FNDC5 Promotes Adipogenic Differentiation of Primary Preadipocytes in Mashen Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Preparation
2.3. Adipogenic Induction and Oil Red O Staining
2.4. RNA Extraction and cDNA Synthesis
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Paraffin Section and HE Staining
2.7. Western Blotting
2.8. Lentiviral-Mediated Transfection
2.9. Statistical Analysis
3. Results
3.1. The Expression Profile of FNDC5 in Mashen Pigs
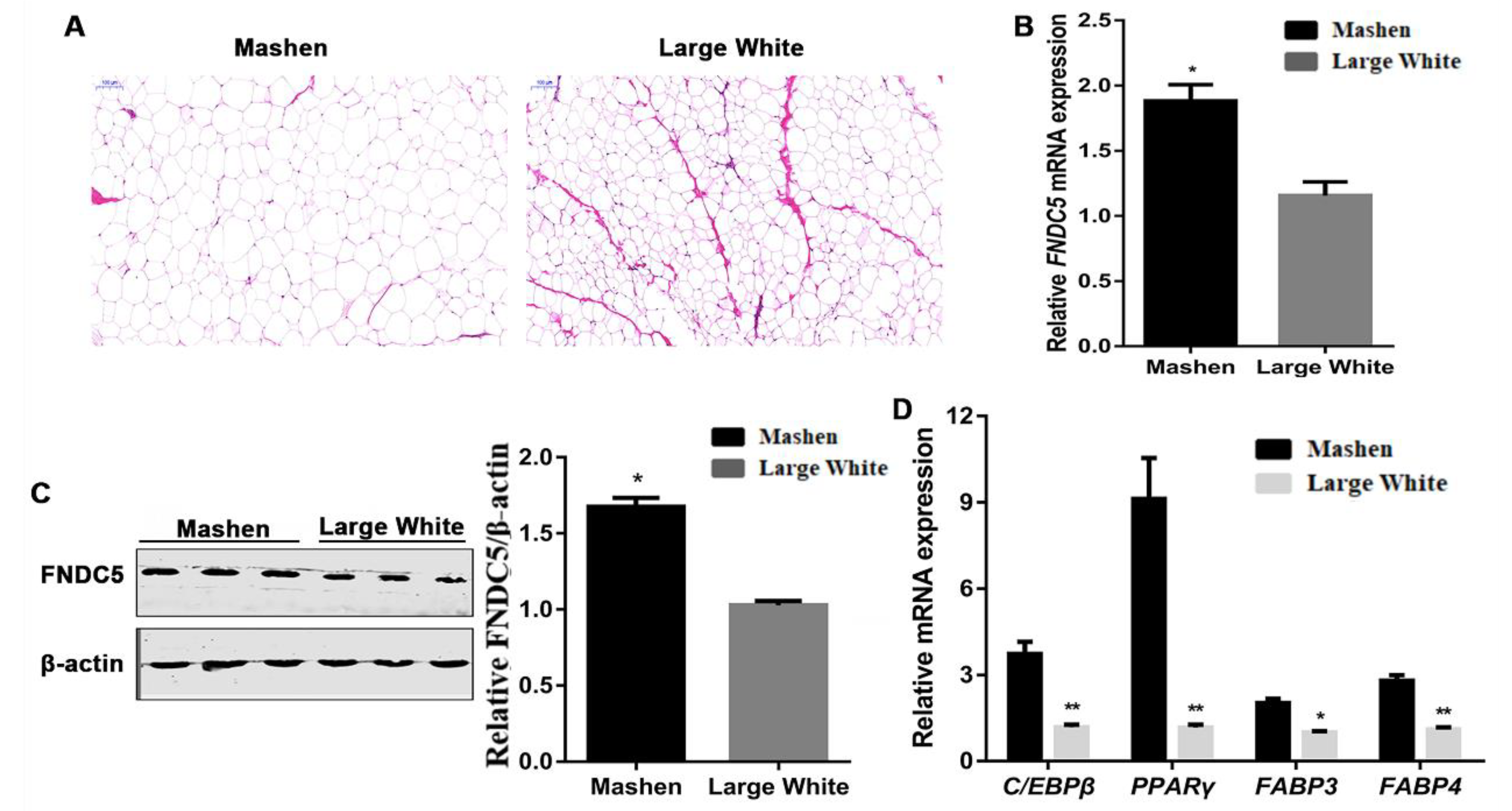
3.2. Difference of Back Fat between Mashen and Large White Pigs
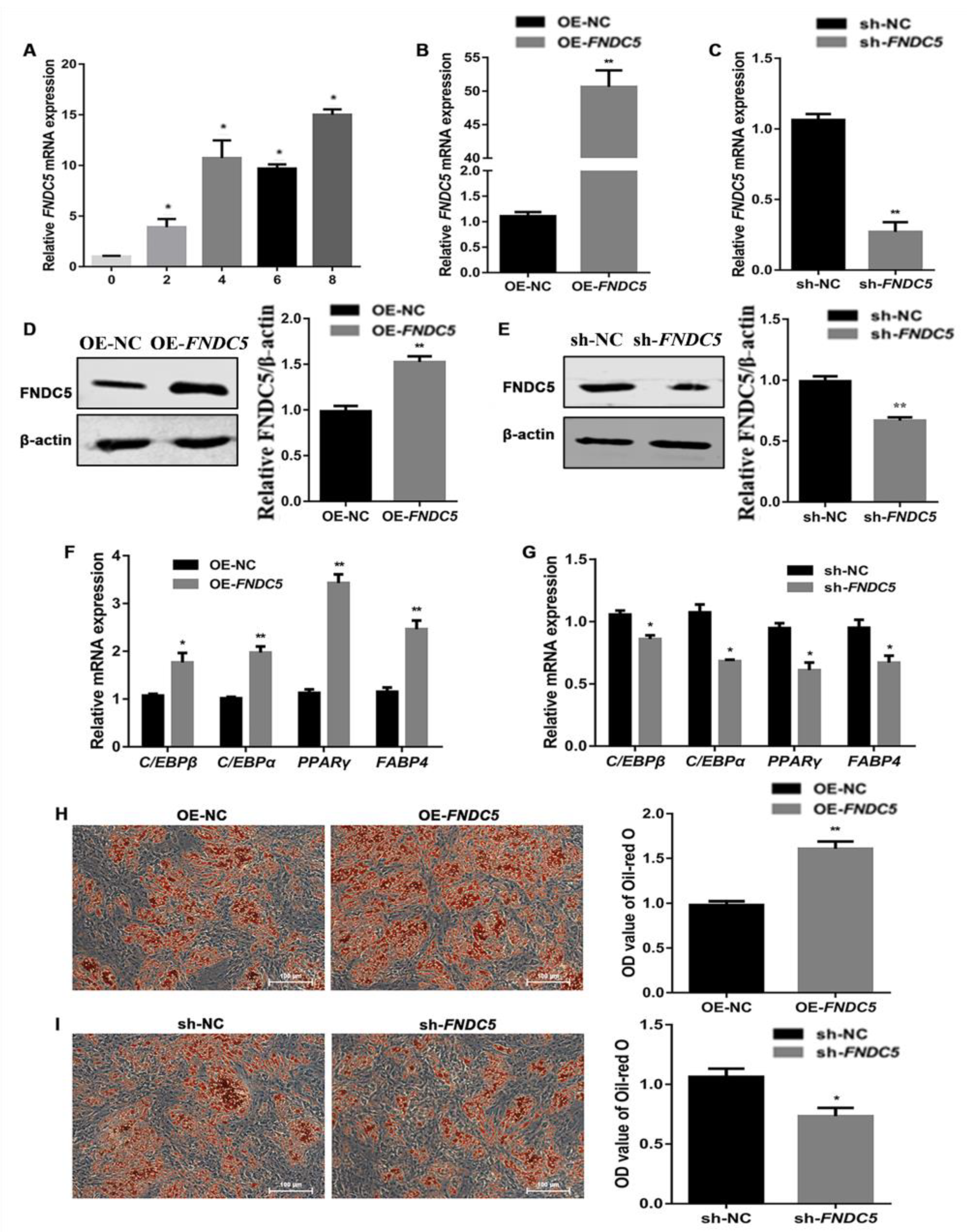
3.3. FNDC5 Promoted the Adipogenic Differentiation of Porcine Preadipocytes in Mashen Pigs
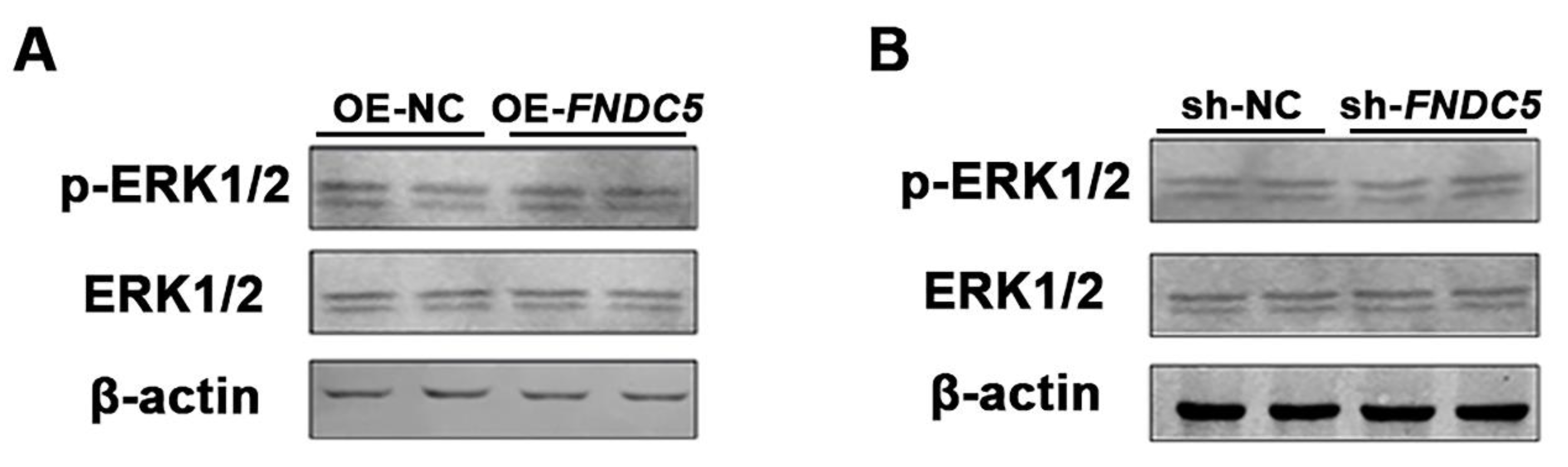
3.4. FNDC5 Had No Effect on ERK1/2 Phosphorylation during Adipogenic Differentiation in Porcine Preadipocytes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monziols, M.; Bonneau, M.; Davenel, A.; Kouba, M. Comparison of the lipid content and fatty acid composition of intermuscular and subcutaneous adipose tissues in pig carcasses. Meat Sci. 2006, 76, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Saetang, J.; Sangkhathat, S. Role of innate lymphoid cells in obesity and metabolic disease (Review). Mol. Med. Rep. 2018, 17, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, C.F.; Sirtori, O.; Franci, A.; Acciaioli, R.; Bozzi, A.; Pezzati, A.; Pugliese, C. Effects of protein restriction on performances and meat quality of cinta senese pig reared in an organic system. Animals 2019, 9, 310. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, R.; Gong, Z.; Zou, Q.; Wang, Y.; Tang, G.; Zhu, L.; Li, X.; Jiang, Y. Evaluation of coat color inheritance and production performance for crossbreed from Chinese indigenous Chenghua pig crossbred with Berkshire. Anim. Biosci. 2022, 35, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qin, B.; Yang, X.; Jia, J.; Niu, J.; Li, M.; Cai, C.; Zhao, Y.; Gao, P.; Du, M.; et al. Comparison of carcass traits, meat quality and expressions of MyHCs in muscles between Mashen and Large White pigs. Ital. J. Anim. Sci. 2019, 18, 1410–1418. [Google Scholar] [CrossRef]
