stresses: nematode infection (at 3, 6, 7, and 9 days after infection; DAI); Ultraviolet (UV) exposure; drought treatments (dry-Down and dehydration); and combined drought imposition and nematode infection (cross). The color key represents differential gene expression magnitude in Log2 fold change (FC) values. Figure S4: Heatmap of cis-acting elements (A) and their corresponding categories (B) in the promoter regions of 20 Arachis stenosperma STS genes. The heatmap colors ranged from red to blue, where red dark indicated increasing values in the numbers of cis-elements and blue scale decreasing values. Cis-acting elements are distributed in categories associated with responses to hormones (HRE), light (LRE), and stress (STRE) and related to tissue specificity and development (TS&DE).
Author Contributions
A.C.M.B., M.A.G. and P.M.G. and contributed to the conception, design of the work, data analysis and interpretation, and drafting and critical revision of the manuscript. B.M.P. and M.N.A. conducted hairy roots transformation and critical revision of the manuscript, A.P.Z.M. conducted bioinformatics analysis and critical revision of the manuscript. A.C.Q.M. conducted gene cloning and critical revision of the manuscript. M.A.S.P. conducted qRT-PCR analysis. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge grants support from Empresa Brasileira de Pesquisa Agropecuária (Embrapa); Brazilian National Council for Scientific and Technological Development (CNPq; project number 403942/2021-7); INCT PlantStress (project number 465480/2014-4); Coordination for the Improvement of Higher Education Personnel (CAPES) and Distrito Federal Research Foundation (FAPDF; project number 00193-00000755/2021-91). Each of the funding bodies granted the funds based on a research proposal. They did not influence the experimental design, data analysis or interpretation, or manuscript writing.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Genome references for the genome and gene annotation data were downloaded from the following NCBI GenBank assembly accessions: Arachis hypogaea (GCF_003086295.2); Arachis duranensis (GCF_000817695.3); Arachis ipaënsis (GCF_000816755.2); and Arachis stenosperma (GCF_014773155.1).
Acknowledgments
The authors would like to thank Regina M.D.G. Carneiro for providing nematode populations and Leandro Mesquita for greenhouse assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CHS = Chalcone Synthase; |
| DAI = Days after Inoculation; |
| FC = Fold-Change; |
| HR = Hypersensitive Response; |
| PKS = Polyketide Synthases |
| qRT-PCR = quantitative Reverse Transcription-Polymerase Chain Reaction; |
| RKN = Root-Knot Nematode; |
| STS = Stilbene Synthase; |
| TE = Transposable Element; |
| UV = Ultraviolet. |
References
- Austin, M.B.; Bowman, M.E.; Ferrer, J.-L.; Schröder, J.; Noel, J.P. An aldol switch discovered in stilbene synthases mediates cyclization specificity of type III polyketide synthases. Chem. Biol. 2004, 11, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, X. Healthy benefits and edible delivery systems of resveratrol: A review. Food Rev. Int. 2021, 39, 3879–3905. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.B.; Pandey, R.P.; Park, Y.I.; Sohng, J.K. Biotechnological advances in resveratrol production and its chemical diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Chen, J.; Ye, W.; Liao, K.; Wang, Z.; Song, X.; Qiao, M. Synthetic biology-driven microbial production of resveratrol: Advances and perspectives. Front. Bioeng. Biotechnol. 