Isolation of Salvia miltiorrhiza Kaurene Synthase-like (KSL) Gene Promoter and Its Regulation by Ethephon and Yeast Extract
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Cultivation
2.2. Isolation of Genomic DNA
2.3. Promoter Isolation and In Silico Characterization
2.4. Microarray and NGS Co-Expression Data Analysis
2.5. Callus Induction and Elicitation by YE and ET
2.6. Calculation of Callus Growth Index
2.7. Tanshinone Extraction Procedure
2.8. UHPLC Analysis
2.9. RNA Isolation and cDNA Synthesis
2.10. Real-Time PCR
2.11. Statistical Analysis
3. Results
3.1. In Silico Analysis of SmKSL Promoter
3.2. Microarray and NGS Co-Expression Data Analysis
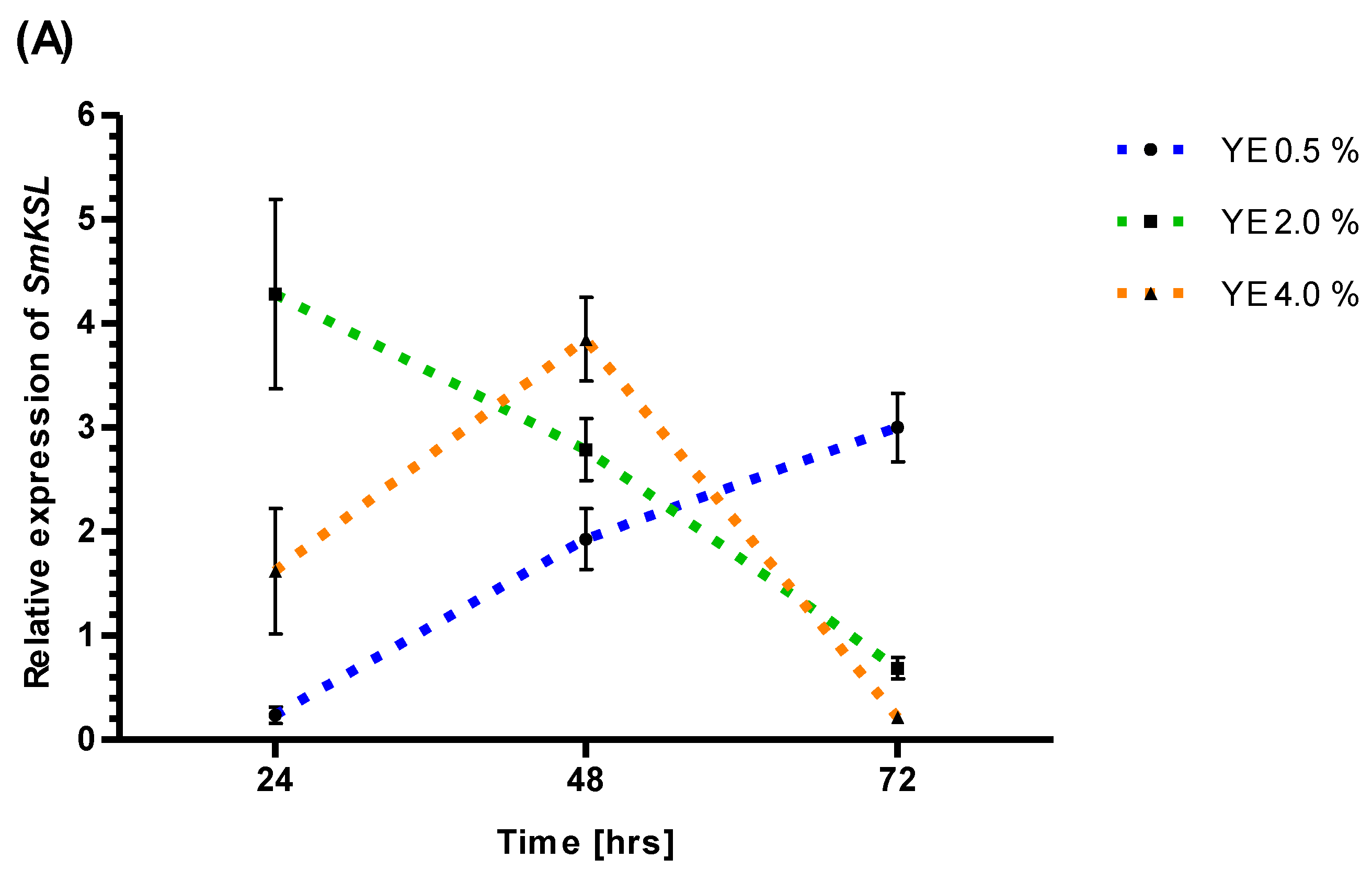
3.3. RT-PCR Analysis of SmKSL Promoter Activity after YE and ET
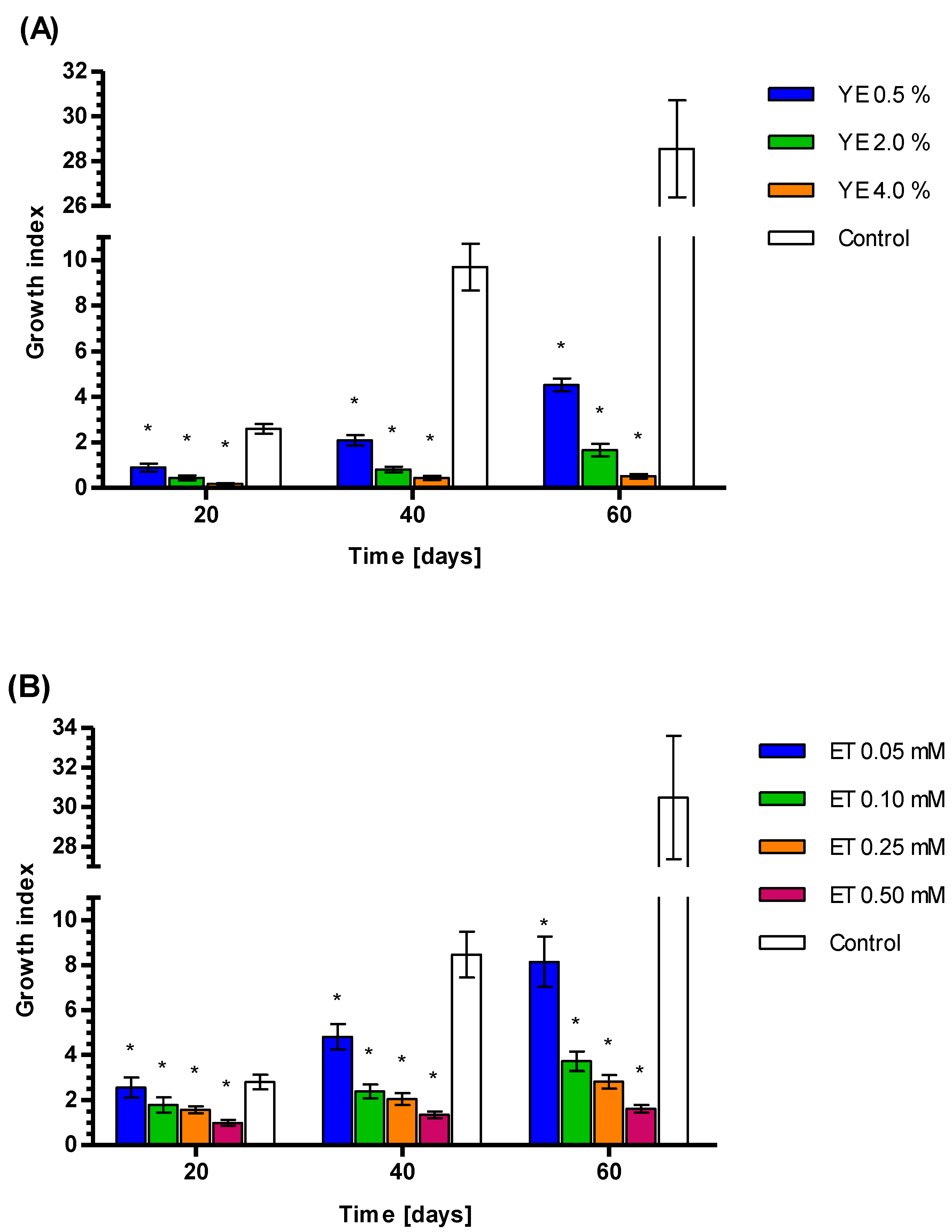
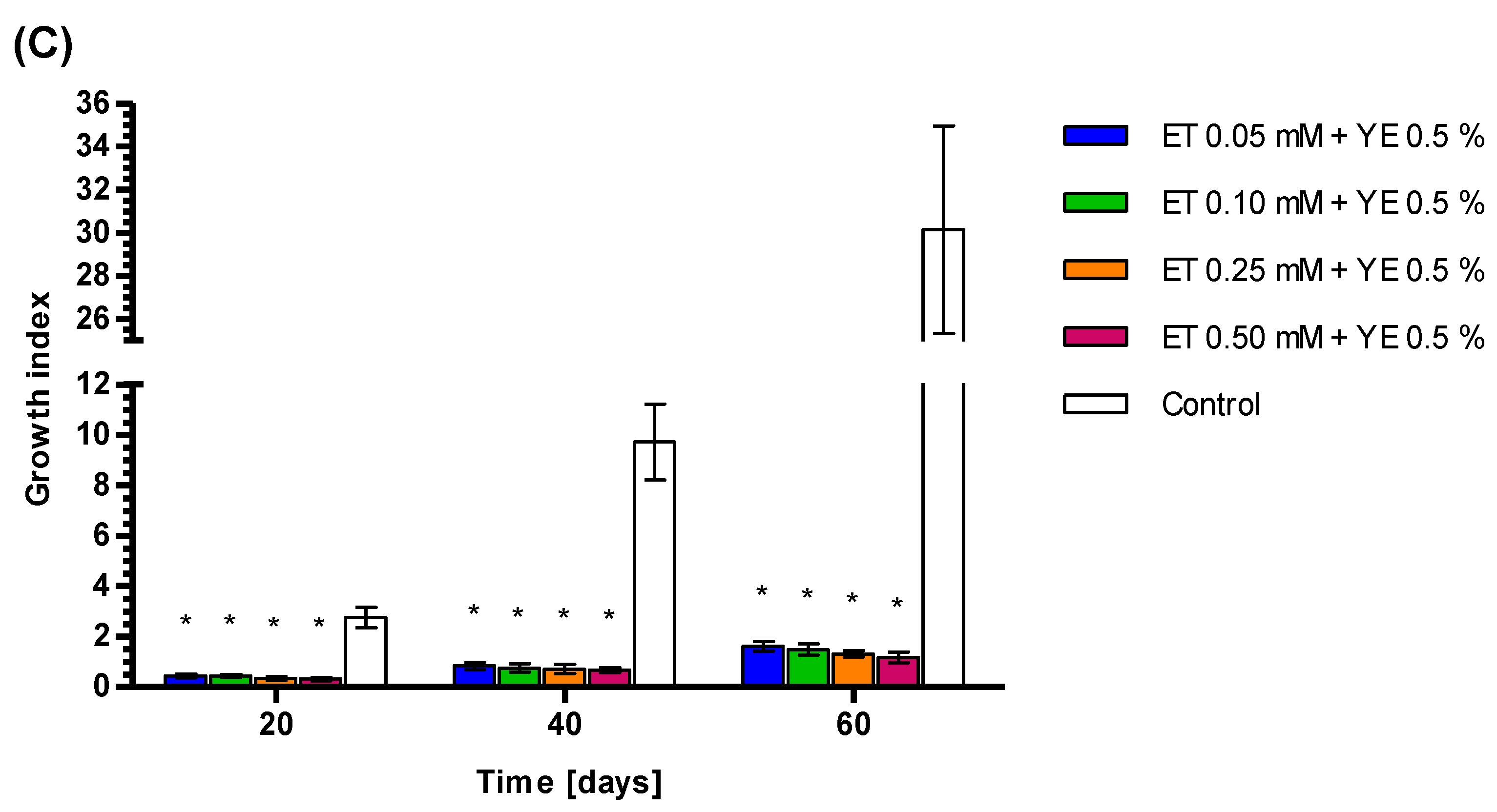
3.4. Growth Index Rates of Callus Treated by YE, ET and YE Combined with ET
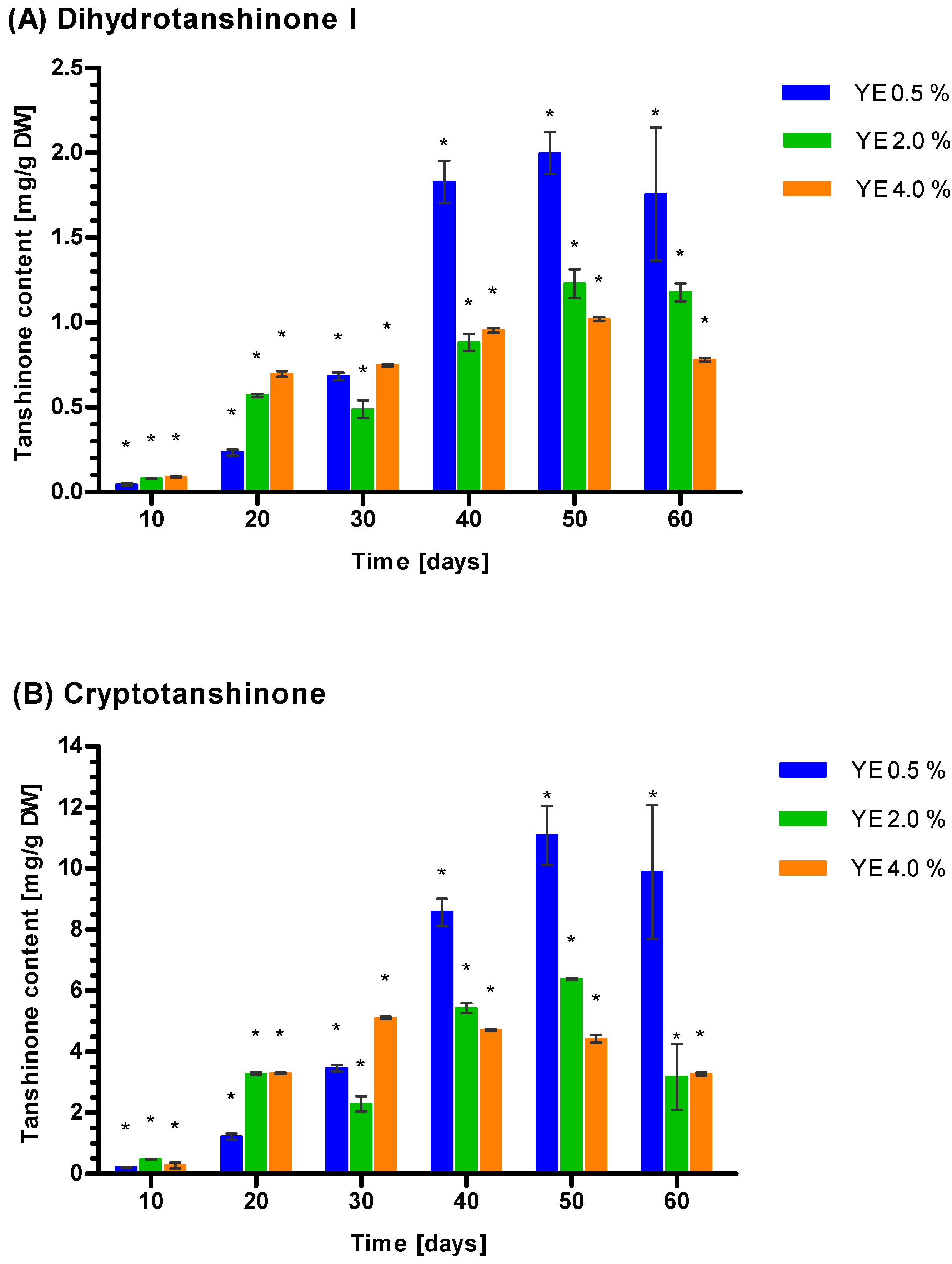
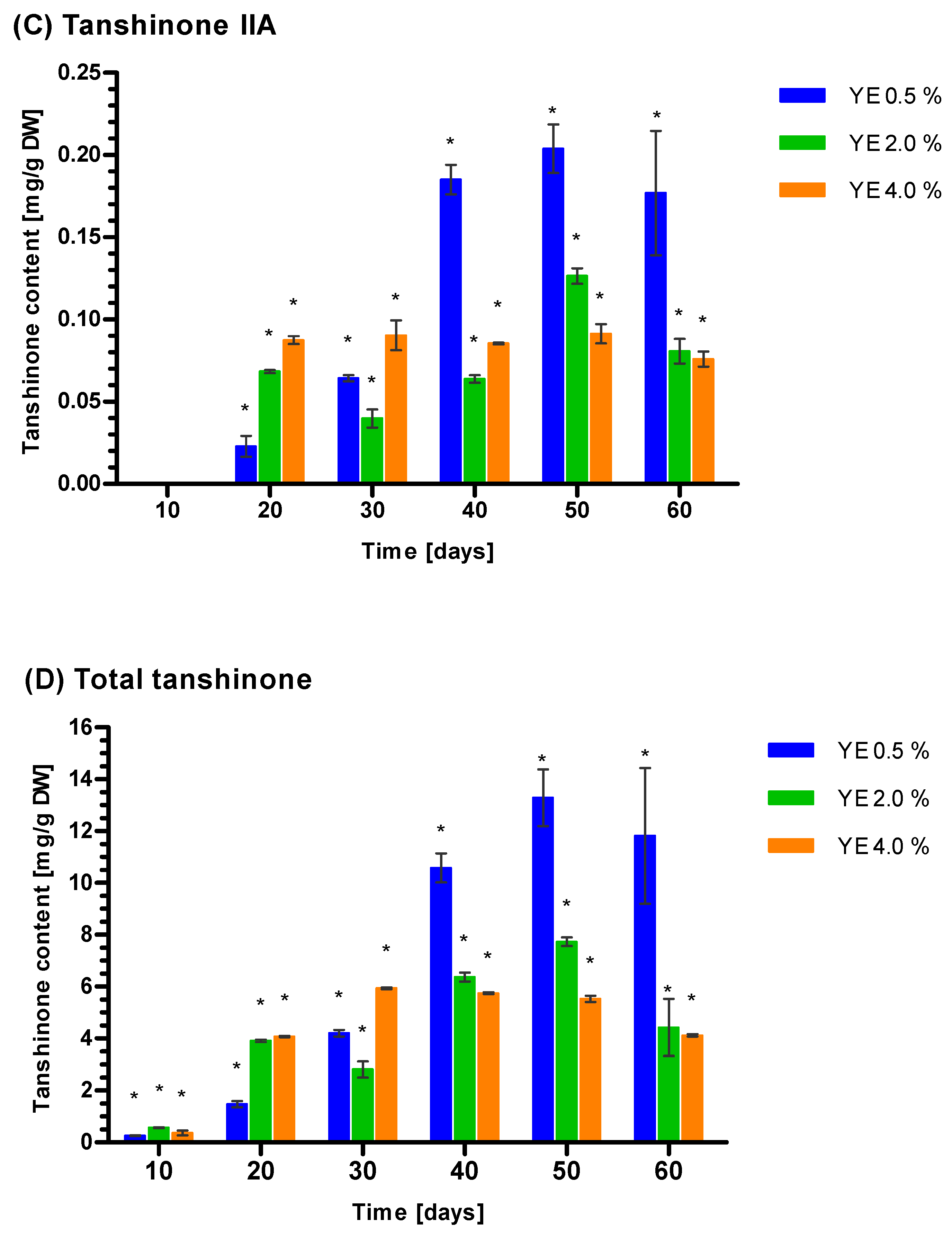
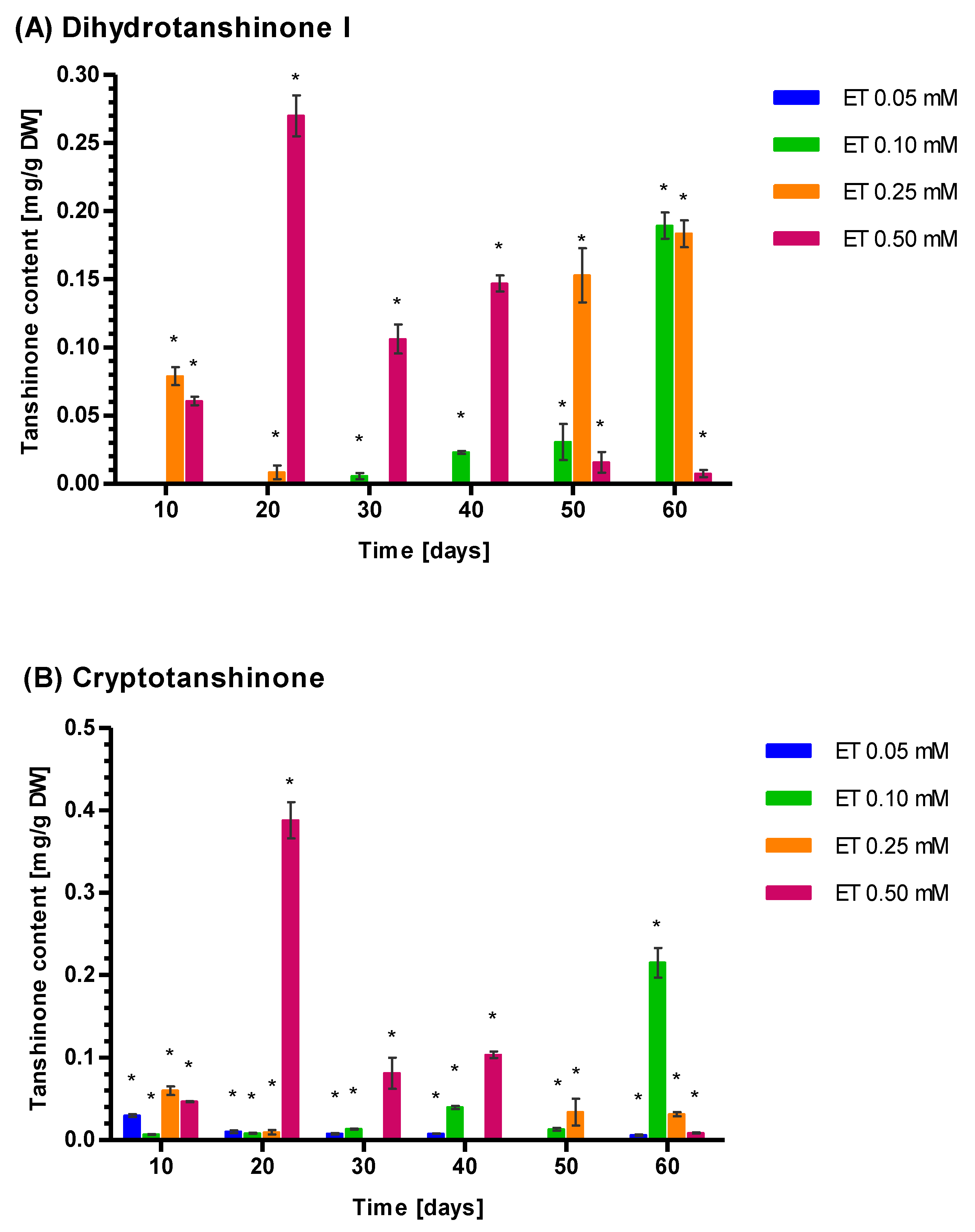
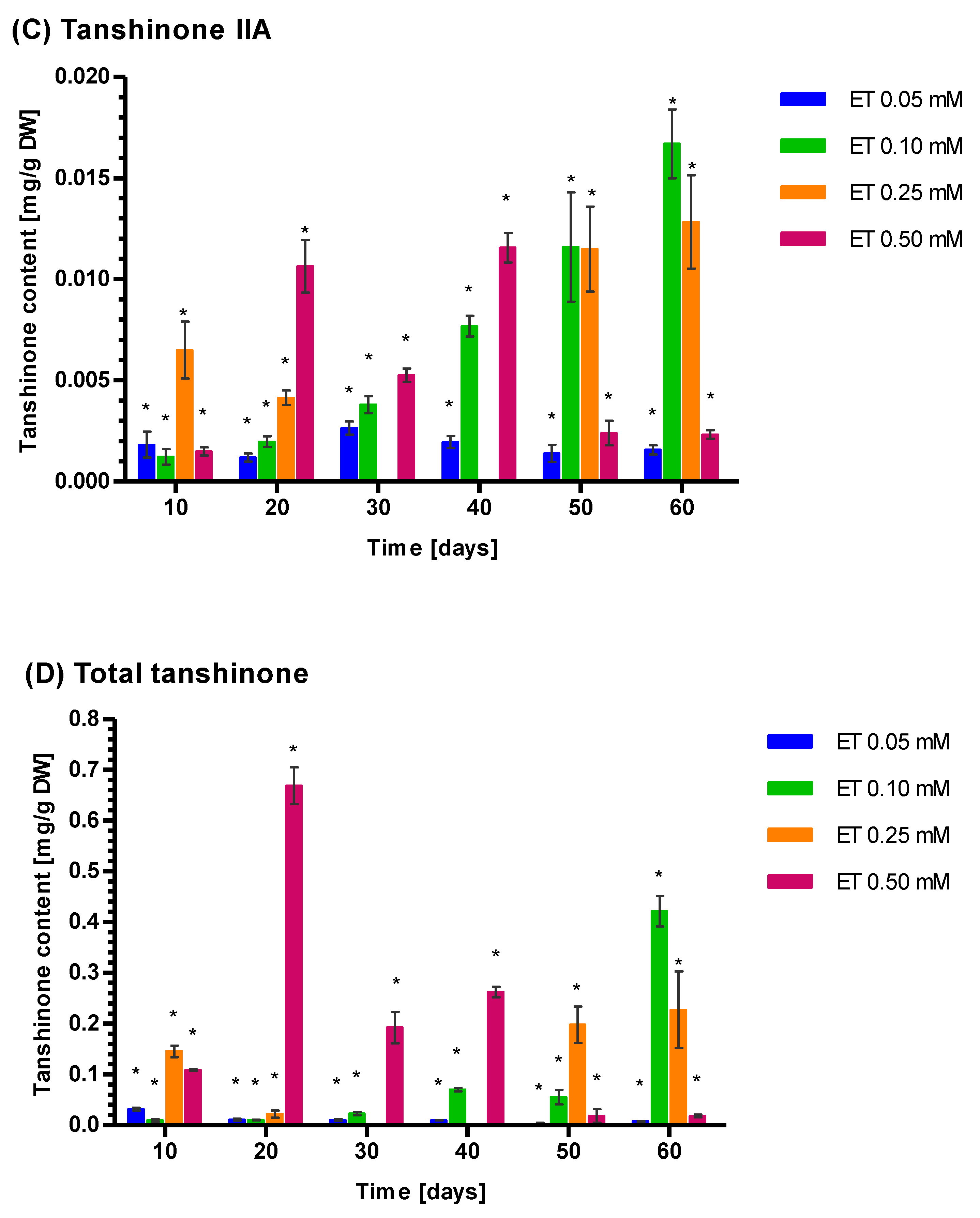
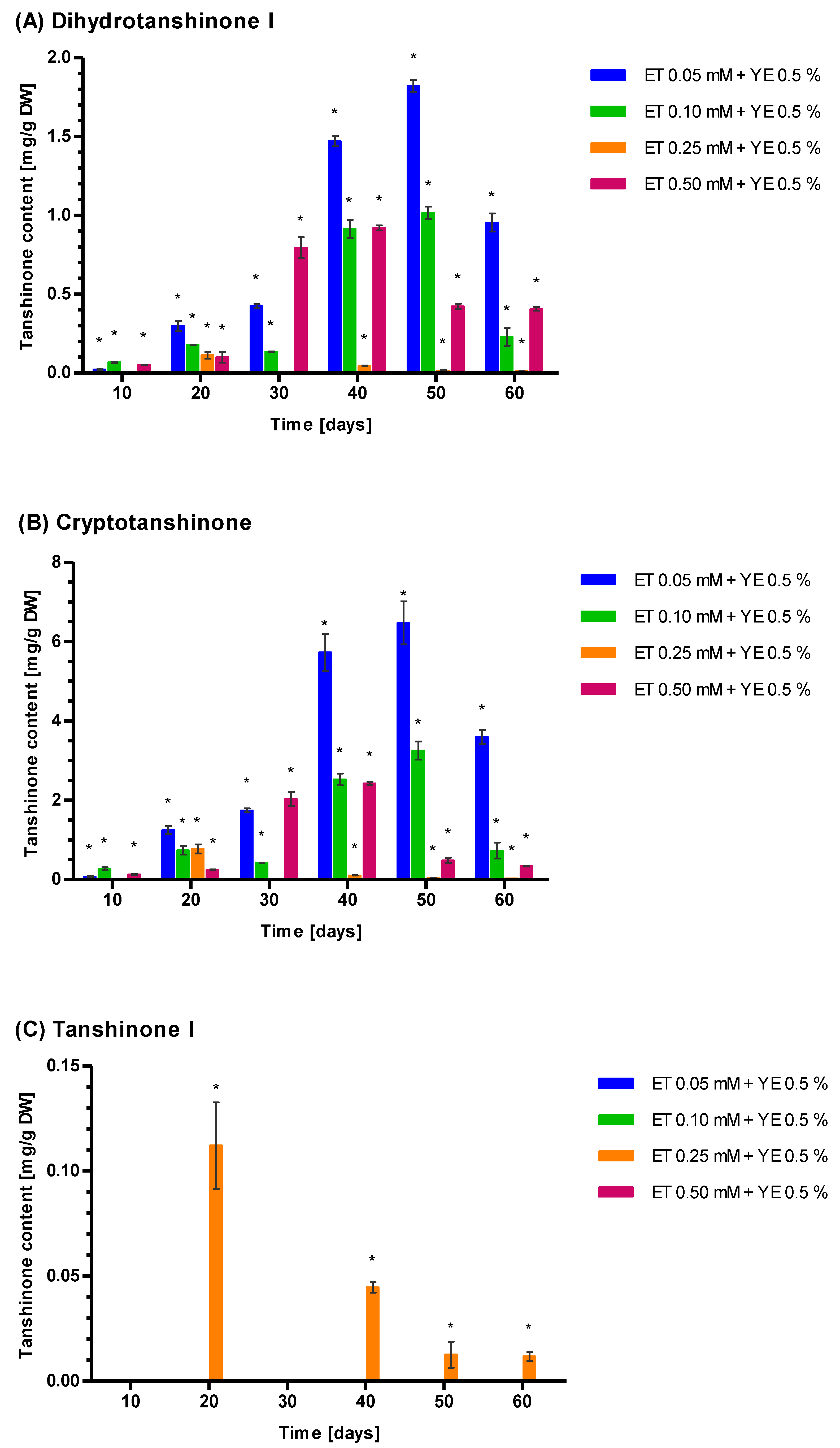
3.5. Induction of Tanshione Biosynthesis by YE, ET and YE Combined with ET
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, C.Y.; Ming, Q.L.; Rahman, K.; Han, T.; Qin, L.P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry and pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Gryszczynska, A.; Opala, B.; Lowicki, Z.; Dreger, M.; Gorska-Paukszta, M.; Szulc, M.; Kaminska, E.; Litwin, E.; Struzik, P.; Dyr, W.; et al. Bioactive compounds determination in the callus and hydroalcoholic extracts from Salvia miltiorrhiza and Salvia przewalskii—Preliminary study on their anti-alcoholic activity effects. Phytochem. Lett. 2015, 11, 399–403. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.; Liu, C.; Liu, H.; Zhu, R.; Guo, S.; Tang, M.; Li, Y.; Niu, J.; Fu, M.; et al. Salvia miltiorrhiza: A potential red light to the development of cardiovascular diseases. Curr. Pharm. Des. 2017, 23, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zuo, Z.; Chow, M.S. Danshen: An overview of its chemistry, pharmacology, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1389. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z. The Genome of Salvia miltiorrhiza. In The Salvia miltiorrhiza Genome, 1st ed.; Lu, S., Ed.; Springer: Cham, Switzerland, 2019; pp. 45–54. [Google Scholar] [CrossRef]
- Sui, C. Salvia miltiorrhiza Resources, Cultivation and Breeding. In The Salvia miltiorrhiza Genome, 1st ed.; Lu, S., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 17–32. [Google Scholar] [CrossRef]
- Krajewska-Patan, A.; Dreger, M.; Górska-Paukszta, M.; Mścisz, A.; Mielcarek, S.; Baraniak, M.; Buchwald, W.; Marecik, R.; Grajek, W.; Mrozikiewicz., P.M. Salvia miltiorrhiza Bunge in vitro cultivation in callus cultures. Herba Pol. 2007, 5, 88–96. [Google Scholar]
