Mulberry MnGolS2 Mediates Resistance to Botrytis cinerea on Transgenic Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Equipment
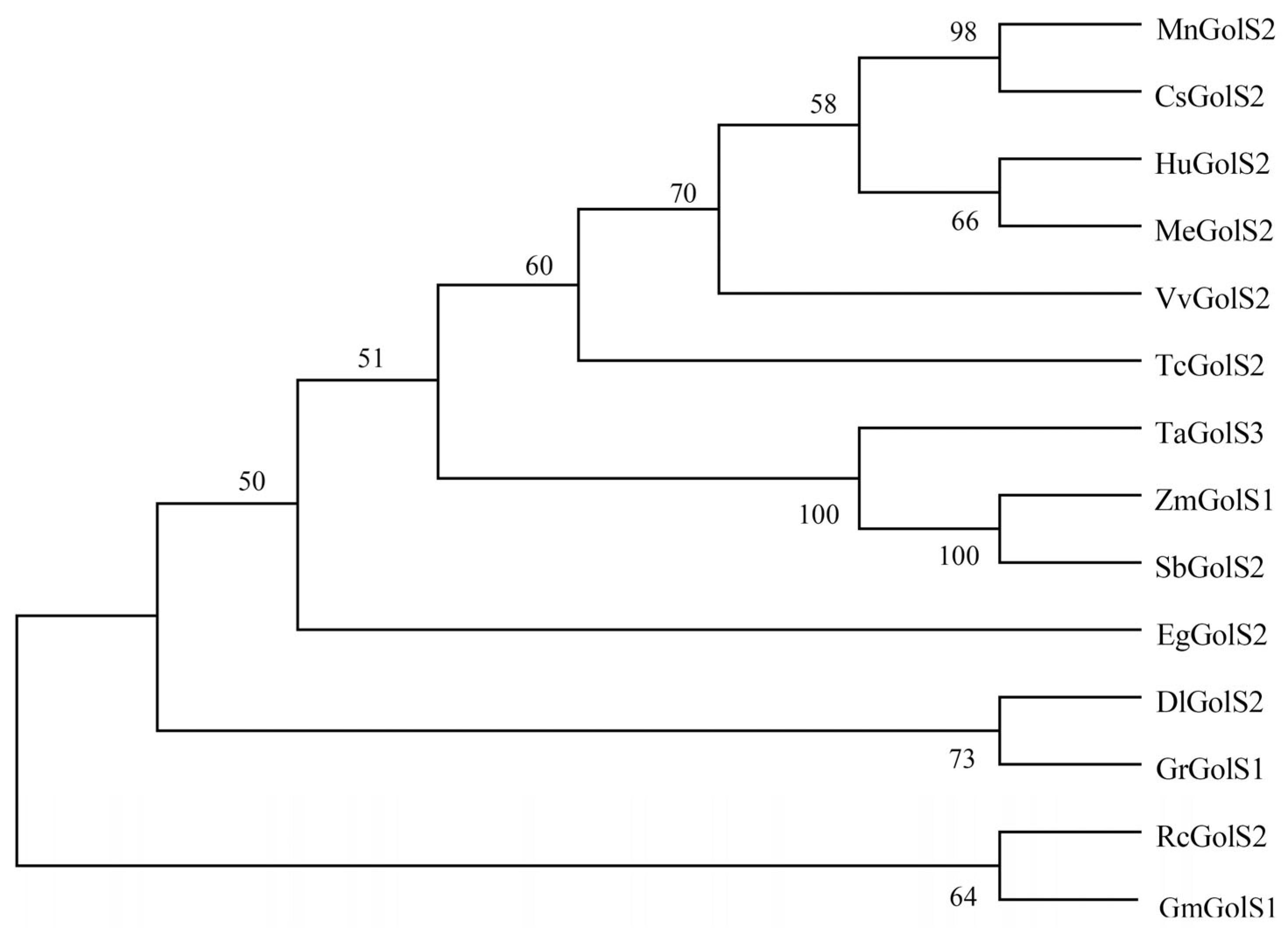
2.2. Phylogenetic Tree of GolSs
2.3. Quantitative Real-Time PCR
2.4. Construction of Expression Vector and Plant Transformation
2.5. Analysis of Resistance of Transgenic Arabidopsis
2.6. Statistical Analysis
3. Results
3.1. GolSs Bioinformatics Analysis
3.2. B. cinerea-Induced MnGolS2 Expression
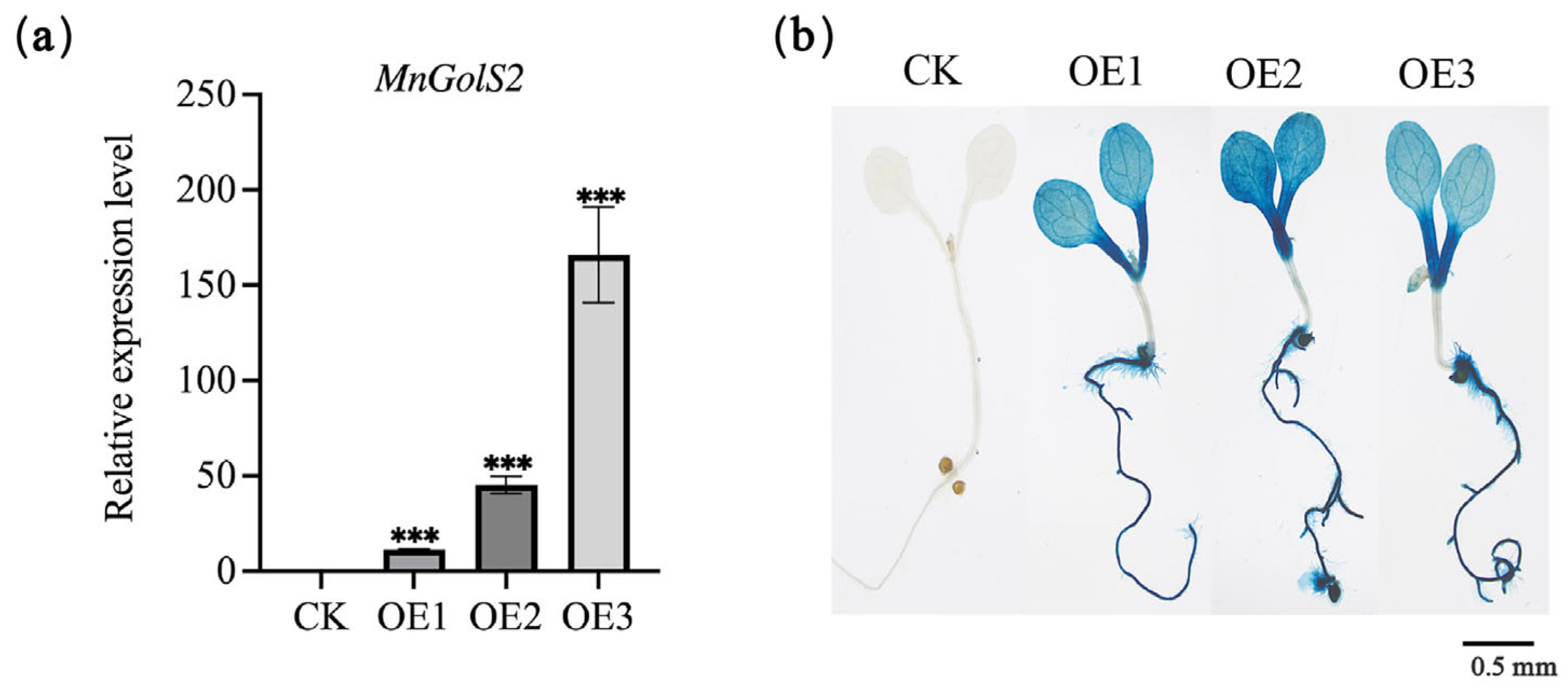
3.3. Ectopic Expression of MnGolS2 in Arabidopsis
3.4. Positive Regulation of MnGolS2 for Resistance
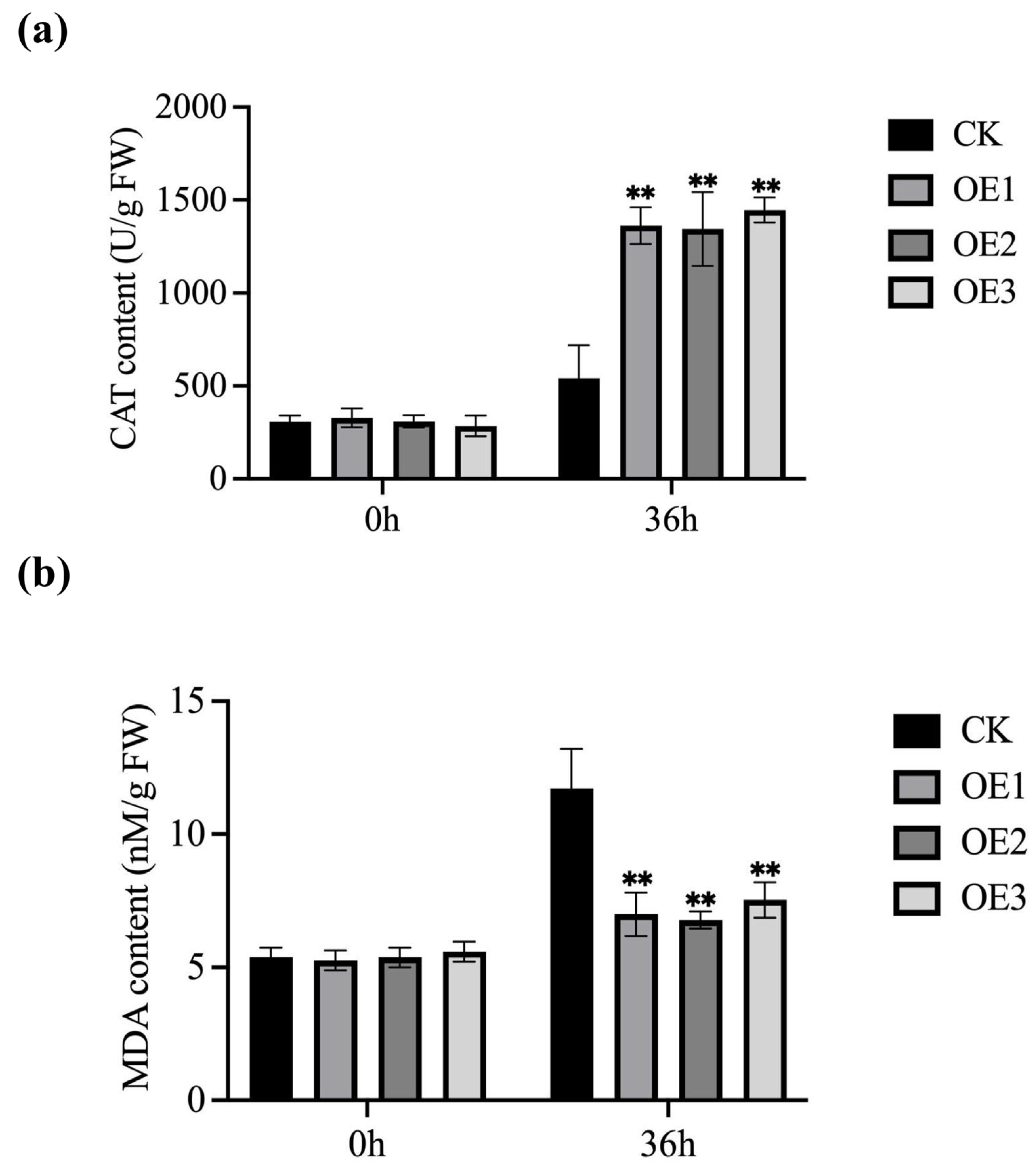
3.5. Biochemical Index Detection
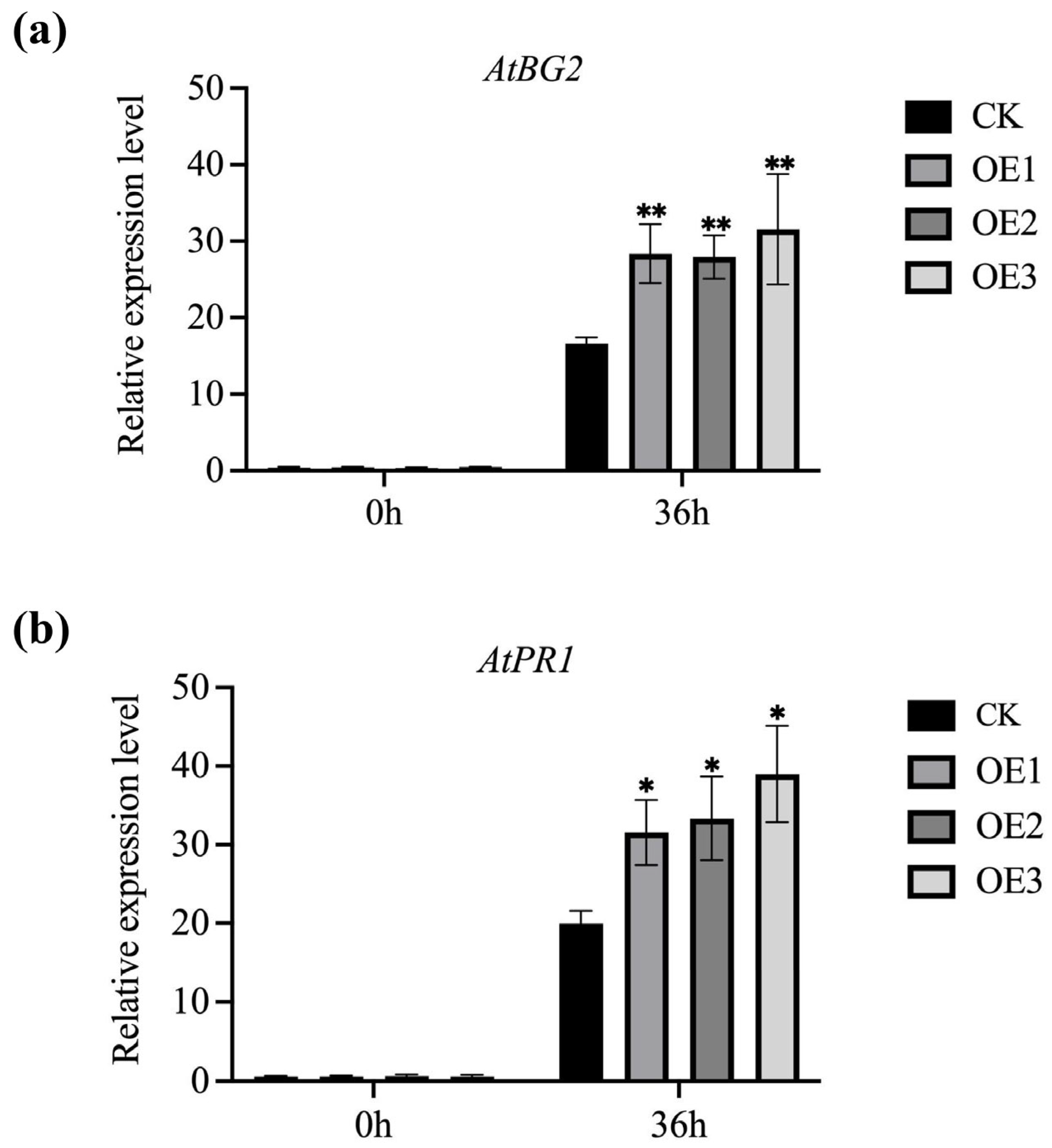
3.6. Transgenic MnGolS2 Plants Enhance the Expression of Resistance-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Egert, A.; Eicher, B.; Keller, F.; Peters, S. Evidence for water deficit-induced mass increases of raffinose family oligosaccharides (RFOs) in the leaves of three Craterostigma resurrection plant species. Front. Physiol. 2015, 6, 206. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Yu, J.; Wang, L.; Yu, X.; Ohtani, M.; Kusano, M.; Saito, K.; Demura, T.; Zhuge, Q. Responses of Populus trichocarpa galactinol synthase genes to abiotic stresses. J. Plant Res. 2013, 127, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Mundree, S.G.; Thomson, J.A.; Farrant, J.M.; Keller, F. Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): Both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. J. Exp. Bot. 2007, 58, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and Raffinose Constitute a Novel Function to Protect Plants from Oxidative Damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Han, Q.; Li, T.; Zhang, L.; Yan, J.; Dirk, L.M.A.; Downie, B.; Zhao, T. Functional Analysis of the 5′-Regulatory Region of the Maize ALKALINE ALPHA-GALACTOSIDASE1 Gene. Plant Mol. Biol. Report. 2015, 33, 1361–1370. [Google Scholar] [CrossRef]
