Genome-Wide Identification and Expression Analysis of Lipoxygenase Genes in Rose (Rosa chinensis)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genome-Wide Identification of LOX Gene Members in Rose
2.2. Phylogenetic and Amino acid Sequence Analysis
2.3. Chromosomal Location and Collinearity Analysis of RcLOX Genes
2.4. Gene Structure and Conserved Motifs Identified
2.5. Secondary and Tertiary Structure Prediction of RcLOX Proteins
2.6. Prediction of Cis-Acting Elements in the Promoter of Rose LOX Genes
2.7. Rose Growth Conditions and Aphid Infestation
2.8. RNA Extraction and Quantitative RT-PCR Analysis
3. Results
3.1. Identification of the LOX Genes in R. chinensis
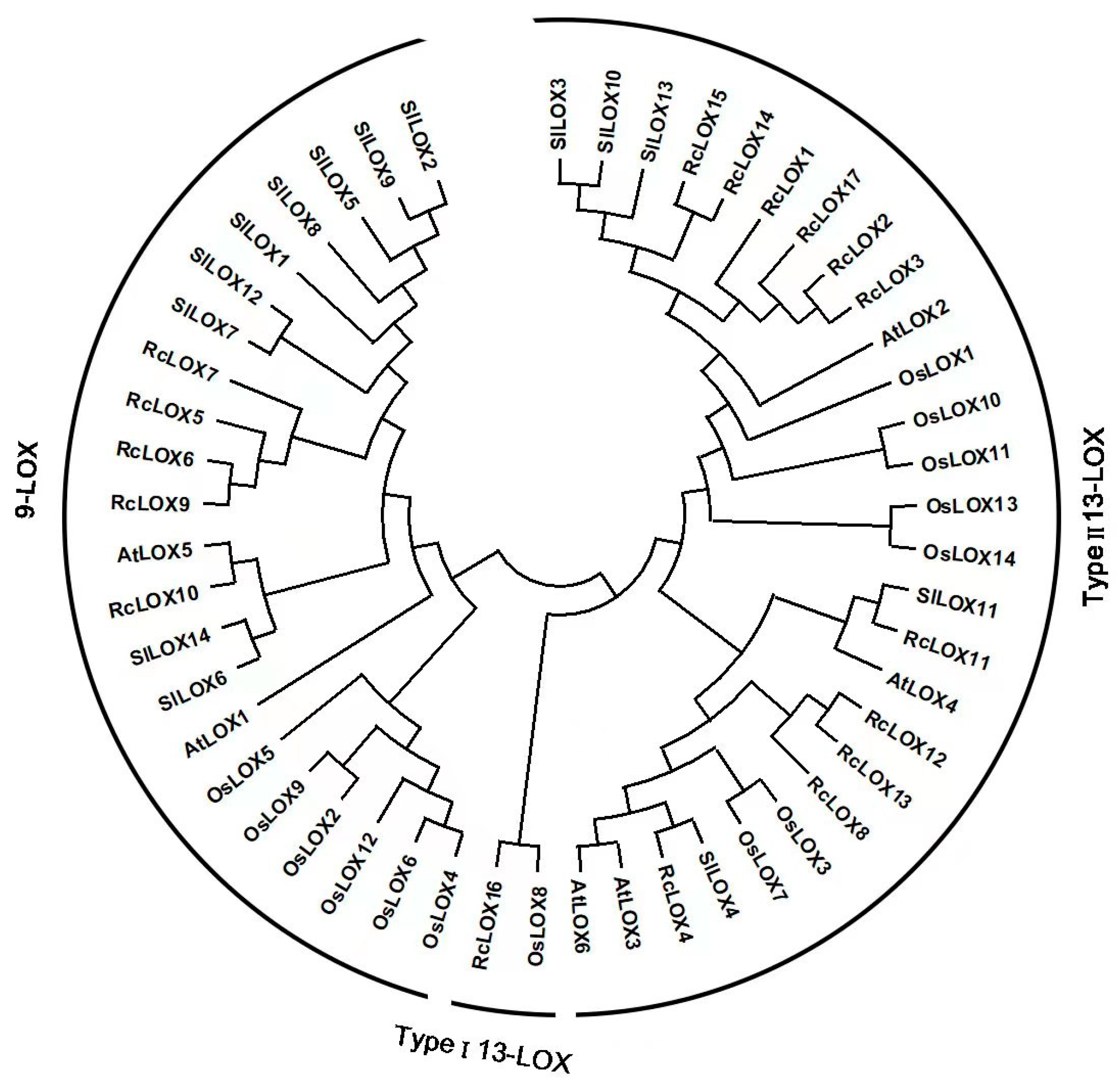
3.2. Phylogenetic Analysis of LOX Members
3.3. Chromosomal Locations and Collinearity Analysis of RcLOX Genes
3.4. Amino Acid Sequence, Conserved Motifs Analysis, and Gene Structure Analysis of RcLOX Genes
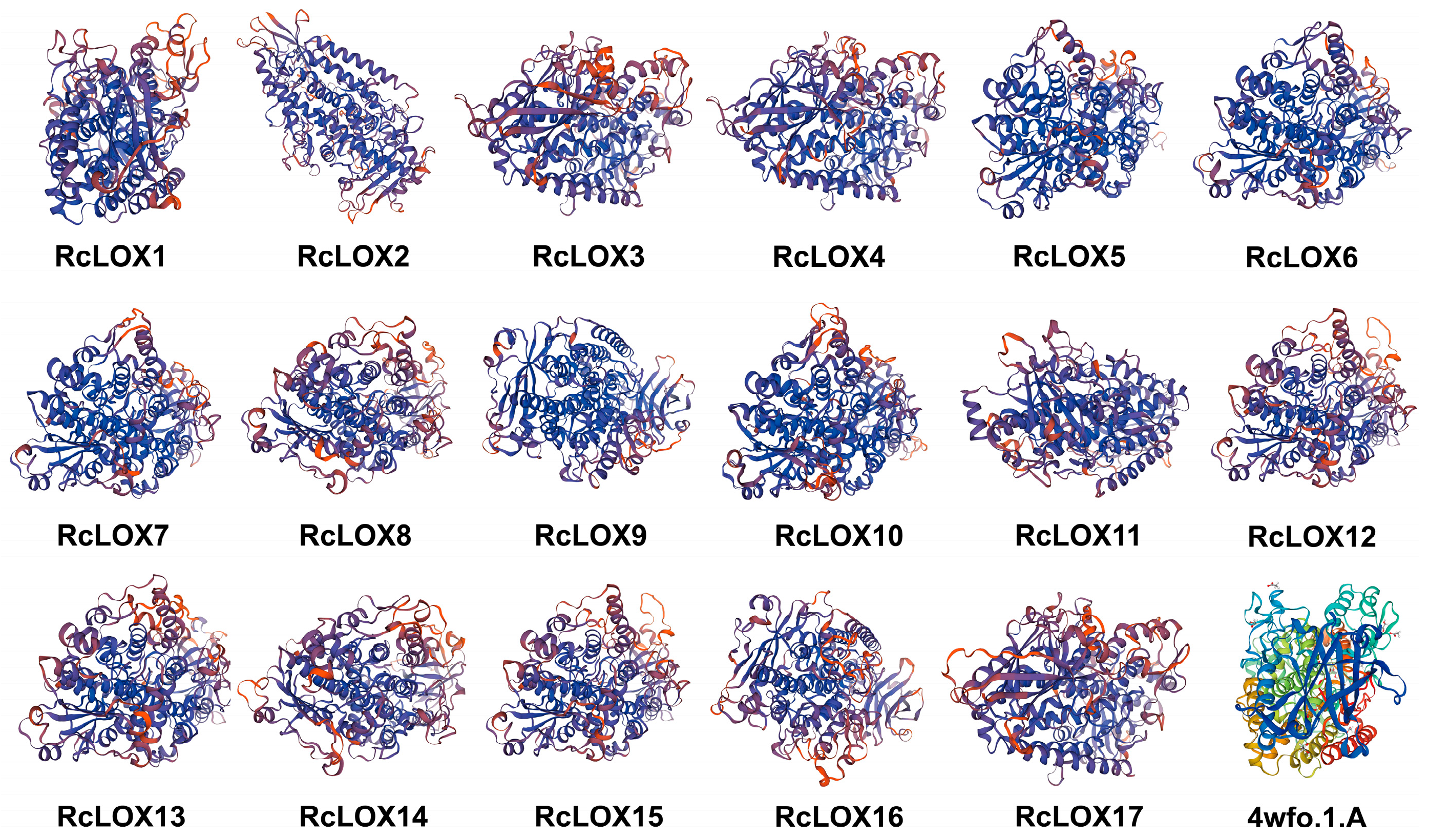
3.5. The Prediction of Secondary and Tertiary Structural Features of RcLOX Proteins
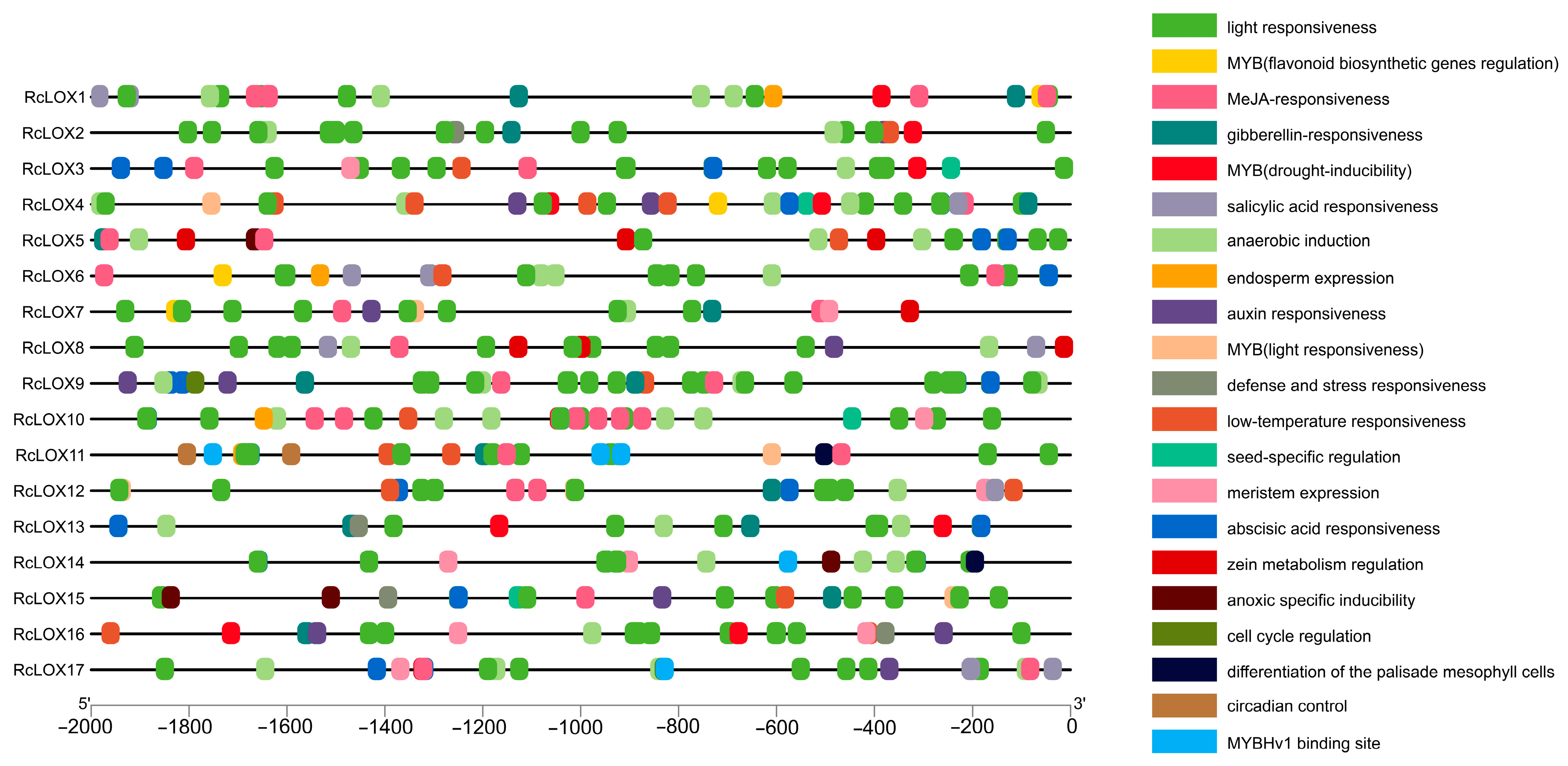
3.6. Cis-Acting Elements Prediction in the Promoter of RcLOX Genes
3.7. The Expression Patterns of RcLOX Genes in Different Tissues
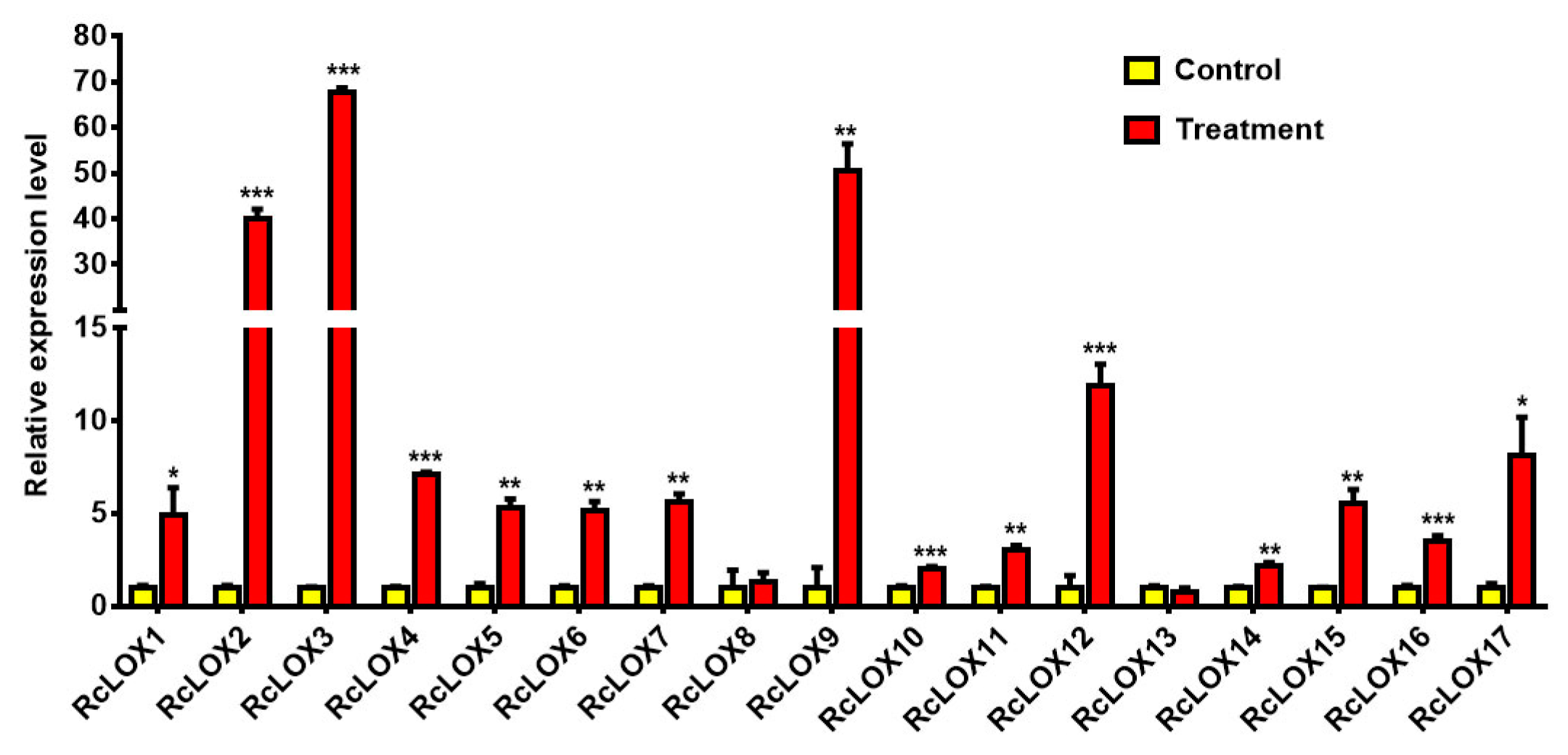
3.8. The Expression Patterns of RcLOX Genes in Rose Leaves after Aphid Infestation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Feussner, I. Lipoxygenases–structure and reaction mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015, 24, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Han, S.-W.; Hwang, I.S.; Kim, D.S.; Hwang, B.K.; Lee, S.C. The pepper lipoxygenase CaLOX1 plays a role in osmotic, drought, and high salinity. Plant Cell Physiol. 2015, 56, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, D.; Zhang, C.; Wang, X.; Yang, K.; Wang, Y.; Wang, X.; You, C. The apple lipoxygenase MdLOX3 regulates salt tolerance and ABA sensitivity. Horticulturae 2022, 8, 651. [Google Scholar] [CrossRef]