- Wang, W.W.; Wu, J.Y. Tanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue cultures. Appl. Microbiol. Biotechnol. 2010, 88, 437–449. [Google Scholar] [CrossRef]
- Wu, C.T.; Mulagabal, V.; Nalawade, S.M.; Chen, C.L.; Yang, T.F.; Tsay, H.S. Isolation and quantitative analysis of cryptotanshinone, an active quinoid diterpene formed in callus of Salvia miltiorrhiza Bunge. Biol. Pharm. Bull. 2003, 26, 845–848. [Google Scholar] [CrossRef]
- Efferth, T.H. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Weber, J.; Maciuk, A. Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl. Microbiol. Biotechnol. 2009, 83, 809–823. [Google Scholar] [CrossRef]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef]
- Tabata, H. Paclitaxel production by plant-cell-culture technology. Adv. Biochem. Eng. Biotechnol. 2004, 87, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, G.A.; Golembo, M.; Shaaltiel, Y. Taliglucerase alfa: An enzyme replacement therapy using plant cell expression technology. Mol. Genet. Metab. 2014, 112, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Wi, S.J. Potential of plants to produce recombinant protein products. J. Plant Biol. 2016, 59, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Top, O.; Geisen, U.; Decker, E.L.; Reski, R. Critical evaluation of strategies for the production of blood coagulation factors in plant-based systems. Front. Plant Sci. 2019, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, F. Induction of phytoalexin formation in crown gall and hairy root culture of Salvia miltiorrhiza by methyl viologen. Biotechnol. Lett. 2000, 22, 715–720. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, J.P.; Chen, F.; Zhang, Y.L.; Song, J.Y. Tanshinone production in Ti transformed Salvia miltiorrhiza cell suspension cultures. J. Biotechnol. 1997, 58, 147–156. [Google Scholar] [CrossRef]
- Cheng, Q.; He, Y.; Li, G.; Liu, Y.; Gao, W.; Huang, L. Effects of combined elicitors on tanshinone metabolic profiling and SmCPS expression in Salvia miltiorrhiza hairy root cultures. Molecules 2013, 18, 7473–7485. [Google Scholar] [CrossRef]
- Hao, X.; Shi, M.; Cui, L.; Xu, C.; Zhang, Y.; Kai, G. Effects of methyl jasmonate and salicylic acid on tanshinone production and biosynthetic gene expression in transgenic Salvia miltiorrhiza hairy roots. Biotechnol. Appl. Biochem. 2015, 62, 24–31. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zhou, L.G.; Wu, J.Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010, 87, 137–144. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, T.; Wu, Y.; Zhou, Y.; Jiang, Y.; Zhang, L. Effect of elicitors on the metabolites in the suspension cell culture of Salvia miltiorrhiza Bunge. Physiol. Mol. Biol. Plants 2019, 25, 229–242. [Google Scholar] [CrossRef]