- Gu, L.; Han, Z.; Zhang, L.; Downie, B.; Zhao, T. Functional analysis of the 5′ regulatory region of the maize GALACTINOL SYNTHASE2 gene. Plant Sci. Int. J. Exp. Plant Biol. 2013, 213, 38–45. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, Z.; Wang, Z.; Zhang, Z.; Kong, W.; Miao, M. Galactinol synthase 1 improves cucumber performance under cold stress by enhancing assimilate translocation. Hortic. Res. 2022, 9, uhab063. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef]
- Wang, H.X.; Liu, H.; Wang, S.; Li, H.; Xin, Q. Overexpression of a Common Wheat Gene GALACTINOL SYNTHASE3 Enhances Tolerance to Zinc in Arabidopsis and Rice Through the Modulation of Reactive Oxygen Species Production. Plant Mol. Biol. Report. 2016, 34, 794–806. [Google Scholar] [CrossRef]
- Martins, C.P.S.; Fernandes, D.; Guimaraes, V.M.; Du, D.; Silva, D.C.; Almeida, A.F.; Gmitter, F.G., Jr.; Otoni, W.C.; Costa, M.G.C. Comprehensive analysis of the GALACTINOL SYNTHASE (GolS) gene family in citrus and the function of CsGolS6 in stress tolerance. PLoS ONE 2022, 17, e0274791. [Google Scholar] [CrossRef]
- Bento dos Santos, T.; Budzinski, I.G.F.; Marur, C.J.; Petkowicz, C.L.O.; Pereira, L.F.P.; Vieira, L.G.E. Expression of three galactinol synthase isoforms in Coffea arabica L. and accumulation of raffinose and stachyose in response to abiotic stresses. Plant Physiol. Biochem. 2011, 49, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Qi, X.; Wang, Z.; Li, P.; Wu, C.; Zhang, H.; Zhao, Y. Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana enhances tolerance to high salinity and osmotic stresses. Plant Physiol. Biochem. 2013, 69, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Taji, T.; Ohsumi, C.; Iuchi, S.; Seki, M.; Shinozaki, K. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002, 29, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ling, C.; Liu, Y.; Zhang, H.; Hussain, Q.; Lyu, S.; Wang, S.; Liu, Y. Genome-Wide Expression Profiling Analysis of Kiwifruit GolS and RFS Genes and Identification of AcRFS4 Function in Raffinose Accumulation. Int. J. Mol. Sci. 2022, 23, 8836. [Google Scholar] [CrossRef]
- Abkhoo, J.; Sabbagh, S.K. Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. J. Appl. Phycol. 2016, 28, 1333–1342. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Yang, S.; Xue, G.; Lu, L. Mulberry extract upregulates cholesterol efflux and inhibits p38 MAPK-NLRP3-mediated inflammation in foam cells. Food Sci. Nutr. 2023, 11, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Choi, Y.J.; Jang, D.S.; Lee, S. 2-O-β-d-Glucopyranosyl-4,6-dihydroxybenzaldehyde Isolated from Morus alba (Mulberry) Fruits Suppresses Damage by Regulating Oxidative and Inflammatory Responses in TNF-α-Induced Human Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23, 14802. [Google Scholar] [CrossRef]
- Li, X.; Hua, Y.; Yang, C.; Liu, S.; Tan, L.; Guo, J.; Li, Y. Polysaccharides extracted from mulberry fruits (Morus nigra L.): Antioxidant effect of ameliorating H2O2-induced liver injury in HepG2 cells. BMC Complement. Med. Ther. 2023, 23, 112. [Google Scholar] [CrossRef]
- Kumkoon, T.; Srisaisap, M.; Boonserm, P. Biosynthesized Silver Nanoparticles Using Morus alba (White Mulberry) Leaf Extract as Potential Antibacterial and Anticancer Agents. Molecules 2023, 28, 1213. [Google Scholar] [CrossRef]
