Complete Mitogenome of the Triplophysa bombifrons: Comparative Analysis and Phylogenetic Relationships among the Members of Triplophysa
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Experimental Fish and Sampling
2.3. DNA Isolation, Library Preparation, and Sequencing
2.4. T. bombifrons Mitogenome Assembly and Annotation
2.5. Sequence Analyses
2.6. Phylogenetic Analyses
3. Results and Discussion
3.1. Genome Structure and Base Composition
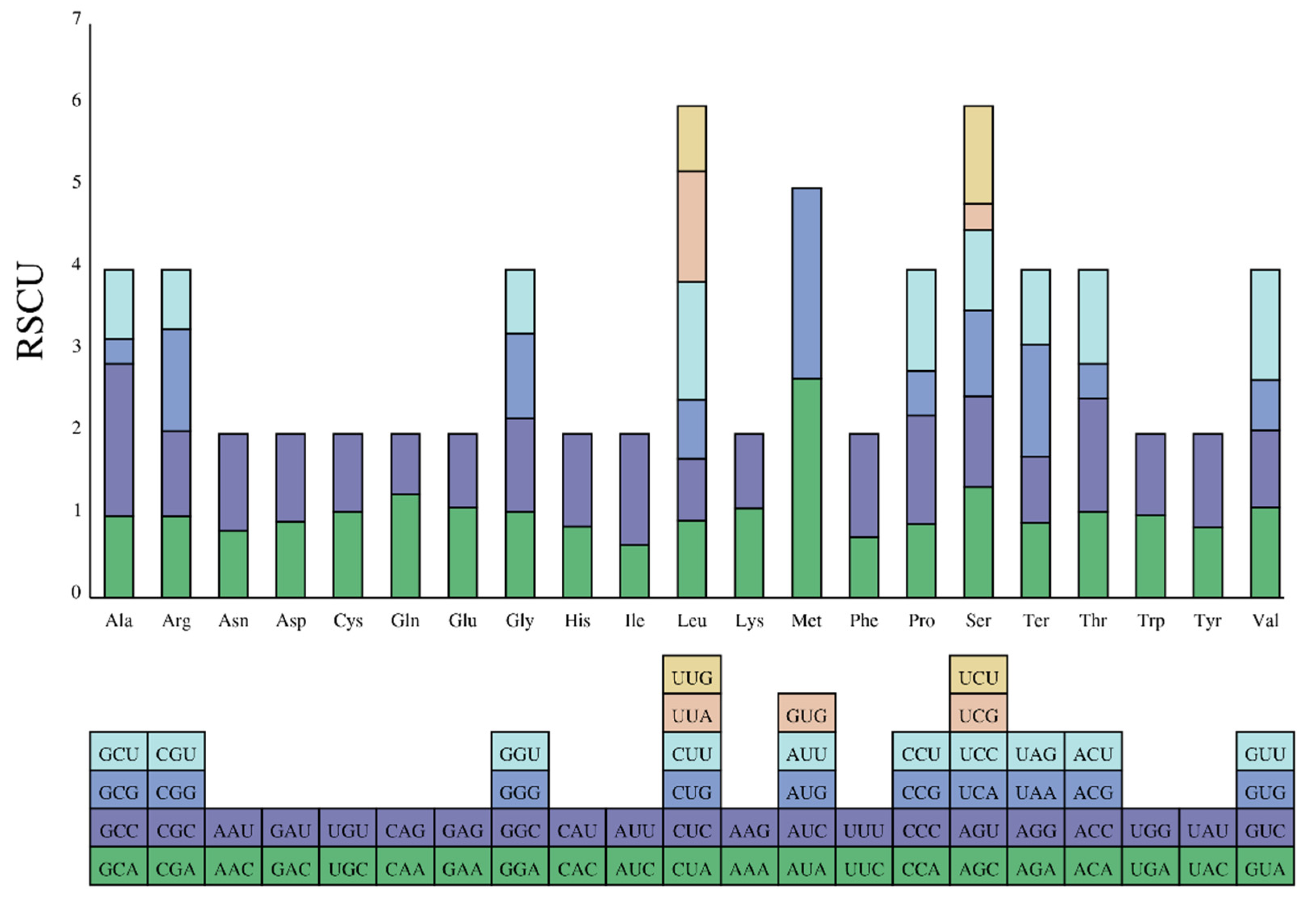
3.2. Description of Protein-Coding Genes (PCGs)
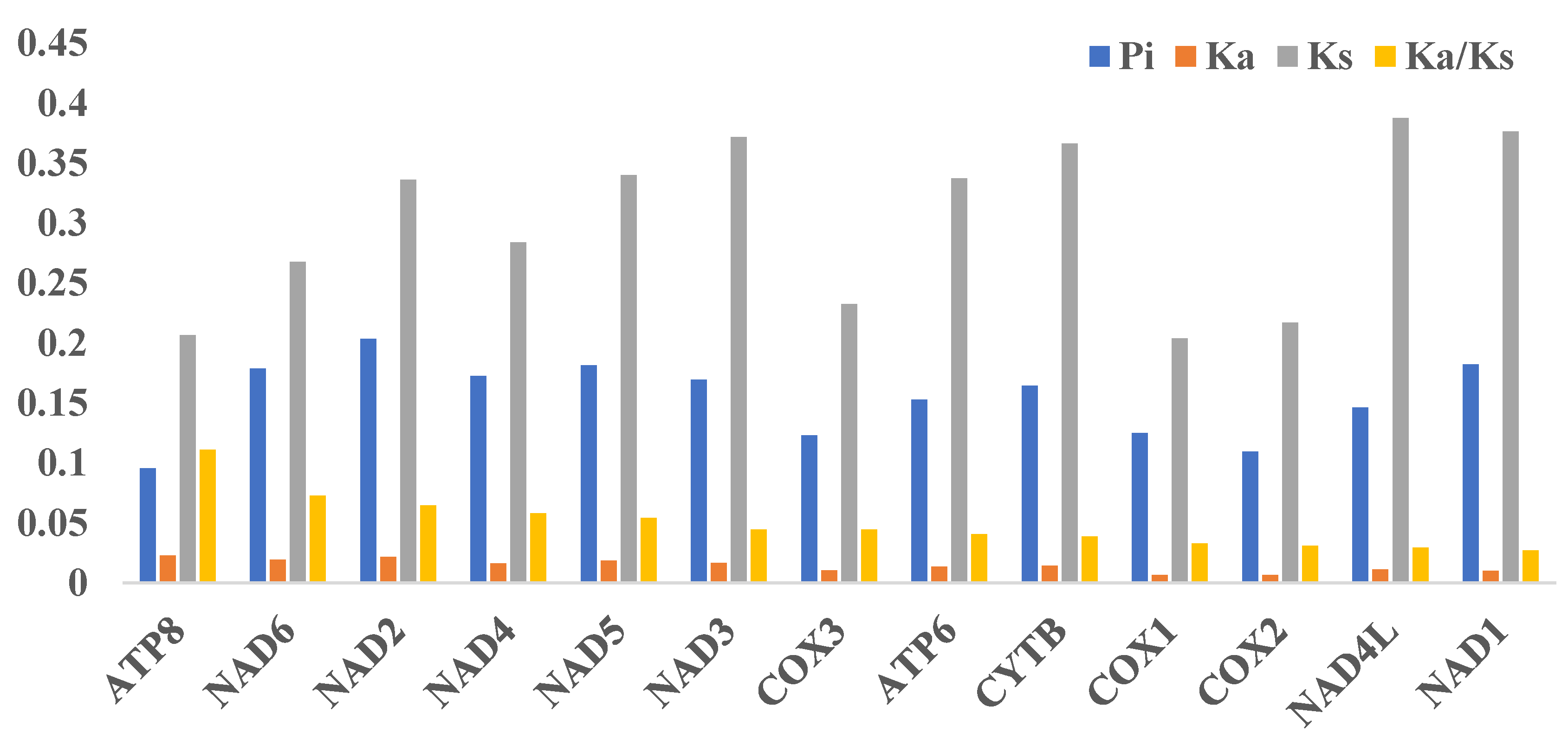
3.3. Sequence Divergence within T. bombifrons Mitogenomes
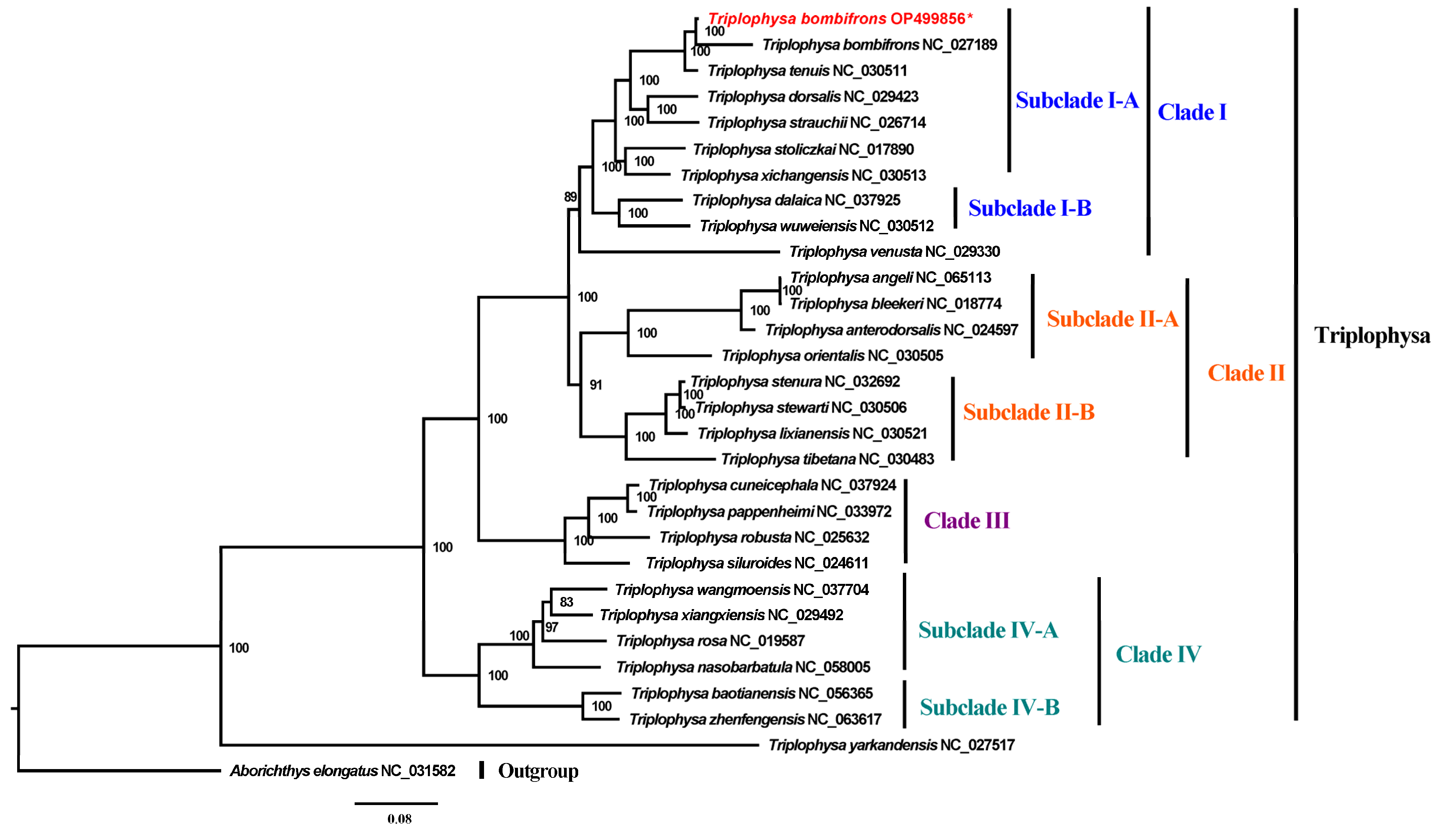
3.4. Phylogenetic Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Galtier, N.; Nabholz, B.; Glémin, S.; Hurst, G. Mitochondrial DNA as a Marker of Molecular Diversity: A Reappraisal. Mol. Ecol. 2009, 18, 4541–4550. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Zhang, Y.-Z.; Sui, Z.-H.; Quan, X.-Q.; Zhang, H.-G.; Liu, L.-X.; Han, Q.-D.; Liu, Y.-G. The Complete Mitochondrial DNA Sequence of Kashgarian Loach (Triplophysa yarkandensis) from Bosten Lake. Mitochondrial DNA Part B 2020, 5, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, N.; Wang, S.; Luo, T.; Yang, X.; Liu, T.; Zhou, J. The Complete Mitochondrial Genome of a Cave-Dwelling Loach Triplophysa baotianensis (Teleostei: Nemacheilidae). Mitochondrial DNA Part B 2021, 6, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-S.; Liu, G.-D.; Prokofiev, A.M. The Complete Mitochondrial Genome of Giant Stone Loach Triplophysa siluroides (Cypriniformes: Balitoridae). Mitochondrial DNA Part A 2016, 27, 998–1000. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Y.; Sui, X.; Chen, Y. The Complete Mitochondrial Genome of Triplophysa cuneicephala (Cypriniformes: Balitoridae) with Phylogenetic Consideration. Mitochondrial DNA Part B 2019, 4, 1239–1240. [Google Scholar] [CrossRef]
- Jing, H.; Yan, P.; Li, W.; Li, X.; Song, Z. The Complete Mitochondrial Genome of Triplophysa lixianensis (Teleostei: Cypriniformes: Balitoridae) with Phylogenetic Consideration. Biochem. Syst. Ecol. 2016, 66, 254–264. [Google Scholar] [CrossRef]
- Liu, T.; You, P. The Complete Mitochondrial Genome of Triplophysa Sp. (Teleostei: Cypriniformes: Balitoridae). Mitochondrial DNA Part A 2016, 27, 4557–4558. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Jin, X.; Wang, P.; Du, Y.; Ma, B. The Complete Mitochondrial Genome of Triplophysa tibetana. Mitochondrial DNA Part B 2019, 4, 1411–1412. [Google Scholar] [CrossRef]