- Keereetaweep, J.; Blancaflor, E.B.; Hornung, E.; Feussner, I.; Chapman, K.D. Lipoxygenase-derived 9-hydro(pero)xides of linoleoylethanolamide interact with ABA signaling to arrest root development during Arabidopsis seedling establishment. Plant J. 2015, 82, 315–327. [Google Scholar] [CrossRef]
- Huang, J.; Cai, M.; Long, Q.; Liu, L.; Lin, Q.; Jiang, L.; Chen, S.; Wan, J. OsLOX2, a rice type I lipoxygenase, confers opposite effects on seed germination and longevity. Transgenic Res. 2014, 23, 643–655. [Google Scholar] [CrossRef]
- Zhou, S.; Li, D.; Cheng, Y.; Guan, J. Characterization of expression and enzyme activity of lipoxygenases during fruit softening and superficial scald development in ‘Wujiuxiang’ pear. J. Appl. Bot. Food Qual. 2016, 89, 307–314. [Google Scholar] [CrossRef]
- Springer, A.; Kang, C.; Rustgi, S.; von Wettstein, D.; Reinbothe, C.; Pollmann, S.; Reinbothe, S. Programmed chloroplast destruction during leaf senescence involves 13-lipoxygenase (13-LOX). Proc. Natl. Acad. Sci. USA 2016, 113, 3383–3388. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Mattoo, A.K. Genome-wide identification of tomato (Solanum lycopersicum L.) lipoxygenases coupled with expression profiles during plant development and in response to methyl-jasmonate and wounding. J. Plant Physiol. 2018, 231, 318–328. [Google Scholar] [CrossRef]
- Rahimi, S.; Kim, Y.J.; Sukweenadhi, J.; Zhang, D.; Yang, D.C. PgLOX6 encoding a lipoxygenase contributes to jasmonic acid biosynthesis and ginsenoside production in Panax ginseng. J. Exp. Bot. 2016, 67, 6007–6019. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shen, W.; Liu, L.; Jiang, L.; Liu, Y.; Su, N.; Wan, J. A novel lipoxygenase gene from developing rice seeds confers dual position specificity and responds to wounding and insect attack. Plant Mol. Biol. 2008, 66, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Lanubile, A.; Scala, V.; Reverberi, M.; Gregori, R.; Falavigna, C.; Dall’asta, C.; Park, Y.S.; Bennett, J.; Borrego, E.J.; et al. Oxylipins from both pathogen and host antagonize jasmonic acid-mediated defence via the 9-lipoxygenase pathway in Fusarium verticillioides infection of maize. Mol. Plant Pathol. 2018, 19, 2162–2176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Qi, J.; Ren, N.; Cheng, J.; Erb, M.; Mao, B.; Lou, Y. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009, 60, 638–648. [Google Scholar] [CrossRef]
- Yan, L.; Zhai, Q.; Wei, J.; Li, S.; Wang, B.; Huang, T.; Du, M.; Sun, J.; Kang, L.; Li, C.-B. Role of tomato lipoxygenase D in wound-induced jasmonate biosynthesis and plant immunity to insect herbivores. PLOS Genet. 2013, 9, e1003964. [Google Scholar] [CrossRef]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.; Vaughn, K.A. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013, 74, 59–73. [Google Scholar] [CrossRef]
- Losvik, A.; Beste, L.; Glinwood, R.; Ivarson, E.; Stephens, J.; Zhu, L.H.; Jonsson, L. Overexpression and Down-Regulation of Barley Lipoxygenase LOX2.2 Affects Jasmonate-Regulated Genes and Aphid Fecundity. Int. J. Mol. Sci. 2017, 18, 2765. [Google Scholar] [CrossRef]
- Pathi, K.M.; Rink, P.; Budhagatapalli, N.; Betz, R.; Saado, I.; Hiekel, S.; Becker, M.; Djamei, A.; Kumlehn, J. Engineering smut resistance in maize by site-directed mutagenesis of LIPOXYGENASE 3. Front. Plant Sci. 2020, 11, 543895. [Google Scholar] [CrossRef]