- Bae, C.R.; Kwon, Y.G. CU06-1004 modulates the adenosine monophosphate (AMP)-associated protein kinase (AMPK) signaling pathway and inhibits lipogenesis in 3T3-L1 adipocytes and high-fat diet-induced obese mice. Life Sci. 2022, 296, 120440. [Google Scholar] [CrossRef]
- Ferrer-Martínez, A.; Ruiz-Lozano, P.; Chien, K.R. Mouse PeP: A novel peroxisomal protein linked to myoblast differentiation and development. Dev. Dyn. 2002, 224, 154–167. [Google Scholar] [CrossRef]
- Varela-Rodríguez, B.M.; Pena-Bello, L.; Juiz-Valiña, P.; Vidal-Bretal, B.; Cordido, F.; Sangiao-Alvarellos, S. FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Sci. Rep. 2016, 19, 29898. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef]
- Men, X.M.; Xu, Z.W.; Tao, X.; Deng, B.; Qi, K.K. FNDC5 expression closely correlates with muscle fiber types in porcine longissimus dorsi muscle and regulates myosin heavy chains (MyHCs) mRNA expression in C2C12 cells. PeerJ 2021, 9, e11065. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Hydrogen Sulfide regulates Irisin and glucose metabolism in myotubes and muscle of HFD-fed diabetic mice. Antioxidants 2022, 11, 1369. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Mohammed, A.K.; Al-Attas, O.S.; Amer, O.E.; Clerici, M.; Alenad, A.; Alokail, M.S. SNPs in FNDC5 (irisin) are associated with obesity and modulation of glucose and lipid metabolism in Saudi subjects. Lipids Health Dis. 2016, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sotelo, D.; Roca-Rivada, A.; Baamonde, I.; Baltar, J.; Castro, A.I.; Domínguez, E.; Collado, M.; Casanueva, F.F.; Pardo, M. Lack of adipocyte-Fndc5/Irisin expression and secretion reduces thermogenesis and enhances adipogenesis. Sci. Rep. 2017, 7, 16289. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.J.; Li, J.; Park, K.; Qian, X.; Ma, J.X.; Zhang, S.X. Pigment epithelium-derived factor suppresses adipogenesis via inhibition of the MAPK/ERK pathway in 3T3-L1 preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2009, 297, e1378–e1387. [Google Scholar] [CrossRef]