- Que, Y.; Liao, X.; Xu, D.; Yang, Z.; Tang, H.; Zhu, B. The Complete Mitochondrial Genome Sequence of Triplophysa anterodorsalis (Teleostei, Balitoridae, Nemacheilinae). Mitochondrial DNA Part A 2016, 27, 937–938. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, Y.; Wang, J.; Huang, J.; Wang, Z.; Peng, Z. The Complete Mitochondrial Genome Sequence of Triplophysa bleekeri (Teleostei, Balitoridae, Nemacheilinae). Mitochondrial DNA 2013, 24, 25–27. [Google Scholar] [CrossRef]
- Yan, Y.; Luo, D. The Complete Mitochondrial Genome Sequence of Triplophysa stenura (Teleostei, Cypriniformes): Genome Characterization and Phylogenetic Analysis. Mitochondrial DNA Part B 2016, 1, 607–608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Cao, L.; Zhang, E. The Complete Mitochondrial Genome Sequence of Triplophysa xiangxiensis (Teleostei: Nemacheilidae). Mitochondrial DNA Part A 2017, 28, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, Q.; Wang, Z.; Zhang, Y.; Wu, Q.; Peng, Z. The Complete Mitogenome Sequence of a Cave Loach Triplophysa rosa (Teleostei, Balitoridae, Nemacheilinae). Mitochondrial