- Muneer, S.; Jeong, H.K.; Park, Y.G.; Jeong, B.R. Proteomic analysis of aphid-resistant and -sensitive rose (Rosa Hybrida) cultivars at two developmental stages. Proteomes 2018, 6, 25. [Google Scholar] [CrossRef]
- Mou, D.-F.; Kundu, P.; Pingault, L.; Puri, H.; Shinde, S.; Louis, J. Monocot crop–aphid interactions: Plant resilience and aphid adaptation. Curr. Opin. Insect Sci. 2023, 57, 101038. [Google Scholar] [CrossRef]
- Radchenko, E.E.; Abdullaev, R.A.; Anisimova, I.N. Genetic resources of cereal crops for aphid resistance. Plants 2022, 11, 1490. [Google Scholar] [CrossRef] [PubMed]
- Umate, P. Genome-wide analysis of lipoxygenase gene family in Arabidopsis and rice. Plant Signal Behav. 2011, 6, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Sarde, S.J.; Kumar, A.; Remme, R.N.; Dicke, M. Genome-wide identification, classification and expression of lipoxygenase gene family in pepper. Plant Mol. Biol. 2018, 98, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liang, Y.; Liao, B.; He, W.; Liu, Q.; Shen, X.; Xu, J.; Chen, S. Genome-wide identification, characterization and expression analysis of lipoxygenase gene family in Artemisia annua L. Plants 2022, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Jia, K.; Zhang, J.; Xiao, Z.; Sha, X.; Gao, J.; Yan, H. Genome-wide identification and expression pattern analysis of lipoxygenase gene family in turnip (Brassica rapa L. subsp. rapa). PeerJ 2022, 10, e13746. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, X.; Guo, L.; Xu, Q.; Zhao, S.; Li, F.; Yan, X.; Liu, S.; Wei, C. Characterization and alternative splicing profiles of the lipoxygenase gene family in tea plant (Camellia sinensis). Plant Cell Physiol. 2018, 59, 1765–1781. [Google Scholar] [CrossRef]