- Shi, M.; Luo, X.; Ju, G.; Yu, X.; Hao, X.; Huang, Q.; Xiao, J.; Cui, L. Increased accumulation of the cardio-cerebrovascular disease treatment drug tanshinone in Salvia miltiorrhiza hairy roots by the enzymes 3-hydroxy-3-methylglutaryl CoA reductase and 1-deoxy-D-xylulose 5-phosphate reductoisomerase. Funct. Integr. Genom. 2014, 14, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Khodakovskaya, M.V.; Glazunov, V.P.; Radchenko, S.V.; Zvereva, E.V.; Fedoreyev, S.A.; Zhuravlev, Y.N. Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J. Biotechnol. 2002, 97, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M. Ethylene signalingin plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Ji, A.J.; Luo, H.M.; Xu, Z.C.; Zhang, X.; Zhu, Y.J.; Liao, B.S.; Yao, H.; Song, J.Y.; Chen, S.L. Genome-Wide Identification of the AP2/ERF Gene Family Involved in Active Constituent Biosynthesis in Salvia miltiorrhiza. Plant Genome 2016, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, A.; Xu, Z.; Luo, H.; Song, J. The AP2/ERF transcription factor SmERF128 positively regulates diterpenoid biosynthesis in Salvia miltiorrhiza. Plant Mol. Biol. 2019, 100, 83–93. [Google Scholar] [CrossRef]
- Huang, Q.; Sun, M.; Yuan, T.; Wang, Y.; Shi, M.; Lu, S.; Tang, B.; Pan, J.; Wang, Y.; Kai, G. The AP2/ERF transcription factor SmERF1L1 regulates the biosynthesis of tanshinones and phenolic acids in Salvia miltiorrhiza. Food Chem. 2019, 274, 368–375. [Google Scholar] [CrossRef]
- Bai, Z.; Li, W.; Jia, Y.; Yue, Z.; Jiao, J.; Huang, W.; Xia, P.; Liang, Z. The ethylene response factor SmERF6 co-regulates the transcription of SmCPS1 and SmKSL1 and is involved in tanshinone biosynthesis in Salvia miltiorrhiza hairy roots. Planta 2018, 248, 243–255. [Google Scholar] [CrossRef]
- Bai, Z.; Wu, J.; Huang, W.; Jiao, J.; Zhang, C.; Hou, Z.; Yan, K.; Zhang, X.; Han, R.; Liang, Z.; et al. The ethylene response factor SmERF8 regulates the expression of SmKSL1 and is involved in tanshinone biosynthesis in Saliva miltiorrhiza hairy roots. J. Plant Physiol. 2020, 244, 153006. [Google Scholar] [CrossRef]
- Zheng, H.; Jing, L.; Jiang, X.; Pu, C.; Zhao, S.; Yang, J.; Guo, J.; Cui, G.; Tang, J.; Ma, Y.; et al. The ERF-VII transcription factor SmERF73 coordinately regulates tanshinone biosynthesis in response to stress elicitors in Salvia miltiorrhiza. New Phytol. 2021, 231, 1940–1955. [Google Scholar] [CrossRef]
- Xie, W.; Ding, C.; Hu, H.; Dong, G.; Zhang, G.; Qian, Q.; Ren, D. Molecular events of rice AP2/ERF transcription factors. Int. J. Mol. Sci. 2022, 23, 12013. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Chen, S.T.; Agnolet, S.; Hegelund, J.N.; Stanstrup, J.; Christensen, J.H.; Müller, R.; Lütken, H. Ethephon-induced changes in antioxidants and phenolic compounds in anthocyanin-producing black carrot hairy root cultures. J. Exp. Bot. 2020, 71, 7030–7045. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Dziurka, M.; Blažević, I.; Đulović, A.; Apola, A.; Ekiert, H.; Szopa, A. Impacts of elicitors on metabolite production and on antioxidant potential and tyrosinase inhibition in watercress microshoot cultures. Appl. Microbiol. Biotechnol. 