Rapid Visual Detection of High Nitrogen-Use Efficiency Gene OsGRF4 in Rice (Oryza sativa L.) Using Loop-Mediated Isothermal Amplification Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Genomic DNA Isolation
2.2. The Primer Designing
2.3. PCR Amplification and Restriction Enzyme Digestion
2.4. LAMP Amplification
2.5. Visual Detection of LAMP Reaction
3. Results
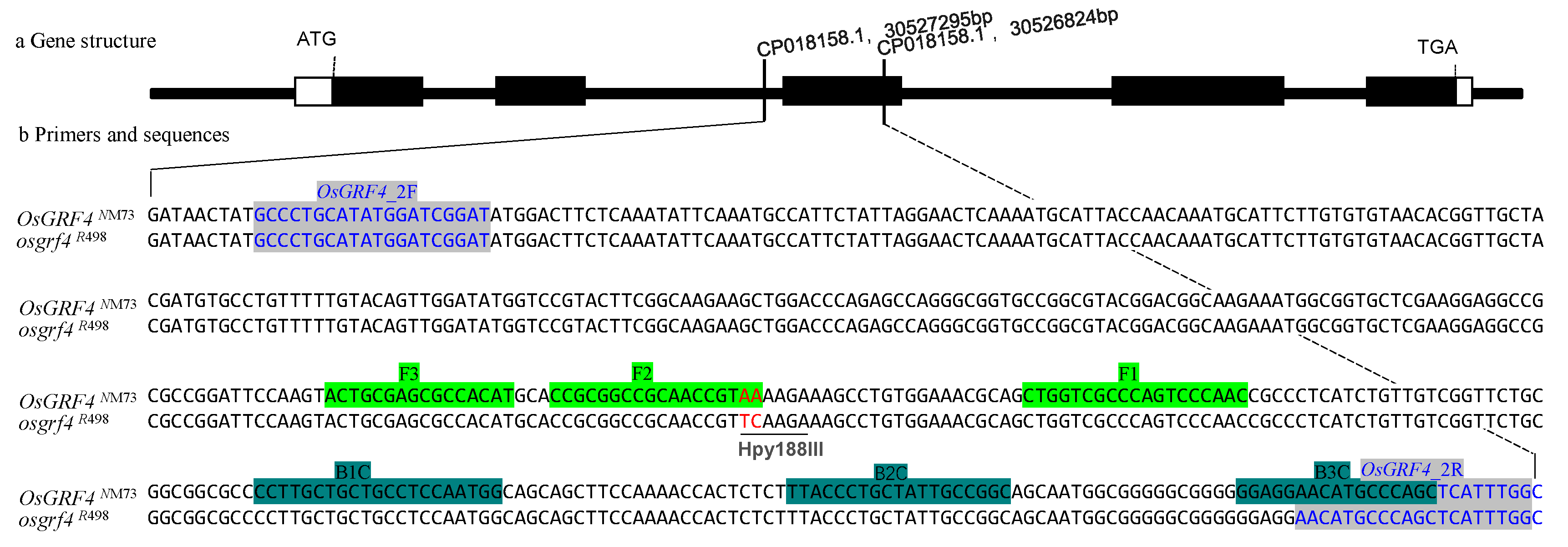
3.1. Designing of CAPs and LAMP Primer Sets
3.2. Optimization of PCR Reaction Conditions
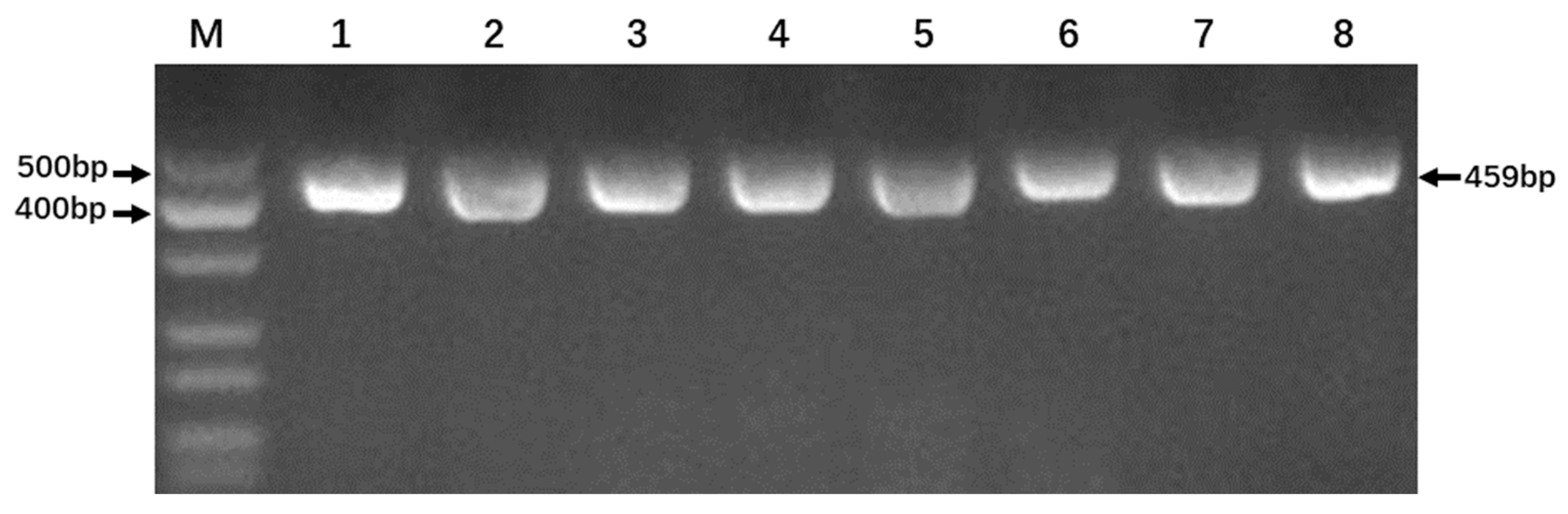
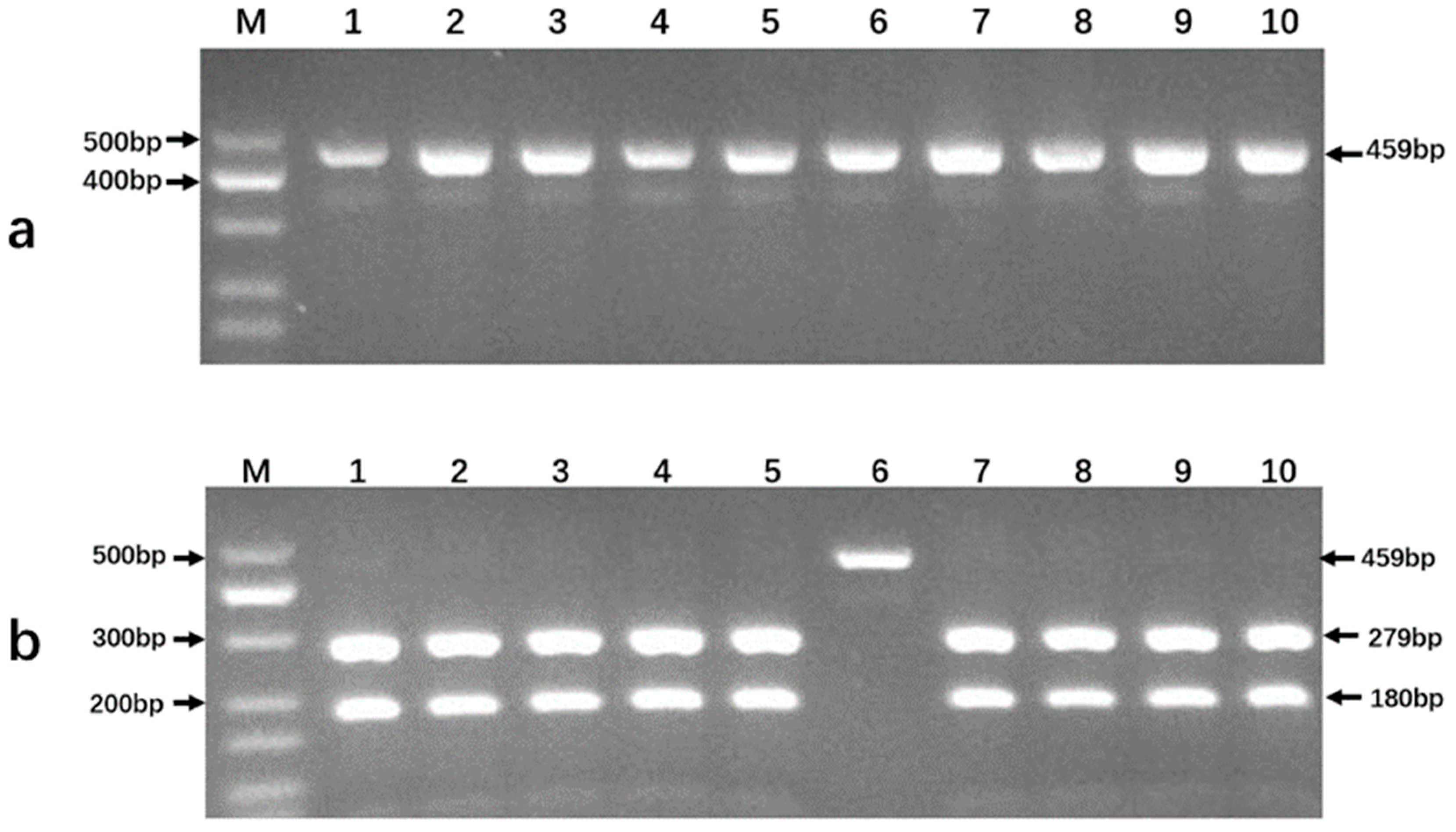
3.3. Product Detection from PCR Amplifications and Endonuclease Digestion Reactions
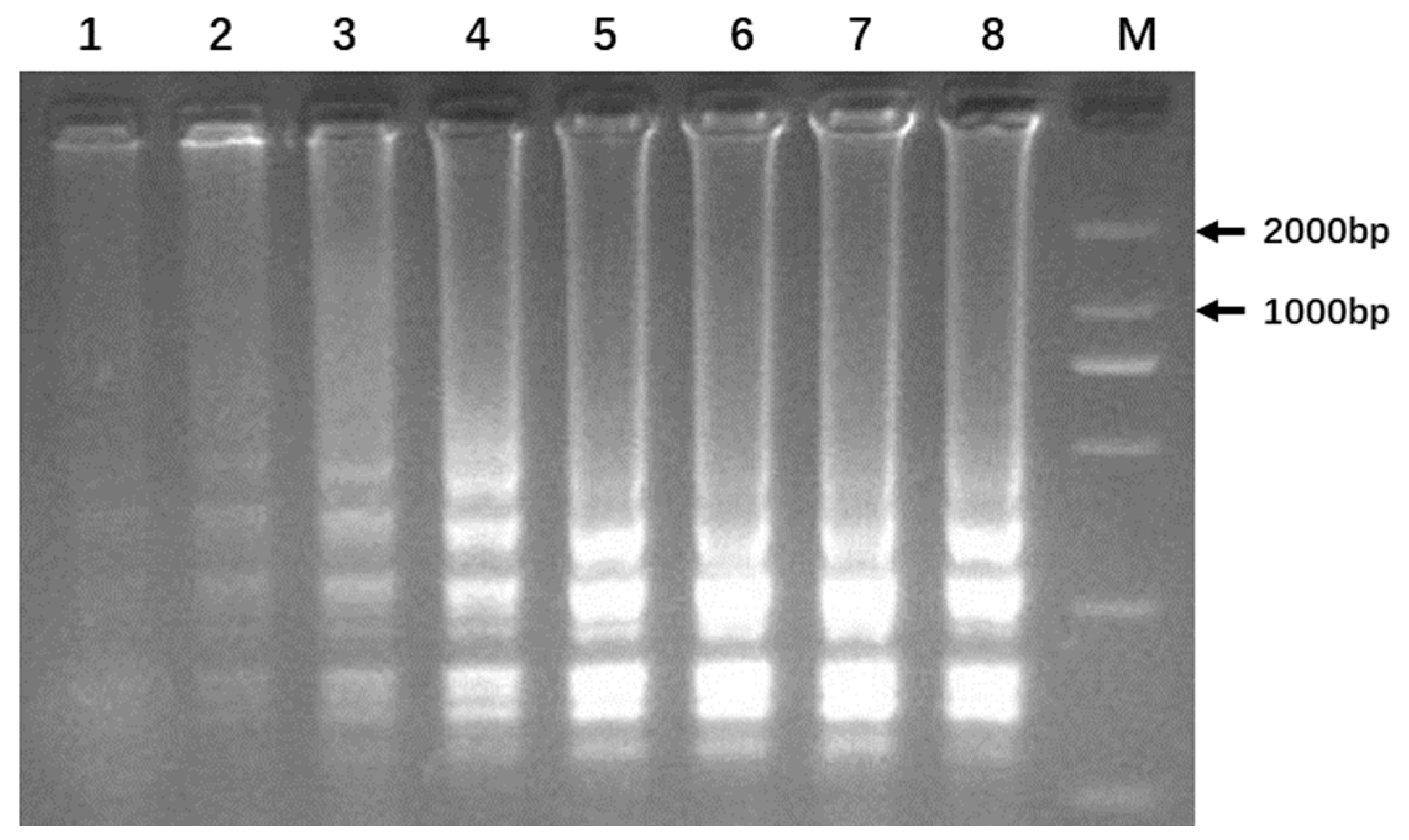
3.4. Optimization of LAMP Reaction
3.5. Colorimetric Detection of LAMP Amplification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Matsuoka, M. Improved nutrient use gives cereal crops a boost. Nature 2018, 560, 563–564. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Wu, K.; Ye, Y.; Yu, J.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; et al. Modulating plant growth–metabolism coordination for sustainable agriculture. Nature 2018, 560, 595–600. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Fang, Y.; Zeng, L.; Xu, J.; Yu, H.; Shi, Z.; Pan, J.; Zhang, D.; Kang, S.; et al. A Rare Allele of GS2 Enhances Grain Size and Grain Yield in Rice. Mol. Plant 2015, 8, 1455–1465. [Google Scholar] [CrossRef]
- Duan, P.; Ni, S.; Wang, J.; Zhang, B.; Xu, R.; Wang, Y.; Chen, H.; Zhu, X.; Li, Y. Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat. Plants 2016, 2, 15203. [Google Scholar] [CrossRef]