- Ogunola, O.F.; Hawkins, L.K.; Mylroie, E.; Kolomiets, M.V.; Borrego, E.; Tang, J.D.; Williams, W.P.; Warburton, M.L. Characterization of the maize lipoxygenase gene family in relation to aflatoxin accumulation resistance. PLoS ONE 2017, 12, e0181265. [Google Scholar] [CrossRef]
- Fukuchi-Mizutani, M.; Ishiguro, K.; Nakayama, T.; Utsunomiya, Y.; Tanaka, Y.; Kusumi, T.; Ueda, T. Molecular and functional characterization of a rose lipoxygenase cDNA related to flower senescence. Plant Sci. 2000, 160, 129–137. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.h.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, İ.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Steinegger, M.; Meier, M.; Mirdita, M.; Vöhringer, H.; Haunsberger, S.J.; Söding, J. HH-suite3 for fast remote homology detection and deep protein annotation. BMC Bioinform. 2019, 20, 473. [Google Scholar] [CrossRef]
- Mou, Y.; Sun, Q.; Yuan, C.; Zhao, X.; Wang, J.; Yan, C.; Li, C.; Shan, S. Identification of the LOX gene family in peanut and functional characterization of AhLOX29 in drought tolerance. Front. Plant Sci. 2022, 13, 832785. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K.; Edelman, M.; Mattoo, A.K. Identification, phylogeny, and comparative expression of the lipoxygenase gene family of the aquatic duckweed, Spirodela polyrhiza, during growth and in response to methyl jasmonate and salt. Int. J. Mol. Sci. 2020, 21, 9527. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Zhang, L.-K.; Zhang, K.; Chen, S.-M.; Hu, J.-B.; Cheng, F. The impact of tandem duplication on gene evolution in Solanaceae species. J. Integr. Agr. 2022, 21, 1004–1014. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Liu, Y.; Yang, L.; Dong, R.; Jiang, L.; Liu, P.; Liu, G.; Wang, Z.; Luo, L. Genome-wide analysis of tandem duplicated genes and their contribution to stress resistance in pigeonpea (Cajanus cajan). Genomics 2021, 113, 728–735. [Google