- Arunakumar, G.S.; Gnanesh, B.N. Evaluation of artificial inoculation methods to determine resistance reaction to dry root rot and black root rot disease in mulberry (Morus spp.). Arch. Phytopathol. Plant Prot. 2023, 56, 49–65. [Google Scholar] [CrossRef]
- Ma, B.; Wang, H.; Liu, J.; Chen, L.; Xia, X.; Wei, W.; Yang, Z.; Yuan, J.; Luo, Y.; He, N. The gap-free genome of mulberry elucidates the architecture and evolution of polycentric chromosomes. Hortic. Res. 2023, 10, uhad111. [Google Scholar] [CrossRef]
- Luo, Y.; Han, Y.; Wei, W.; Han, Y.; Yuan, J.; He, N. Transcriptome and metabolome analyses reveal the efficiency of in vitro regeneration by TDZ pretreatment in mulberry. Sci. Hortic. 2023, 310, 111678. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, Y.; Xia, X.; He, J.; Zhang, J.; Zeng, Q.; Li, D.; Ma, B.; Zhang, S.; Zhai, C.; et al. Dehydrogenase MnGutB1 catalyzes 1-deoxynojirimycin biosynthesis in mulberry. Plant Physiol. 2023, 192, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yu, M.; Zhao, R.; Sheng, J.; Li, Y.; Shen, L. Ethylene Perception Is Associated with Methyl-Jasmonate-Mediated Immune Response against Botrytis cinerea in Tomato Fruit. J. Agric. Food Chem. 2019, 67, 6725–6735. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gong, N.; Liu, C.; Li, S.; Guo, Z.; Wang, G.; Shang, Q.; Wang, D.; Ji, X.; Xin, Y. MnASI1 Mediates Resistance to Botrytis cinerea in Mulberry (Morus notabilis). Int. J. Mol. Sci. 2022, 23, 13372. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Margosan, D.; Michailides, T.J.; Xiao, C.L. Botrytis californica, a new cryptic species in the B. cinerea species complex causing gray mold in blueberries and table grapes. Mycologia 2016, 108, 330–343. [Google Scholar] [CrossRef]