- Che, R.; Tong, H.; Shi, B.; Liu, Y.; Fang, S.; Liu, D.; Xiao, Y.; Hu, B.; Liu, L.; Wang, H.; et al. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat. Plants 2015, 2, 15195. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, Z.; Zhang, L.; Li, S.; Wang, S.; Yang, H.; Liu, X.; Zeng, D.; Liu, Q.; Qian, Q.; et al. MYB61 is regulated by GRF4 and promotes nitrogen utilization and biomass production in rice. Nat. Commun. 2020, 11, 5219. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, W.; Wang, Y.; He, Q.; Shu, F.; Liu, H.; Wang, J.; Wang, J.; Yuan, L.; Deng, H. OsGRF4 controls grain shape, panicle length and seed shattering in rice. J. Integr. Plant biol. 2016, 58, 836–847. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, L.; Zheng, J.; Chen, F.; Wang, T.; Wang, M.; Tao, Y.; Wang, H.; Hong, Z.; Huang, Y.; et al. A missense mutation in Large Grain Size 1 increases grain size and enhances cold tolerance in rice. J. Exp. Bot. 2019, 70, 3851–3866. [Google Scholar] [CrossRef]
- Liu, J.; Ye, N.; Xiao, X.; Ding, X.; Yi, X.; Xiao, Y.; Cao, Y. Auxin regulator OsGRF4 simultaneously regulates rice grain shape and blast resistance. Chin. J. Rice Sci. 2021, 35, 629–638. [Google Scholar] [CrossRef]
- Kawasaki, E.; Wenjing, D.; Sawada, A.; Nakajima, M.; Momose, K.; Yoshino, T.; Amano, T.; Endoh, D.; Nakajima, N.; Teraoka, H. Conventional gel electrophoresis-resolvable insertion/deletion markers for individual identification and analysis of population genetics in red-crowned cranes in eastern Hokkaido, Japan. Animals 2022, 12, 2293. [Google Scholar] [CrossRef]
- Pan, G.; Li, Z.; Huang, S.; Tao, J.; Shi, Y.; Chen, A.; Li, J.; Tang, H.; Chang, L.; Deng, Y.; et al. Genome-wide development of insertion-deletion (InDel) markers for Cannabis and its uses in genetic structure analysis of Chinese germplasm and sex-linked marker identification. BMC Genom. 2021, 22, 595. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.; Melchinger, A.E.; Lübberstedt, T. Genetic diversity in Arabidopsis thaliana L. Heynh. investigated by cleaved amplified polymorphic sequence (CAPS) and inter-simple sequence repeat (ISSR) markers. Mol. Ecol. 2002, 11, 495–505. [Google Scholar] [CrossRef]