2022, 106, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Xia, P.; Wang, R.; Jiao, J.; Ru, M.; Liu, J.; Liang, Z. Molecular cloning and characterization of five SmGRAS genes associated with tanshinone biosynthesis in Salvia miltiorrhiza hairy roots. PLoS ONE 2017, 12, e0185322. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, F.; Chiu, F.C.; Lo, C.M. The effect of yeast elicitor on the growth and secondary metabolism of hairy root cultures of Salvia miltiorrhiza. Enzyme Microb. Technol. 2001, 28, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, B.; Li, H.; Peng, L.; Ru, M.; Liang, Z.; Yan, X.; Zhu, Y. Establishment of Salvia castanea Diels f. Tomentosa Stib. hairy root cultures and the promotion of tanshinone accumulation and gene expression with Ag⁺, methyl jasmonate, and yeast extract elicitation. Protoplasma 2016, 253, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef]
- Fonseca, J.P.; Menossi, M.; Thibaud-Nissen, F.; Town, C.D. Functional analysis of a TGA factor-binding site located in the promoter region controlling salicylic acid-induced NIMIN-1 expression in Arabidopsis. Genet. Mol. Res. 2010, 9, 167–175. [Google Scholar] [CrossRef]
- Sun, T.; Busta, L.; Zhang, Q.; Ding, P.; Jetter, R.; Zhang, Y. TGACG-BINDING FACTOR 1 (TGA1) and TGA4 regulate salicylic acid and pipecolic acid biosynthesis by modulating the expression of SYSTEMIC ACQUIRED RESISTANCE DEFICIENT 1 (SARD1) and CALMODULIN-BINDING PROTEIN 60g (CBP60g). New Phytol. 2018, 217, 344–354. [Google Scholar] [CrossRef]
- Liu, X.; Song, Y.; Xing, F.; Wang, N.; Wen, F.; Zhu, C. GhWRKY25, a group I WRKY gene from cotton, confers differential tolerance to abiotic and biotic stresses in transgenic Nicotiana benthamiana. Protoplasma 2016, 253, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, Y.; Shi, M.; Hao, X.; Zhao, W.; Wang, Y.; Ren, J.; Kai, G. Transcription factor SmWRKY1 positively promotes the biosynthesis of tanshinones in Salvia miltiorrhiza. Front. Plant Sci. 2018, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Hao, X.; Shi, M.; Fu, R.; Wang, Y.; Zhang, Y.; Zhou, W.; Feng, Y.; Makunga, N.P.; Kai, G. Tanshinone production could be increased by the expression of SmWRKY2 in Salvia miltiorrhiza hairy roots. Plant Sci. 2019, 284, 1–8. [Google Scholar] [CrossRef]
- Zhou, J.M.; Trifa, Y.; Silva, H.; Pontier, D.; Lam, E.; Shah, J.; Klessig, D.F. NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol. Plant Microbe. Interact. 2000, 13, 191–202. [Google Scholar] [CrossRef]
- Blanco, F.; Garretón, V.; Frey, N.; Dominguez, C.; Pérez-Acle, T.; Van der Straeten, D.; Jordana, X.; Holuigue, L. Identification of NPR1-dependent and independent genes early induced by salicylic acid treatment in Arabidopsis. Plant Mol. Biol. 2005, 59, 927–944. [Google Scholar] [CrossRef]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef]