- Xin, Y.; Ma, B.; Zeng, Q.; He, W.; Qin, M.; He, N. Dynamic changes in transposable element and gene methylation in mulberry (Morus notabilis) in response to Botrytis cinerea. Hortic. Res. 2021, 8, 154. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Parween, S.; Majumder, A.L. Galactinol synthase across evolutionary diverse taxa: Functional preference for higher plants? FEBS Lett. 2012, 586, 1488–1496. [Google Scholar] [CrossRef]
- Meyer, T.; Vigouroux, A.; Aumont-Nicaise, M.; Comte, G.; Vial, L.; Lavire, C.; Moréra, S. The plant defense signal galactinol is specifically used as a nutrient by the bacterial pathogen Agrobacterium fabrum. J. Biol. Chem. 2018, 293, 7930–7941. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, H.; Wang, Z.; Feng, T.; Yu, L.; Zhang, J.; Gao, W.; Zhou, Y.; Sun, M.; Liu, P.; et al. Wheat yellow mosaic virus NIb targets TaVTC2 to elicit broad-spectrum pathogen resistance in wheat. Plant Biotechnol. J. 2023, 21, 1073–1088. [Google Scholar] [CrossRef]
- He, F.; Xu, J.; Jian, Y.; Duan, S.; Hu, J.; Jin, L.; Li, G. Overexpression of galactinol synthase 1 from Solanum commersonii (ScGolS1) confers freezing tolerance in transgenic potato. Hortic. Plant J. 2023, 9, 541–552. [Google Scholar] [CrossRef]
- Ranjan, A.; Gautam, S.; Michael, R.; Shukla, T.; Trivedi, P.K. Arsenic-induced galactinol synthase1 gene, AtGolS1, provides arsenic stress tolerance in Arabidopsis thaliana. Environ. Exp. Bot. 2023, 207, 105217. [Google Scholar] [CrossRef]
- Shikakura, Y.; Oguchi, T.; Yu, X.; Ohtani, M.; Demura, T.; Kikuchi, A.; Watanabe, K.N. Transgenic poplar trees overexpressing AtGolS2, a stress-responsive galactinol synthase gene derived from Arabidopsis thaliana, improved drought tolerance in a confined field. Transgenic Res. 2022, 31, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, S.