2022, 10, 833920. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wang, H.; Zhang, S.; Lan, T. The type III polyketide synthase supergene family in plants: Complex evolutionary history and functional divergence. Plant J. 2022, 112, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Naake, T.; Maeda, H.A.; Proost, S.; Tohge, T.; Fernie, A.R. Kingdom-wide analysis of the evolution of the plant type III polyketide synthase superfamily. Plant Physiol. 2021, 185, 857–875. [Google Scholar] [CrossRef] [PubMed]
- Tropf, S.; Lanz, T.; Rensing, S.A.; Schröder, J.; Schröder, G. Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J. Mol. Evol. 1994, 38, 610–618. [Google Scholar] [CrossRef]
- Teka, T.; Lele, Z.; Xiaoyan, G.; Li, Y.; Lifeng, H.; Xiaohui, Y. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- Hasan, M.M.; Cha, M.; Bajpai, V.K.; Baek, K.-H. Production of a major stilbene phytoalexin, resveratrol in peanut (Arachis hypogaea) and peanut products: A mini review. Rev. Environ. Sci. Bio Technol. 2013, 12, 209–221. [Google Scholar] [CrossRef]
- Carvalho, P.A.S.V.; Brasileiro, A.C.M.; Leal-Bertioli, S.C.M.; Bertioli, D.J.; Silva, J.P.; Agostini-Costa, T.S.; Gimenes, M.A. Coupled transcript and metabolite identification: Insights on induction and synthesis of resveratrol in peanut, wild relatives and synthetic allotetraploid. Genet. Mol. Res. 2017, 16, gmr16039802. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.A.S.V.; de Carvalho Moretzsohn, M.; Brasileiro, A.C.M.; Guimaraes, P.M.; Agostini-Costa, T.S.; da Silva, J.P.; Gimenes, M.A. Presence of resveratrol in wild Arachis species adds new value to this overlooked genetic resource. Sci. Rep. 2020, 10, 12787. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.V.; de Carvalho Moretzsohn, M.; Brasileiro, A.C.M.; Guimarães, P.M.; Costa, T.d.S.A.; da Silva, J.P.; Gimenes, M.A. Evidences that polyploidization and hybridization affected resveratrol content in Arachis interspecific hybrids. J. Plant Breed. Crop. Sci. 2019, 11, 265–270. [Google Scholar]
- Lopes, R.M.; Silveira, D.; Gimenes, M.A.; Vasconcelos, P.A.S.; Alves, R.B.N.; Silva, J.P.; Agostini-Costa, T.S. Characterization of resveratrol content in ten wild species of section Arachis, genus Arachis. Genet. Resour. Crop. Evol. 2013, 60, 2219–2226. [Google Scholar] [CrossRef]
- Sousa-Machado, I.B.; Felippe, T.; Garcia, R.; Pacheco, G.; Moreira, D.; Mansur, E. Total phenolics, resveratrol content and antioxidant activity of seeds and calluses of pinto peanut (Arachis pintoi Krapov. & WC Greg.). Plant Cell Tissue Organ Cult. 2018, 134, 491–502. [Google Scholar]
- Fang, L.; Yang, T.; Medina-Bolivar, F. Production of prenylated stilbenoids in hairy root cultures of peanut (Arachis hypogaea) and its wild relatives A. ipaënsis and A. duranensis via an optimized elicitation procedure. Molecules 2020, 25, 509. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Dubrovina, A.S. Overexpression of stilbene synthase genes to modulate the properties of plants and plant cell cultures. Biotechnol. Appl. Biochem. 2021, 68, 13–19. [Google Scholar] [CrossRef]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef]
- Li, H.; Liang, J.; Chen, H.; Ding, G.; Ma, B.; He, N. Evolutionary and functional analysis of mulberry type III polyketide synthases. BMC Genom. 2016, 17, 540. [Google Scholar] [CrossRef]