- Antolín-Llovera, M.; Leivar, P.; Arró, M.; Ferrer, A.; Boronat, A.; Campos, N. Modulation of plant HMG-CoA reductase by protein phosphatase 2A: Positive and negative control at a key node of metabolism. Plant Signal Behav. 2011, 8, 1127–1131. [Google Scholar] [CrossRef]
- Hemmerlin, A. Post-translational events and modifications regulating plant enzymes involved in isoprenoid precursor biosynthesis. Plant Sci. 2013, 203–204, 41–54. [Google Scholar] [CrossRef]
- Xiao, Y.; Savchenko, T.; Baidoo, E.E.K.; Chehab, W.E.; Hayden, D.M.; Tolstikov, V.; Corvin, J.A.; Kliebenstein, D.J.; Keasling, J.D.; Dehesh, K. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 2012, 149, 1525–1535. [Google Scholar] [CrossRef]
- Kirby, J.; Keasling, J.D. Biosynthesis of Plant Isoprenoids: Perspectives for Microbial Engineering. Annu. Rev. Plant Biol. 2009, 60, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Calisto, B.M.; Perez-Gil, J.; Bergua, M.; Querol-Audi, J.; Fita, I.; Imperial, S. Biosynthesis of isoprenoids in plants: Structure of the 2C-methyl-D-erythritol 2,4-cyclodiphosphate synthase from Arabidopsis thaliana. Comparison with bacterial enzymes. Protein Sci. 2007, 16, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Du, X.; Liang, X.; Han, R.; Liang, Z.; Liu, Y.; Liu, F.; Zhao, J. Different roles of mevalonate and methylerythritol phosphate pathways in cell growth and tanshinone production in Salvia miltiorrhiza hairy roots. PLoS ONE 2012, 7, e46797. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef]
- Ma, X.H.; Ma, Y.; Tang, J.F.; He, Y.L.; Liu, Y.C.; Ma, X.J.; Shen, Y.; Cui, G.H.; Lin, H.X.; Rong, Q.X.; et al. The Biosynthetic Pathways of Tanshinones and Phenolic Acids in Salvia miltiorrhiza. Molecules 2015, 20, 16235–16254. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, Q.; Wu, X.; Zhou, Z.; Ding, M.; Shi, M.; Huang, F.; Li, S.; Wang, Y.; Kai, G. Comprehensive transcriptome profiling of Salvia miltiorrhiza for discovery of genes associated with the biosynthesis of tanshinones and phenolic acids. Sci. Rep. 2017, 7, 10554. [Google Scholar] [CrossRef]
- Cui, G.; Duan, L.; Jin, B.; Qian, J.; Xue, Z.; Shen, G.; Snyder, J.H.; Song, J.; Chen, S.; Huang, L.; et al. Functional Divergence of Diterpene Syntheses in the Medicinal Plant Salvia miltiorrhiza. Plant Physiol. 2015, 169, 1607–1618. [Google Scholar] [CrossRef]
- Xu, Z.; Peters, R.J.; Weirather, J.; Luo, H.; Liao, B.; Zhang, X.; Zhu, Y.; Ji, A.; Zhang, B.; Hu, S.; et al. Full-length transcriptome sequences and splice variants obtained by a combination of sequencing platforms applied to different root tissues of Salvia miltiorrhiza and tanshinone biosynthesis. Plant J. 2015, 82, 951–961. [Google Scholar] [CrossRef]
- Sallaud, C.; Giacalone, C.; Töpfer, R.; Goepfert, S.; Bakaher, N.; Rösti, S.; Tissier, A. Characterization of two genes for the biosynthesis of the labdane diterpene Z-abienol in tobacco (Nicotiana tabacum) glandular trichomes. Plant J. 2012, 72, 1–17. [Google Scholar] [CrossRef]