- Sun, P. Cloning and Functional Analysis of Panicle Traits Gene OsGRF4 in Rice; Hunan Agricultural University: Changsha, China, 2017. [Google Scholar]
- Zhang, L.; Ma, B.; Bian, Z.; Li, X.; Zhang, C.; Liu, J.; Li, Q.; Liu, Q.; He, Z. Grain size selection using novel functional markers targeting 14 genes in rice. Rice 2020, 13, 63. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.; Liu, X.; Zhang, H.; Wang, Q.; Li, N.; Ding, H.; Yao, F. Development and application of intragenic markers for 14 nitrogen-use efficiency genes in rice (Oryza sativa L.). Front. Plant Sci. 2022, 13, 891860. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y. Mining superior alleles in crop germplasm resources: Advances and perspectives. Chin. J. Plant Genet. Resour. 2019, 20, 1380–1389. [Google Scholar] [CrossRef]
- Zhang, F.; Li, H.; Zhou, X.; Wang, C.; Zhou, D.; Lai, S.; Chen, L.; Zhou, S. Approach to rice breeding by molecular design in grain yield. Molecular Plant Breeding. 2013, 11, 663–672. [Google Scholar]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Almasi, M.A.; Hosseyni-Dehabadi, S.M.; Aghapour-Ojaghkandi, M. Comparison and evaluation of three diagnostic methods for detection of beet curly top virus in sugar beet using different visualizing systems. Appl. Biochem. Biotechnol. 2014, 173, 1836–1848. [Google Scholar] [CrossRef]
- Cuadros, J.; Pérez-Tanoira, R.; Prieto-Pérez, L.; Martin-Martin, I.; Berzosa, P.; González, V.; Tisiano, G.; Balcha, S.; Ramos, J.M.; Górgolas, M. Field evaluation of malaria microscopy, rapid malaria tests and loop-mediated isothermal amplification in a rural hospital in south western Ethiopia. PLoS ONE 2015, 10, e0142842. [Google Scholar] [CrossRef] [PubMed]