- Parage, C.; Tavares, R.; Réty, S.; Baltenweck-Guyot, R.; Poutaraud, A.; Renault, L.; Heintz, D.; Lugan, R.; Marais, G.A.B.; Aubourg, S. Structural, functional, and evolutionary analysis of the unusually large stilbene synthase gene family in grapevine. Plant Physiol. 2012, 160, 1407–1419. [Google Scholar] [CrossRef]
- Bertioli, D.J.; Cannon, S.B.; Froenicke, L.; Huang, G.; Farmer, A.D.; Cannon, E.K.S.; Liu, X.; Gao, D.; Clevenger, J.; Dash, S.; et al. The genome sequences of Arachis duranensis and Arachis ipaënsis, the diploid ancestors of cultivated peanut. Nat. Genet. 2016, 48, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Pandey, M.K.; Yang, Q.; Wang, X.; Garg, V.; Li, H.; Chi, X.; Doddamani, D.; Hong, Y.; et al. Draft genome of the peanut A-genome progenitor (Arachis duranensis) provides insights into geocarpy, oil biosynthesis, and allergens. Proc. Natl. Acad. Sci. USA 2016, 113, 6785–6790. [Google Scholar] [CrossRef] [PubMed]
- Bertioli, D.J.; Jenkins, J.; Clevenger, J.; Dudchenko, O.; Gao, D.; Seijo, G.; Leal-Bertioli, S.C.M.; Ren, L.; Farmer, A.D.; Pandey, M.K. The genome sequence of segmental allotetraploid peanut Arachis hypogaea. Nat. Genet. 2019, 51, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Q.; Liu, H.; Zhang, J.; Hong, Y.; Lan, H.; Li, H.; Wang, J.; Liu, H.; Li, S. Sequencing of cultivated peanut, Arachis hypogaea, yields insights into genome evolution and oil improvement. Mol. Plant 2019, 12, 920–934. [Google Scholar] [CrossRef]
- Zhuang, W.; Chen, H.; Yang, M.; Wang, J.; Pandey, M.K.; Zhang, C.; Chang, W.-C.; Zhang, L.; Zhang, X.; Tang, R. The genome of cultivated peanut provides insight into legume karyotypes, polyploid evolution and crop domestication. Nat. Genet. 2019, 51, 865–876. [Google Scholar] [CrossRef]
- Shomura, Y.; Torayama, I.; Suh, D.; Xiang, T.; Kita, A.; Sankawa, U.; Miki, K. Crystal structure of stilbene synthase from Arachis hypogaea. Proteins Struct. Funct. Bioinforma. 2005, 60, 803–806. [Google Scholar] [CrossRef]
- Kong, X.; Khan, A.; Li, Z.; You, J.; Munsif, F.; Kang, H.; Zhou, R. Identification of chalcone synthase genes and their expression patterns reveal pollen abortion in cotton. Saudi J. Biol. Sci. 2020, 27, 3691–3699. [Google Scholar] [CrossRef]
- Hu, H.; Shi, B.; Zhu, W.; Zheng, B.; Zhou, K.; Qian, M.; Wu, H. Genome-wide identification, characterization and expression analysis of mango (Mangifera indica L.) chalcone synthase (CHS) genes in response to light. Horticulturae 2022, 8, 968. [Google Scholar] [CrossRef]
- Vadivel, A.K.A.; Krysiak, K.; Tian, G.; Dhaubhadel, S. Genome-wide identification and localization of chalcone synthase family in soybean (Glycine max [L] Merr). BMC Plant Biol. 2018, 18, 325. [Google Scholar]
- Vinson, C.C.; Mota, A.P.Z.; Porto, B.N.; Oliveira, T.N.; Sampaio, I.; Lacerda, A.L.; Danchin, E.G.J.; Guimarães, P.M.; Williams, T.C.R.; Brasileiro, A.C.M. Characterization of raffinose metabolism genes uncovers a wild Arachis galactinol synthase conferring tolerance to abiotic stresses. Sci. Rep. 2020, 10, 15258. [Google Scholar] [CrossRef]