- Khan, S.; Qureshi, M.I.; Kamaluddin, A.T.; Abdin, M.Z. Protocol for isolation of genomic DNA from fresh and dry roots of medicinal plants suitable for RAPD and restriction digestion. Afr. J. Biotechnol. 2007, 6, 175–178. [Google Scholar] [CrossRef]
- Szymczyk, P.; Szymańska, G.; Kuźma, Ł.; Jeleń, A.; Balcerczak, E. Methyl Jasmonate Activates the 2C Methyl-D-erithrytol 2,4-cyclodiphosphate Synthase Gene and Stimulates Tanshinone Accumulation in Salvia miltiorrhiza Solid Callus Cultures. Molecules 2022, 27, 1772. [Google Scholar] [CrossRef] [PubMed]
- Kibbe, W.A. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res. 2007, 35, W43–W46. [Google Scholar] [CrossRef] [PubMed]
- Birnboim, H.C.; Doly, J. A Rapid Alkaline Extraction Procedure for Screening Recombinant Plasmid DNA. Nucleic Acids Res. 1979, 7, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.N.; Lee, T.Y.; Hung, Y.C.; Li, G.Z.; Tseng, K.C.; Liu, Y.H.; Kuo, P.L.; Zheng, H.Q.; Chang, W.C. PlantPAN3.0: A new and updated resource for reconstructing transcriptional regulatory networks from ChIP-seq experiments in plants. Nucleic Acids Res. 2019, 47, D1155–D1163. [Google Scholar] [CrossRef] [PubMed]
- Shahmuradov, I.A.; Gammerman, A.J.; Hancock, J.M.; Bramley, P.M.; Solovyev, V.V. PlantProm: A database of plant promoter sequences. Nucleic Acids Res. 2003, 31, 114–117. [Google Scholar] [CrossRef]
- Shahmuradov, I.A.; Solovyev, V.V.; Gammerman, A.J. Plant promoter prediction with confidence estimation. Nucleic Acids Res. 2005, 33, 1069–1076. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Toufighi, K.; Brady, S.M.; Austin, R.; Ly, E.; Provart, N.J. The Botany Array Resource: E-Northerns, Expression Angling, and promoter analyses. Plant J. 2005, 43, 153–163. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Yu, Y.; Feng, L.; Jia, J.; Liu, B.; Li, B.; Guo, H.; Zhai, J. A Comprehensive Online Database for Exploring ~20,000 Public Arabidopsis RNA-Seq Libraries. Mol. Plant 2020, 13, 1231–1233. [Google Scholar] [CrossRef]
- Usadel, B.; Obayashi, T.; Mutwil, M.; Giorgi, F.M.; Bassel, G.W.; Tanimoto, M.; Chow, A.; Steinhauser, D.; Persson, S.; Provart, N.J. Co-expression tools for plant biology: Opportunities for hypothesis generation and caveats. Plant Cell Environ. 2009, 32, 1633–1651. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays for tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Foster, K.R.; Reid, D.M.; Pharis, R.P. Ethylene Biosynthesis and Ethephon Metabolism and Transport in Barley. Crop Sci. 1992, 32, 1345–1352. [Google Scholar] [CrossRef]
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An Introduction to Terminology and Methodology of Chemical Synergy—Perspectives from Across Disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef]