- Soroka, M.; Wasowicz, B.; Rymaszewska, A. Loop-mediated isothermal amplification (LAMP): The better sibling of PCR? Cells 2021, 10, 1931. [Google Scholar] [CrossRef]
- Becherer, L.; Borst, N.; Bakheit, M.; Frischmann, S.; Zengerle, R.; von Stetten, F. Loop-mediated isothermal amplification (LAMP)—Review and classification of methods for sequence-specific detection. Anal. Methods 2020, 12, 717–746. [Google Scholar] [CrossRef]
- Fellner, M.D.; Bonaventura, R.; Basiletti, J.; Avaro, M.; Benedetti, E.; Campos, A.; Dattero, M.E.; Russo, M.; Vladmirsky, S.; Molina, V.; et al. Evaluation of RT-qPCR and Loop-Mediated Isothermal Amplification (LAMP) Assays for the Detection of SARS-CoV-2 in Argentina. Genes 2021, 12, 659. [Google Scholar] [CrossRef]
- Narushima, J.; Kimata, S.; Soga, K.; Sugano, Y.; Kishine, M.; Takabatake, R.; Mano, J.; Kitta, K.; Kanamaru, S.; Shirakawa, N.; et al. Rapid DNA template preparation directly from a rice sample without purification for loop-mediated isothermal amplification (LAMP) of rice genes. Biosci. Biotechnol. Biochem. 2019, 84, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Prasannakumar, M.K.; Parivallal, B.P.; Manjunatha, C.; Pramesh, D.; Narayan, K.S.; Venkatesh, G.; Banakar, S.N.; Mahesh, H.B.; Vemanna, R.S.; Rangaswamy, K.T. Rapid genotyping of bacterial leaf blight resistant genes of rice using loop-mediated isothermal amplification assay. Mol. Biol. Rep. 2021, 48, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Rai, P.; Yadav, J.; Verma, S.; Chakdar, H.; Goswami, S.K.; Srivastava, A.K.; Kashyap, P.L.; Saxena, A.K. A rapid colorimetric LAMP assay for detection of Rhizoctonia solani AG-1 IA causing sheath blight of rice. Sci. Rep. 2020, 10, 22022. [Google Scholar] [CrossRef] [PubMed]