Scholar] [CrossRef]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant lipoxygenases and their role in plant physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, J.; Xing, D. Phytochrome B-mediated activation of lipoxygenase modulates an excess red light-induced defence response in Arabidopsis. J. Exp. Bot. 2014, 65, 4907–4918. [Google Scholar] [CrossRef]
- Yi, R.; Yan, J.; Xie, D. Light promotes jasmonate biosynthesis to regulate photomorphogenesis in Arabidopsis. Sci. China Life Sci. 2020, 63, 943–952. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. The interplay between light and jasmonate signalling during defence and development. J. Exp. Bot. 2011, 62, 4087–4100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, K.; Wang, Z.; Li, X.; Li, M.; Zhu, F.; Majeed, Z.; Lan, X.; Guan, Q. The lipoxygenase gene AfLOX4 of Amorpha fruticosa L. is a potential regulator of drought stress tolerance pathways under saline and alkaline conditions. Acta Physiol. Plant. 2023, 45, 72. [Google Scholar] [CrossRef]
- Xing, Q.; Zhang, X.; Li, Y.; Shao, Q.; Cao, S.; Wang, F.; Qi, H. The lipoxygenase CmLOX13 from oriental melon enhanced severe drought tolerance via regulating ABA accumulation and stomatal closure in Arabidopsis. Environ. Exp. Bot. 2019, 167, 103815. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Y.; Yu, J.; Kou, X.; Xie, L.; Deng, P.; Li, T.; Chen, C.; Ji, W.; Liu, X. Genome-wide identification and characterization of lipoxygenase genes related to the English grain aphid infestation response in wheat. Planta 2023, 257, 84. [Google Scholar] [CrossRef] [PubMed]
- Ilarduya, O.M.d.; Xie, Q.; Kaloshian, I. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant Microbe Interact. 2003, 16, 699–708. [Google Scholar] [CrossRef]