- Mota, A.P.Z.; Vidigal, B.; Danchin, E.G.J.; Togawa, R.C.; Leal-Bertioli, S.C.M.; Bertioli, D.J.; Araujo, A.C.G.; Brasileiro, A.C.M.; Guimaraes, P.M. Comparative root transcriptome of wild Arachis reveals NBS-LRR genes related to nematode resistance. BMC Plant Biol. 2018, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, L.A.; Mota, A.P.Z.; Araujo, A.C.G.; Figueiredo, L.F.A.; Pereira, B.M.; Passos Saraiva, M.A.; Silva, R.B.; Danchin, E.G.J.J.; Guimaraes, P.M.; Brasileiro, A.C.M. Genome-wide analysis of expansin superfamily in wild Arachis discloses a stress-responsive expansin-like B gene. Plant Mol. Biol. 2017, 94, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Fliegmann, J.; Schröder, G.; Schanz, S.; Britsch, L.; Schröder, J. Molecular analysis of chalcone and dihydropinosylvin synthase from Scots pine (Pinus sylvestris), and differential regulation of these and related enzyme activities in stressed plants. Plant Mol. Biol. 1992, 18, 489–503. [Google Scholar] [CrossRef]
- Muccilli, V.; Licciardello, C.; Fontanini, D.; Russo, M.P.; Cunsolo, V.; Saletti, R.; Recupero, G.R.; Foti, S. Proteome analysis of Citrus sinensis L.(Osbeck) flesh at ripening time. J. Proteom. 2009, 73, 134–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Z.; Yang, Y.; Xu, H.; Bi, Q.; Wang, L. Genome-wide identification and expression analysis of the KCS gene family in yellow horn reveal their putative function on abiotic stress responses and wax accumulation. Horticulturae 2022, 9, 25. [Google Scholar] [CrossRef]
- Martins, A.C.Q.; Mota, A.P.Z.; Carvalho, P.A.S.V.; Passos, M.A.S.; Gimenes, M.A.; Guimaraes, P.M.; Brasileiro, A.C.M. Transcriptome responses of wild Arachis to UV-C exposure reveal genes involved in general plant defense and priming. Plants 2022, 11, 408. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Van Der Straeten, D.; De Coninck, B.; Vandenbussche, F. Ultraviolet radiation from a plant perspective: The plant-microorganism context. Front. Plant Sci. 2020, 11, 1984. [Google Scholar] [CrossRef]
- Pereira, B.M.; Guimaraes, L.A.; Souza, N.O.S.; Saraiva, M.A.P.; Guimaraes, P.M.; Brasileiro, A.C.M. Overexpression of wild Arachis lipocalin enhances root-knot nematode resistance in peanut hairy roots. Plant Mol. Biol. Report 2019, 37, 74–86. [Google Scholar] [CrossRef]
- Guimaraes, L.A.; Pereira, B.M.; Araujo, A.C.G.; Guimaraes, P.M.; Brasileiro, A.C.M. Ex vitro hairy root induction in detached peanut leaves for plant-nematode interaction studies. Plant Methods 2017, 13, 25. [Google Scholar] [CrossRef]
- Araujo, A.C.G.; Guimaraes, P.M.; Mota, A.P.Z.; Guimaraes, L.A.; Pereira, B.M.; Vinson, C.C.; Lacerda, A.L.; Martins, A.C.Q.; Brasileiro, A.C.M. Overexpression of DUF538 from wild Arachis enhances plant resistance to Meloidogyne spp. Agronomy 2021, 11, 559. [Google Scholar] [CrossRef]
- Medina-Bolivar, F.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S.; Khan, S.I.; Tabanca, N.; Wedge, D.E.; Manly, S.P.; Cutler, S.J.; Coy, M.R.; Becnel, J.J.; Neff, S.A.; Gloer, J.B. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic stilbenoids. J. Agric. Food Chem. 2011, 59, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, Z.; Noureen, F.; Khan, M.; Khan, M.; Haider, G.; Munir, F.; Gul, A.; Amir, R. Identification and expression analysis of stilbene synthase genes in Arachis hypogaea in response to methyl Jasmonate and salicylic acid induction. Plants 2022, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Han, J.; Liu, S.; Chen, X.; Varshney, R.K.; Liang, X. Cloning, expression pattern analysis and subcellular localization of resveratrol synthase gene in peanut (Arachis hypogaea L.). Am. J. Plant Sci. 2014, 5, 3619–3631. [Google Scholar] [CrossRef][Green Version]