- Prasannakumar, M.K.; Parivallal, P.B.; Pramesh, D.; Mahesh, H.B.; Raj, E. LAMP-based foldable microdevice platform for the rapid detection of Magnaporthe oryzae and Sarocladium oryzae in rice seed. Sci. Rep. 2021, 11, 178. [Google Scholar] [CrossRef]
- Konieczny, A.; Ausubel, F.M. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993, 4, 403–410. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. ISPMB 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Vincze, T.; Posfai, J.; Roberts, R.J. NEBcutter: A program to cleave DNA with restriction enzymes. Nucleic Acids Res. 2003, 31, 3688–3691. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K.-I. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 2009, 46, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Srividya, A.; Maiti, B.; Chakraborty, A.; Chakraborty, G. Loop mediated isothermal amplification: A promising tool for screening genetic mutations. Mol. Diagn. Ther. 2019, 23, 723–733. [Google Scholar] [CrossRef]
- Shang, L.; Li, X.; He, H.; Yuan, Q.; Song, Y.; Wei, Z.; Lin, H.; Hu, M.; Zhao, F.; Zhang, C.; et al. A super pan-genomic landscape of rice. Cell Res. 2022, 32, 878–896. [Google Scholar] [CrossRef]
- Qin, P.; Lu, H.; Du, H.; Wang, H.; Chen, W.; Chen, Z.; He, Q.; Ou, S.; Zhang, H.; Li, X.; et al. Pan-genome analysis of 33 genetically diverse rice accessions reveals hidden genomic variations. Cell 2021, 184, 3542–3558. [Google Scholar] [CrossRef]
- Khush, G.S. Green revolution: The way forward. Nat. Rev. Genet. 2001, 2, 815–822. [Google Scholar] [CrossRef]
- Gooding, M.J.; Addisu, M.; Uppal, R.K.; Snape, J.W.; Jones, H.E. Effect of wheat dwarfing genes on nitrogen-use efficiency. J. Agric. Sci. 2012, 150, 3–22. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Prasad, R. Fertilizer urea, food security, health and the environment. Curr. Sci. 1998, 75, 677–683. [Google Scholar]