-I.; Nishiguchi, M.; Kogawara, S.; Tahara, K.; Mohri, T.; Kakegawa, K.; Yokota, S.; Nanjo, T. Isolation of the drought- and salt-responsive galactinol synthase (GolS) gene from black poplar leaves and analysis of the transformants overexpressing GolS. Bull. For. For. Prod. Res. Inst. 2017, 16, 77–86. [Google Scholar] [CrossRef]
- You, J.; Wang, Y.; Zhang, Y.; Dossa, K.; Li, D.; Zhou, R.; Wang, L.; Zhang, X. Genome-wide identification and expression analyses of genes involved in raffinose accumulation in sesame. Sci. Rep. 2018, 8, 4331. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.; Kamble, N.U.; Majee, M. Ectopic over-expression of ABA-responsive Chickpea galactinol synthase (CaGolS) gene results in improved tolerance to dehydration stress by modulating ROS scavenging. Environ. Exp. Bot. 2020, 171, 103957. [Google Scholar] [CrossRef]
- Ma, S.; Lv, J.; Li, X.; Ji, T.; Zhang, Z.; Gao, L. Galactinol synthase gene 4 (CsGolS4) increases cold and drought tolerance in Cucumis sativus L. by inducing RFO accumulation and ROS scavenging. Environ. Exp. Bot. 2021, 185, 104406. [Google Scholar] [CrossRef]
- Salvi, P.; Saxena, S.C.; Petla, B.P.; Kamble, N.U.; Kaur, H.; Verma, P.; Rao, V.; Ghosh, S.; Majee, M. Differentially expressed galactinol synthase(s) in chickpea are implicated in seedvigor and longevity by limiting the age induced ROS accumulation. Sci. Rep. 2016, 6, 35088. [Google Scholar] [CrossRef]
- Santarius, K.A.; Milde, H. Sugar compartmentation in frost-hardy and partially dehardened cabbage leaf cells. Planta 1977, 136, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Panikulangara, T.J.; Eggers-Schumacher, G.; Wunderlich, M.; Stransky, H.; Schöffl, F. Galactinol synthase1. A Novel Heat Shock Factor Target Gene Responsible for Heat-Induced Synthesis of Raffinose Family Oligosaccharides in Arabidopsis. Plant Physiol. 2004, 136, 3148–3158. [Google Scholar] [CrossRef]