- Bost, F.; Aouadi, M.; Caron, L.; Even, P.; Belmonte, N.; Prot, M.; Dani, C.; Hofman, P.; Pagès, G.; Pouysségur, J.; et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes 2005, 54, 402–411. [Google Scholar] [CrossRef]
- Liao, Q.C.; Li, Y.L.; Qin, Y.F.; Quarles, L.D.; Xu, K.K.; Li, R.; Zhou, H.H.; Xiao, Z.S. Inhibition of adipocyte differentiation by phytoestrogen genistein through a potential downregulation of extracellular signal-regulated kinases 1/2 activity. J. Cell Biochem. 2008, 104, 1853–1864. [Google Scholar] [CrossRef]
- Jiang, S.; Wei, H.; Song, T.; Yang, Y.; Zhang, F.; Zhou, Y.; Peng, J.; Jiang, S. KLF13 promotes porcine adipocyte differentiation through PPARγ activation. Cell Biosci. 2015, 5, 28. [Google Scholar] [CrossRef]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisløff, U.; Tjønna, A.E.; Raastad, T.; et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, Y.J.; Song, I.S.; Noh, Y.H.; Seo, K.W.; Kim, M.; Han, J. Glucocorticoid receptor positively regulates transcription of FNDC5 in the liver. Sci. Rep. 2017, 7, 43296. [Google Scholar] [CrossRef]
- Xue, C.; Li, X.; Ba, L.; Shen, Y.; Sun, Z.; Gu, J.; Yang, Y.; Han, Q.; Zhao, R.C. Irisin mediates beiging of adipose-derived mesenchymal stem cells through binding to TRPC3. BMC Biol. 2022, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Donelan, W.; Wang, F.; Zhang, P.; Yang, L.; Ding, Y.; Tang, D.; Li, S. GLP-1 induces the expression of FNDC5 derivatives that execute lipolytic actions. Front. Cell Dev. Biol. 2021, 9, 777026. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, S.; Tao, J.; Zhang, X.; Zhang, J.; Yang, J.; Liang, H.; Liu, D.; Cang, M. Fibronectin type III domain-containing protein 5 promotes proliferation and differentiation of goat adipose-derived stem cells. Res. Vet. Sci. 2019, 125, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Ates, I.; Arikan, M.F.; Erdogan, K.; Kaplan, M.; Yuksel, M.; Topcuoglu, C.; Yilmaz, N.; Guler, S. Factors associated with increased irisin levels in the type 1 diabetes mellitus. Endocr. Regul. 2017, 51, 1–7. [Google Scholar] [CrossRef]