- Yan, X.; Xia, L.; Ti, C. Win-win nitrogen management practices for improving crop yield and environmental sustainability. Bull. Chin. Acad. Sci. 2018, 33, 177–183. [Google Scholar] [CrossRef]
- Martinez, D.A.; Loening, U.E.; Graham, M.C.; Gathorne-Hardy, A. When the medicine feeds the problem; do nitrogen fertilisers and pesticides enhance the nutritional quality of crops for their pests and pathogens? Front. Sustain. Food Syst. 2021, 5, 701310. [Google Scholar] [CrossRef]
- Cai, S.; Zhao, X.; Pittelkow, C.M.; Fan, M.; Zhang, X.; Yan, X. Optimal nitrogen rate strategy for sustainable rice production in China. Nature 2023, 615, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Shuqin, J.; Fang, Z. Zero growth of chemical fertilizer and pesticide use: China’s objectives, progress and challenges. J. Resour. Ecol. 2018, 9, 50–58. [Google Scholar] [CrossRef]
- Wu, B.; Hu, W.; Xing, Y. The history and prospect of rice genetic breeding in China. Yi Chuan 2018, 40, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Huang, J.; Lan, H.; Wang, C.; Yin, C.; Wu, Y.; Tang, H.; Qian, Q.; Li, J.; et al. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc. Natl. Acad. Sci. USA 2012, 109, 21534–21539. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Anumalla, M.; Murugaiyan, V.; Li, Z. Green Super Rice (GSR) traits: Breeding and genetics for multiple biotic and abiotic stress tolerance in rice. In Rice Improvement: Physiological, Molecular Breeding and Genetic Perspectives; Ali, J., Wani, S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 59–97. [Google Scholar]
- Vieira, M.B.; Faustino, M.V.; Lourenço, T.F.; Oliveira, M.M. DNA-based tools to certify authenticity of rice varieties-an overview. Foods 2022, 11, 258. [Google Scholar] [CrossRef]