- Grover, S.; Puri, H.; Xin, Z.; Sattler, S.E.; Louis, J. Dichotomous role of jasmonic acid in modulating sorghum defense against aphids. Mol. Plant Microbe Interact. 2022, 35, 755–767. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Ensemble ID | Chr Location | Subcellular Localization | Isoelectric Point | Molecular Weight (Da) | Protein Length (aa) |
|---|---|---|---|---|---|---|---|
| RcLOX1 | A0A2P6S733_ROSCH | RchiOBHm_Chr1g0314111 | Chr 1: 1,398,352–1,403,844 reverse | cytoplasm | 5.68 | 89,452.09 | 784 |
| RcLOX2 | A0A2P6S713_ROSCH | RchiOBHm_Chr1g0314131 | Chr 1: 1,418,573–1,424,284 reverse | chloroplast | 6.19 | 102,764.18 | 913 |
| RcLOX3 | A0A2P6S725_ROSCH | RchiOBHm_Chr1g0314151 | Chr 1: 1,462,401–1,469,489 reverse | chloroplast | 6.22 | 102,838.21 | 913 |
| RcLOX4 | A0A2P6RYR4_ROSCH | RchiOBHm_Chr2g0145961 | Chr 2: 63,721,112–63,725,566 reverse | cytoplasm | 7.33 | 103,318.16 | 914 |
| RcLOX5 | A0A2P6R6Y4_ROSCH | RchiOBHm_Chr3g0455091 | Chr 3: 4,945,765–4,949,386 forward | cytoplasm | 6.17 | 99,007.46 | 870 |
| RcLOX6 | A0A2P6R6Z5_ROSCH | RchiOBHm_Chr3g0455111 | Chr 3: 4,971,165–4,976,556 forward | cytoplasm | 5.62 | 104,221.55 | 919 |
| RcLOX7 | A0A2P6R730_ROSCH | RchiOBHm_Chr3g0455121 | Chr 3: 4,979,448–4,983,533 forward | chloroplast | 6.82 | 104,342.04 | 927 |
| RcLOX8 | A0A2P6RBM1_ROSCH | RchiOBHm_Chr3g0472481 | Chr 3: 18,384,687–18,388,356 forward | nucleus | 6.27 | 98,052.53 | 864 |
| RcLOX9 | A0A2P6QTX2_ROSCH | RchiOBHm_Chr4g0404751 | Chr 4: 24,642,061–24,646,849 forward | cytoplasm | 6.41 | 97,561.23 | 862 |
| RcLOX10 | A0A2P6QWV3_ROSCH | RchiOBHm_Chr4g0416591 | Chr 4: 41,590,100–41,594,705 reverse | cytoplasm | 5.69 | 100,734.62 | 884 |
| RcLOX11 | A0A2P6R0J4_ROSCH | RchiOBHm_Chr4g0430941 | Chr 4: 55,302,376–55,306,479 forward | chloroplast | 8.19 | 103,725.45 | 920 |