- Godoy-Hernández, G.; Vázquez-Flota, F.A. Growth Measurements. In Plant Cell Culture Protocols. Methods in Molecular Biology; Loyola-Vargas, V.M., Vázquez-Flota, F., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 318, pp. 51–58. [Google Scholar] [CrossRef]
- Wan, X.; Wang, Y.; Row, K.H. Separation of tanshinone I, tanshinone IIA, and cryptotanshinone from Salvia miltiorrhiza Bunge by normal phase HPLC. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 544–552. [Google Scholar] [CrossRef]
- Majewska, M.; Szymczyk, P.; Gomulski, J.; Jeleń, A.; Grąbkowska, R.; Balcerczak, E.; Kuźma, Ł. The Expression Profiles of the Salvia miltiorrhiza 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase 4 Gene and Its Influence on the Biosynthesis of Tanshinones. Molecules 2022, 27, 4354. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, S.; Cui, G.; Chen, S.; Wei, J.; Huang, L. Characterization of reference genes for quantitative real time PCR analysis in various tissues of Salvia miltiorrhiza. Mol. Biol. Rep. 2010, 35, 507–513. [Google Scholar] [CrossRef]
- Szymczyk, P.; Grąbkowska, R.; Skała, E.; Żebrowska, M.; Balcerczak, E.; Jeleń, A. Isolation and characterization of a 3-hydroxy-3-methylglutaryl coenzyme A reductase 2 promoter from Salvia miltiorrhiza. J. Plant Biochem. Biotechnol. 2018, 27, 223–236. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excell-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Kiran, K.; Ansari, S.A.; Srivastava, R.; Lodhi, N.; Chaturvedi, C.P.; Sawant, S.V.; Tuli, R. The TATA-box sequence in the basal promoter contributes to determining light-dependent gene expression in plants. Plant Physiol. 2006, 142, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.; Grotewold, E. Genome wide analysis of Arabidopsis core promoters. BMC Genom. 2005, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Saulierè, J.; Sureau, A.; Expert-Bezancon, A.; Marie, J. The polypyrimidine tract binding protein (PTB) represses splicing of exon 6B from the tropomyosin pre-mRNA by directly interfering with the binding of the U2AF65 subunit. Mol. Cell. Biol. 2006, 26, 8755–8769. [Google Scholar] [CrossRef] [PubMed]
- Wachter, A.; Rühl, C.; Stauffer, E. The role of polypyrimidine tract-binding proteins and other hnRNP proteins in plant splicing regulation. Front. Plant Sci. 2012, 3, 81. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J.K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell. Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Szymczyk, P.; Szymańska, G.; Kochan, E.; Szemraj, J.; Grąbkowska, R. Elicitation of solid callus cultures of Salvia miltiorrhiza Bunge with salicylic acid and a synthetic auxin (1-naphthalenecetic acid). Plant Cell. Tiss. Organ Cult. 2021, 147, 491–502. [Google Scholar] [CrossRef]