- Edwards, K.; Johnstone, C.; Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991, 19, 1349. [Google Scholar] [CrossRef]

| Name | Sequence | Usage |
|---|---|---|
| OsGRF4_2F | GCCCTGCATATGGATCGGAT | Forward primer for CAPs marker |
| OsGRF4_2R | AATGAGCTGGGCATGTTCCT | Backward primer for CAPs marker |
| F3 | ACTGCGAGCGCCACAT | Forward inner primer for LAMP marker |
| B3 | GCTGGGCATGTTCCTCC | Backward inner primer for LAMP marker |
| FIP (F1C-F2) | GTTGGGACTGGGCGACCAGCCGCGGCCGCAACCGTAA | Forward outer primer for LAMP marker |
| BIP (B1C-B2) | CCTTGCTGCTGCCTCCAATGGGCCGGCAATAGCAGGGTAA | Backward outer primer for LAMP marker |
| Primer Name | start | end | len | tm | gc% | any_th | 3′_th | hp | COMPL | PS |
|---|---|---|---|---|---|---|---|---|---|---|
| OsGRF4_2F | 2499 | 2519 | 20 | 59.75 | 55.00 | 12.91 | 0 | 0 | 9.33 | 459 |
| OsGRF4_2R | 2957 | 2938 | 20 | 59.67 | 50.00 | 0 | 0 | 0 |
| Marker Name | Type | Seq (5′–3′) | References |
|---|---|---|---|
| PT2 | ARMS | AA-R: cgtttccacaggctttctttt | [13] |
| TC-R: cgtttccacaggctttcttga | |||
| PT2-F: ctgtgaaccaacaccctg | |||
| PT2-R: cggcaatagcagggtaaa | |||
| GS2_SNP | dCAPs | GS2_SNP-F: gagcgccacatgcaccgcggccgcatacgt (SnaBI) | [14] |
| GS2_SNP-R: ttgcctgttccaccaccaacagc | |||
| NGR2 5U S+Tag II | CAPs | F: tcattgacctacggttgc | [15] |
| R: gctgctccaacatcttct |
| Primer/Region Name | 5′pos | 3′pos | len | Tm | 5′dG | 3′dG | GCrate |
|---|---|---|---|---|---|---|---|
| F3 | 2741 | 2756 | 16 | 60.83 | −6.57 | −5.05 | 0.63 |
| B3 | 2952 | 2936 | 17 | 59.23 | −6.69 | −5.55 | 0.65 |
| FIP | 37 | ||||||
| BIP | 40 | ||||||
| F2 | 2760 | 2775 | 18 | 67.78 | −7.87 | −5.02 | 0.72 |
| F1c | 2818 | 2800 | 19 | 64.6 | −5.61 | −5.35 | 0.68 |
| B2 | 2916 | 2898 | 19 | 61.88 | −7.94 | −4.69 | 0.58 |