- Lee, C.; Hong, W.-J.; Jung, K.-H.; Hong, H.-C.; Kim, D.-Y.; Ok, H.-C.; Choi, M.-S.; Park, S.-K.; Kim, J.; Koh, H.-J. Arachis hypogaea resveratrol synthase 3 alters the expression pattern of UDP-glycosyltransferase genes in developing rice seeds. PLoS ONE 2021, 16, e0245446. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-P.; Yu, S.-L.; Chen, C.-J.; Li, H.; Wu, Y.-L.; Li, H.-H. Cloning a peanut resveratrol synthase gene and its expression in purple sweet potato. Plant Cell Rep. 2012, 31, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, Y.; Wang, S.; Yang, J.; Ai, X.; Zhang, N.; Zhao, X.; Liu, X.; Zhong, C.; Yu, H. Genome-wide characterization of phospholipase D family genes in allotetraploid peanut and its diploid progenitors revealed their crucial roles in growth and abiotic stress responses. Front. Plant Sci. 2023, 14, 1102200. [Google Scholar] [CrossRef]
- Imran, M.; Shafiq, S.; Naeem, M.K.; Widemann, E.; Munir, M.Z.; Jensen, K.B.; Wang, R.R.-C. Histone deacetylase (HDAC) gene family in allotetraploid cotton and its diploid progenitors: In silico identification, molecular characterization, and gene expression analysis under multiple abiotic stresses, DNA damage and phytohormone treatments. Int. J. Mol. Sci. 2020, 21, 321. [Google Scholar] [CrossRef]
- Li, M.; Wang, R.; Liang, Z.; Wu, X.; Wang, J. Genome-wide identification and analysis of the EIN3/EIL gene family in allotetraploid Brassica napus reveal its potential advantages during polyploidization. BMC Plant Biol. 2019, 19, 110. [Google Scholar] [CrossRef]
- Sun, W.; Li, M.; Wang, J. Genome-wide identification and characterization of the RCI2 gene family in allotetraploid Brassica napus compared with its diploid progenitors. Int. J. Mol. Sci. 2022, 23, 614. [Google Scholar] [CrossRef]
- Dubin, M.J.; Scheid, O.M.; Becker, C. Transposons: A blessing curse. Curr. Opin. Plant Biol. 2018, 42, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Samoluk, S.S.; Chalup, L.M.I.; Chavarro, C.; Robledo, G.; Bertioli, D.J.; Jackson, S.A.; Seijo, G. Heterochromatin evolution in Arachis investigated through genome-wide analysis of repetitive DNA. Planta 2019, 249, 1405–1415. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Kumar, S.R.; Sharma, A.; Sathishkumar, R. Plant metabolic gene clusters: Evolution, organization, and their applications in synthetic biology. Front. Plant Sci. 2021, 12, 697318. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Shen, S.; Yang, C.; Liu, Z.; Fernie, A.R.; Graham, I.A.; Luo, J. Plant metabolic gene clusters in the multi-omics era. Trends Plant Sci. 2022, 27, 981–1001. [Google Scholar] [CrossRef] [PubMed]
- Marand, A.P.; Eveland, A.L.; Kaufmann, K.; Springer, N.M. cis-Regulatory elements in plant development, adaptation, and evolution. Annu. Rev. Plant Biol. 2023, 74, 111–137. [Google Scholar] [CrossRef] [PubMed]
- Sheshadri, S.A.; Nishanth, M.J.; Simon, B. Stress-mediated cis-element transcription factor interactions interconnecting primary and specialized metabolism in planta. Front. Plant Sci. 2016, 7, 1725. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y. Transcription factor VqERF114 regulates stilbene synthesis in Chinese wild Vitis quinquangularis by interacting with VqMYB35. Plant Cell Rep. 2019, 38, 1347–1360. [Google Scholar] [CrossRef]
- Proite, K.; Carneiro, R.; Falcao, R.; Gomes, A.; Leal-Bertioli, S.; Guimarães, P.; Bertioli, D. Post-infection development and histopathology of Meloidogyne arenaria race 1 on Arachis spp. Plant Pathol. 2008, 57, 974–980. [Google Scholar] [CrossRef]