- Chen, H.; Qi, Y.; He, X.; Xu, L.; Zhang, W.; Lv, X.; Zhang, H.; Yang, D.; Zhu, Y.; Liang, Z. Endophytic fungus Mucorcircinelloides DF20 promote tanshinone biosynthesis and accumulation in Salvia miltiorrhiza root. Plant Sci. 2021, 307, 110898. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, L.; Lu, S.; Xue, Y.; Wei, X.; Lu, J.; Zhang, Y. Transcriptome sequencing of Salvia miltiorrhiza after infection by its endophytic fungi and identification of genes related to tanshinone biosynthesis. Pharm. Biol. 2019, 57, 760–769. [Google Scholar] [CrossRef]
- Yang, D.; Fang, Y.; Xia, P.; Zhang, X.; Liang, Z. Diverse responses of tanshinone biosynthesis to biotic and abiotic elicitors in hairy root cultures of Salvia miltiorrhiza and Salvia castanea Diels f. tomentosa. Gene 2018, 643, 61–67. [Google Scholar] [CrossRef]
- Li, M.H.; Peng, Y.; Xiao, P.G. Distribution of tanshinones in the genus Salvia (family Lamiaceae) from China and its systematic significance. J. Syst. Evol. 2010, 48, 118–122. [Google Scholar] [CrossRef]
- Shi, M.; Kwok, K.W.; Wu, J.Y. Enhancement of tanshinone production in Salvia miltiorrhiza Bunge (red or Chinese sage) hairy-root culture by hyperosmotic stress and yeast elicitor. Biotechnol. Appl. Biochem. 2007, 46, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ma, Y. Biosynthetic pathway of tanshinones in Salvia miltiorhiza. In The Salvia miltiorrhiza Genome, 1st ed.; Lu, S., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 129–140. [Google Scholar] [CrossRef]
- Luo, H.; Zhu, Y.; Song, J.; Xu, L.; Sun, C.; Zhang, X.; Xu, Y.; He, L.; Sun, W.; Xu, H.; et al. Transcriptional data mining of Salvia miltiorrhiza in response to methyl jasmonate to examine the mechanism of bioactive compound biosynthesis and regulation. Physiol. Plant. 2014, 152, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Ma, P.; Ding, K.D.; Liu, S.; Jia, Y.; Ru, M.; Dong, J.; Liang, Z. SmJAZ8 acts as a core repressor regulating JA-induced biosynthesis of salvianolic acid and tanshinones in Salvia miltiorrhiza hairy roots. J. Exp. Bot. 2018, 69, 1663–1678. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczyk, P.; Kuźma, Ł.; Jeleń, A.; Balcerczak, E.; Majewska, M. Isolation of Salvia miltiorrhiza Kaurene Synthase-like (KSL) Gene Promoter and Its Regulation by Ethephon and Yeast Extract. Genes 2023, 14, 54. https://doi.org/10.3390/genes14010054
Szymczyk P, Kuźma Ł, Jeleń A, Balcerczak E, Majewska M. Isolation of Salvia miltiorrhiza Kaurene Synthase-like (KSL) Gene Promoter and Its Regulation by Ethephon and Yeast Extract. Genes. 2023; 14(1):54. https://doi.org/10.3390/genes14010054
Chicago/Turabian StyleSzymczyk, Piotr, Łukasz Kuźma, Agnieszka Jeleń, Ewa Balcerczak, and Małgorzata Majewska. 2023. "Isolation of Salvia miltiorrhiza Kaurene Synthase-like (KSL) Gene Promoter and Its Regulation by Ethephon and Yeast Extract" Genes 14, no. 1: 54. https://doi.org/10.3390/genes14010054
APA StyleSzymczyk, P., Kuźma, Ł., Jeleń, A., Balcerczak, E., & Majewska, M. (2023). Isolation of Salvia miltiorrhiza Kaurene Synthase-like (KSL) Gene Promoter and Its Regulation by Ethephon and Yeast Extract. Genes, 14(1), 54. https://doi.org/10.3390/genes14010054