| B1c | 2853 | 2873 | 21 | 65.96 | −5.85 | −4.66 | 0.62 |
| Variety Name | Sub-Population | GenBank Assembly Accession | Reference | DOI |
|---|---|---|---|---|
| Nipponbare | Japonica | GCA_004295705.1 | ||
| WYJ7 | Japonica | [36] | 10.1038/s41422-022-00685-z | |
| R498 | Indica | GCA_002151415.1 | ||
| ZH11(ZH11-SPL14) | Japonica | GCA_014526345.1 | ||
| 9311 | Indica | GCA_014636015.1 | ||
| 02428 | Japonica | [37] | 10.1016/j.cell.2021.04.046 | |
| YX1B | Indica | [37] | 10.1016/j.cell.2021.04.046 | |
| II-32B | Indica | [37] | 10.1016/j.cell.2021.04.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Ye, W.; Liang, X.; Xu, P.; Wu, X.; Fu, X.; Chin, Y.; Liao, Y. Rapid Visual Detection of High Nitrogen-Use Efficiency Gene OsGRF4 in Rice (Oryza sativa L.) Using Loop-Mediated Isothermal Amplification Method. Genes 2023, 14, 1850. https://doi.org/10.3390/genes14101850
Tian Y, Ye W, Liang X, Xu P, Wu X, Fu X, Chin Y, Liao Y. Rapid Visual Detection of High Nitrogen-Use Efficiency Gene OsGRF4 in Rice (Oryza sativa L.) Using Loop-Mediated Isothermal Amplification Method. Genes. 2023; 14(10):1850. https://doi.org/10.3390/genes14101850
Chicago/Turabian StyleTian, Yonghang, Wenwei Ye, Xiangshuai Liang, Peizhou Xu, Xianjun Wu, Xiangdong Fu, Yaoxian Chin, and Yongxiang Liao. 2023. "Rapid Visual Detection of High Nitrogen-Use Efficiency Gene OsGRF4 in Rice (Oryza sativa L.) Using Loop-Mediated Isothermal Amplification Method" Genes 14, no. 10: 1850. https://doi.org/10.3390/genes14101850
APA StyleTian, Y., Ye, W., Liang, X., Xu, P., Wu, X., Fu, X., Chin, Y., & Liao, Y. (2023). Rapid Visual Detection of High Nitrogen-Use Efficiency Gene OsGRF4 in Rice (Oryza sativa L.) Using Loop-Mediated Isothermal Amplification Method. Genes, 14(10), 1850. https://doi.org/10.3390/genes14101850