- Ma, E.B.; Sahar, N.E.; Jeong, M.; Huh, J.Y. Irisin exerts inhibitory effect on adipogenesis through regulation of Wnt signaling. Front. Physiol. 2019, 10, 1085. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Wang, F.; Donelan, W.; Zona, M.C.; Li, S.; Reeves, W.; Ding, Y.; Tang, D.; Yang, L. Effects of irisin on the differentiation and browning of human visceral white adipocytes. Am. J. Transl. Res. 2019, 11, 7410–7421. [Google Scholar]
- Yang, H.; Cheng, J.; Song, Z.; Li, X.; Zhang, Z.; Mai, Y.; Pang, W.; Shi, X.; Yang, G. The anti-adipogenic effect of PGRN on porcine preadipocytes involves ERK1,2 mediated PPARγ phosphorylation. Mol. Biol. Rep. 2013, 40, 6863–6872. [Google Scholar] [CrossRef]
- Almalki, S.G.; Agrawal, D.K. ERK signaling is required for VEGF-A/VEGFR2-induced differentiation of porcine adipose-derived mesenchymal stem cells into endothelial cells. Stem Cell Res. Ther. 2017, 8, 113. [Google Scholar] [CrossRef]
- Sun, Y.M.; Qin, J.; Liu, S.G.; Cai, R.; Chen, X.C.; Wang, X.M.; Pang, W.J. PDGFRα regulated by miR-34a and FoxO1 promotes adipogenesis in porcine intramuscular preadipocytes through Erk signaling pathway. Int. J. Mol. Sci. 2017, 18, 2424. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Song, H.; Zhang, Y.; Zhang, Y.; Mu, Q.; Jiang, M.; Wang, F.; Zhang, W.; Li, L.; Li, H.; et al. Irisin induces angiogenesis in human umbilical vein endothelial cells in vitro and in zebrafish embryos in vivo via activation of the ERK signaling pathway. PLoS ONE 2015, 10, e0134662. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, M.V.; Freitas, G.B.; Raony, Í.; Ferreira, S.T.; De Felice, F.G. Irisin stimulates protective signaling pathways in rat hippocampal neurons. Front. Cell Neurosci. 2022, 16, 953991. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.; Kim, Y.H.; Lee, N.H.; Song, K.M. Irisin promotes C2C12 myoblast proliferation via ERK-dependent CCL7 upregulation. PLoS ONE 2019, 14, e0222559. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Kang, Y.; Xu, X.; Wang, B.; Du, M.; Zhu, M.J. Raspberry supplementation improves insulin signaling and promotes brown-like adipocyte development in white adipose tissue of obese mice. Mol. Nutr. Food Res. 2018, 62, 1701035. [Google Scholar] [CrossRef]

| Genes | Primer Sequence (5’-3’) | GenBank No. |
|---|---|---|
| FNDC5 | F: TGCAGGCCATCTCCATTCAG | XM_021095830.1 |
| R: ATATTGGCGGCAGAAGAGGG | ||
| FABP3 | F: TGACACTGGATGGAGGCAAA | NM_001099931.1 |
| R: TGGGTGAGTGTCAGGATGAGT | ||
| PPARγ | F: CTATTCCATGCTGTCATGGGTG | NM_214379.1 |
| R: ACCATGGTCACCTCTTGTGA | ||
| FABP4 | F: TGGTACAGGTGCAGAAGTGG | NM_001002817.1 |
| R: TTCTGGTAGCCGTGACACCT | ||
| C/EBPα | F: AGAACAGCAACGAGTACCGG | NM_001123135.1 |
| R: GCTCCAGCACCTTCTGTTGA | ||
| C/EBPβ | F: CTGGAGACGCAGCATAAGGT | NM_001199889.1 |
| R: TGCTTGAACAAGTTCCGCAG | ||
| 18S | F: ATGCCAGAGTCTCGTTCGTTAT | NR_046261.1 |
| R: CGGACAGGATTGACAGATTGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hei, W.; You, Z.; An, J.; Zhao, T.; Li, J.; Zhang, W.; Li, M.; Yang, Y.; Gao, P.; Cao, G.; et al. FNDC5 Promotes Adipogenic Differentiation of Primary Preadipocytes in Mashen Pigs. Genes 2023, 14, 90. https://doi.org/10.3390/genes14010090
Hei W, You Z, An J, Zhao T, Li J, Zhang W, Li M, Yang Y, Gao P, Cao G, et al. FNDC5 Promotes Adipogenic Differentiation of Primary Preadipocytes in Mashen Pigs. Genes. 2023; 14(1):90. https://doi.org/10.3390/genes14010090
Chicago/Turabian StyleHei, Wei, Ziwei You, Jiaqi An, Tianzhi Zhao, Jiao Li, Wanfeng Zhang, Meng Li, Yang Yang, Pengfei Gao, Guoqing Cao, and et al. 2023. "FNDC5 Promotes Adipogenic Differentiation of Primary Preadipocytes in Mashen Pigs" Genes 14, no. 1: 90. https://doi.org/10.3390/genes14010090
APA StyleHei, W., You, Z., An, J., Zhao, T., Li, J., Zhang, W., Li, M., Yang, Y., Gao, P., Cao, G., Guo, X., Cai, C., & Li, B. (2023). FNDC5 Promotes Adipogenic Differentiation of Primary Preadipocytes in Mashen Pigs. Genes, 14(1), 90. https://doi.org/10.3390/genes14010090