- Liu, L.; Wu, X.; Sun, W.; Yu, X.; Demura, T.; Li, D.; Zhuge, Q. Galactinol synthase confers salt-stress tolerance by regulating the synthesis of galactinol and raffinose family oligosaccharides in poplar. Ind. Crops Prod. 2021, 165, 113432. [Google Scholar] [CrossRef]
- Yamada, T.; Matsuda, F.; Kasai, K.; Fukuoka, S.; Kitamura, K.; Tozawa, Y.; Miyagawa, H.; Wakasa, K. Mutation of a Rice Gene Encoding a Phenylalanine Biosynthetic Enzyme Results in Accumulation of Phenylalanine and Tryptophan. Plant Cell 2008, 20, 1316–1329. [Google Scholar] [CrossRef]
- Wang, W.; Withers, J.; Li, H.; Zwack, P.J.; Rusnac, D.-V.; Shi, H.; Liu, L.; Yan, S.; Hinds, T.R.; Guttman, M.; et al. Structural basis of salicylic acid perception by Arabidopsis NPR proteins. Nature 2020, 586, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Loon, L.C.V.; Rep, M.; Pieterse, C.M.J. Significance of Inducible Defense-related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Laird, J.; Armengaud, P.; Giuntini, P.; Laval, V.; Milner, J.J. Inappropriate annotation of a key defence marker in Arabidopsis: Will the real PR-1 please stand up? Planta 2004, 219, 1089–1092. [Google Scholar] [CrossRef]
- Sarowar, S.; Kim, Y.J.; Kim, E.N.; Kim, K.D.; Hwang, B.K.; Islam, R.; Shin, J.S. Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep. 2005, 24, 216–224. [Google Scholar] [CrossRef]
- Dai, R.; Yang, M.; Zhao, J.; Liu, X.; Zhou, Y.; Kang, L.; Zhang, W.; Lyu, L.; Yuan, S.; Liu, Z. The extracellular β-glucosidase BGL2 has two variants with different molecular sizes and hydrolytic activities in the stipe or pilei of Coprinopsis cinerea. Microbiology 2021, 167, 1100. [Google Scholar] [CrossRef]