| RcLOX12 | A0A2P6Q438_ROSCH | RchiOBHm_Chr5g0008501 | Chr 5: 5,436,496–5,440,087 forward | chloroplast | 6.56 | 104,212.73 | 916 |
| RcLOX13 | A0A2P6Q441_ROSCH | RchiOBHm_Chr5g0008511 | Chr 5: 5,459,688–5,464,027 forward | cytoplasm | 6.78 | 107,499.38 | 950 |
| RcLOX14 | A0A2P6QM50_ROSCH | RchiOBHm_Chr5g0078061 | Chr 5: 83,928,147–83,935,447 reverse | chloroplast | 5.77 | 108,405.14 | 958 |
| RcLOX15 | A0A2P6QM53_ROSCH | RchiOBHm_Chr5g0078091 | Chr 5: 83,993,484–83,998,908 reverse | chloroplast | 5.48 | 110,595.97 | 981 |
| RcLOX16 | A0A2P6PTU3_ROSCH | RchiOBHm_Chr6g0282631 | Chr 6: 45,898,666–45,902,121 forward | chloroplast | 7.9 | 99,420.98 | 875 |
| RcLOX17 | A0A2P6PBW3_ROSCH | RchiOBHm_Chr7g0216951 | Chr 7: 34,853,149–34,859,784 reverse | cytoplasm | 5.64 | 94,662.09 | 891 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Jiao, B.; Wang, J.; Sun, L.; Li, S.; Wu, Z.; Gao, J.; Zhou, S. Genome-Wide Identification and Expression Analysis of Lipoxygenase Genes in Rose (Rosa chinensis). Genes 2023, 14, 1957. https://doi.org/10.3390/genes14101957
Dong W, Jiao B, Wang J, Sun L, Li S, Wu Z, Gao J, Zhou S. Genome-Wide Identification and Expression Analysis of Lipoxygenase Genes in Rose (Rosa chinensis). Genes. 2023; 14(10):1957. https://doi.org/10.3390/genes14101957
Chicago/Turabian StyleDong, Wenqi, Bo Jiao, Jiao Wang, Lei Sun, Songshuo Li, Zhiming Wu, Junping Gao, and Shuo Zhou. 2023. "Genome-Wide Identification and Expression Analysis of Lipoxygenase Genes in Rose (Rosa chinensis)" Genes 14, no. 10: 1957. https://doi.org/10.3390/genes14101957
APA StyleDong, W., Jiao, B., Wang, J., Sun, L., Li, S., Wu, Z., Gao, J., & Zhou, S. (2023). Genome-Wide Identification and Expression Analysis of Lipoxygenase Genes in Rose (Rosa chinensis). Genes, 14(10), 1957. https://doi.org/10.3390/genes14101957