- Michelotto, M.D.; Barioni, W.; De Resende, M.D.V.; De Godoy, I.J.; Leonardecz, E.; Favero, A.P. Identification of fungus resistant wild accessions and interspecific hybrids of the genus Arachis. PLoS ONE 2015, 10, e0128811. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Wu, X.; Wang, Y.; Lin, Y.; Wu, D.; Zhang, H.; Qin, J. Molecular analysis of UV-C induced resveratrol accumulation in Polygonum cuspidatum leaves. Int. J. Mol. Sci. 2019, 20, 6185. [Google Scholar] [CrossRef]
- Ciaffi, M.; Paolacci, A.R.; Paolocci, M.; Alicandri, E.; Bigini, V.; Badiani, M.; Muganu, M. Transcriptional regulation of stilbene synthases in grapevine germplasm differentially susceptible to downy mildew. BMC Plant Biol. 2019, 19, 404. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Branch, W.D.; Brenneman, T.B. Registration of ‘Georgia-SP/RKN’peanut. J. Plant Regist. 2023, 17, 47–55. [Google Scholar] [CrossRef]
- Guimaraes, P.M.; Guimaraes, L.A.; Morgante, C.V.; Silva, O.B.; Araújo, A.C.G.; Martins, A.C.Q.; Saraiva, M.A.P.; Oliveira, T.N.; Togawa, R.C.; Leal-Bertioli, S.C.M.; et al. Root transcriptome analysis of wild peanut reveals candidate genes for nematode resistance. PLoS ONE 2015, 10, e0140937. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Heene, E.; Qiao, F.; Nick, P. The phytoalexin resveratrol regulates the initiation of hypersensitive cell death in Vitis cell. PLoS ONE 2011, 6, e26405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Vinson, C.C.; Mota, A.P.Z.; Oliveira, T.N.; Guimarães, L.A.; Leal-Bertioli, S.C.M.; Williams, T.C.R.; Nepomuceno, A.L.; Saraiva, M.A.P.; Araújo, A.C.G.; Guimarães, P.M.; et al. Early responses to dehydration in contrasting wild Arachis species. PLoS ONE 2018, 13, e0198191. [Google Scholar] [CrossRef]
- Mota, A.P.Z.; Brasileiro, A.C.M.; Vidigal, B.; Oliveira, T.N.; Martins, A.d.C.Q.; Saraiva, M.A.P.; Araújo, A.C.G.; Togawa, R.C.; Grossi-de-Sá, M.F.; Guimarães, P.M. Defining the combined stress response in wild. Arachis. Sci. Rep. 2021, 11, 11097. [Google Scholar] [CrossRef]
- Zijlstra, C.; Donkers-Venne, D.T.H.M.; Fargette, M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology 2000, 2, 847–853. [Google Scholar]
- Pereira, B.M.; Arraes, F.; Martins, A.C.Q.; Alves, N.S.F.; Melo, B.P.; Morgante, C.V.; Saraiva, M.A.P.; Grossi-de-Sá, M.F.; Guimaraes, P.M.; Brasileiro, A.C.M. A novel soybean hairy root system for gene functional validation. PLoS ONE 2023, 18, e0285504. [Google Scholar] [CrossRef] [PubMed]
- Morgante, C.V.; Brasileiro, A.C.M.; Roberts, P.A.; Guimaraes, L.A.; Araujo, A.C.G.; Fonseca, L.N.; Leal-Bertioli, S.C.M.; Bertioli, D.J.; Guimaraes, P.M. A survey of genes involved in Arachis stenosperma resistance to Meloidogyne arenaria race 1. Funct. Plant Biol. 2013, 40, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fernald, R.D. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Rancurel, C.; van Tran, T.; Elie, C.; Hilliou, F. SATQPCR: Website for statistical analysis of real-time quantitative PCR data. Mol. Cell Probes 2019, 46, 101418. [Google Scholar] [CrossRef]
- Morgante, C.V.; Guimaraes, P.M.; Martins, A.; Araujo, A.C.G.; Leal-Bertioli, S.C.M.; Bertioli, D.J.; Brasileiro, A.C.M. Reference genes for quantitative reverse transcription-polymerase chain reaction expression studies in wild and cultivated peanut. BMC Res. Notes 2011, 4, 339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).