- Asghari, M.; Hasanlooe, A.R. Interaction effects of salicylic acid and methyl jasmonate on total antioxidant content, catalase and peroxidase enzymes activity in “Sabrosa” strawberry fruit during storage. Sci. Hortic. 2015, 197, 490–495. [Google Scholar] [CrossRef]
- Roshdy, A.E.-D.; Alebidi, A.; Almutairi, K.; Al-Obeed, R.; Elsabagh, A. The Effect of Salicylic Acid on the Performances of Salt Stressed Strawberry Plants, Enzymes Activity, and Salt Tolerance Index. Agronomy 2021, 11, 775. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Vieira, L.G.E. Involvement of the galactinol synthase gene in abiotic and biotic stress responses: A review on current knowledge. Plant Gene 2020, 24, 100258. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, Z.; Qin, Y.; Zhang, S.; Yang, L.; Shang, Q.; Ji, X.; Xin, Y.; Li, X. Mulberry MnGolS2 Mediates Resistance to Botrytis cinerea on Transgenic Plants. Genes 2023, 14, 1912. https://doi.org/10.3390/genes14101912
Wang D, Liu Z, Qin Y, Zhang S, Yang L, Shang Q, Ji X, Xin Y, Li X. Mulberry MnGolS2 Mediates Resistance to Botrytis cinerea on Transgenic Plants. Genes. 2023; 14(10):1912. https://doi.org/10.3390/genes14101912
Chicago/Turabian StyleWang, Donghao, Zixuan Liu, Yue Qin, Shihao Zhang, Lulu Yang, Qiqi Shang, Xianling Ji, Youchao Xin, and Xiaodong Li. 2023. "Mulberry MnGolS2 Mediates Resistance to Botrytis cinerea on Transgenic Plants" Genes 14, no. 10: 1912. https://doi.org/10.3390/genes14101912
APA StyleWang, D., Liu, Z., Qin, Y., Zhang, S., Yang, L., Shang, Q., Ji, X., Xin, Y., & Li, X. (2023). Mulberry MnGolS2 Mediates Resistance to Botrytis cinerea on Transgenic Plants. Genes, 14(10), 1912. https://doi.org/10.3390/genes14101912