DNA 2012, 23, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Conteh Kanu, U.; Zhao, G.; Xie, P.; Yuan, H.; Li, Y.; Niu, J.; Ma, X. The Complete MtDNA Genome of Triplophysa dorsalis (Cypriniformes, Balitoridae, Cobitoidea): Genome Characterization and Phylogenetic Analysis. Mitochondrial DNA Part A 2016, 27, 3745–3746. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liang, Y.-Q.; Li, M.; Zhang, Y.; Shen, Z.-J.; Jiang, Z.-W. Complete Mitochondrial DNA Genome of Triplophysa venusta (Cypriniformes: Cobitida). Mitochondrial DNA Part A 2016, 27, 4617–4619. [Google Scholar] [CrossRef]
- Ming Han, M.; Lu, J.; Wang, L.; Mahboob, S.; Al-Ghanim, K.A.; Sun, X.-W. Complete Mitochondrial Genome of the Triplophysa bombifrons and Triplophysa strauchii. Mitochondrial DNA Part A 2016, 27, 4710–4711. [Google Scholar] [CrossRef]
- Yang, X.; Wen, H.; Luo, T.; Zhou, J. Complete Mitochondrial Genome of Triplophysa nasobarbatula. Mitochondrial DNA Part B 2020, 5, 3771–3772. [Google Scholar] [CrossRef]
- Yan, P.; Li, J.; Ma, Q.; Deng, Y.; Song, Z. Complete Mitochondrial Genome of Triplophysa robusta (Teleostei: Cypriniformes: Balitoridae). Mitochondrial DNA Part A 2016, 27, 1715–1716. [Google Scholar]
- Sun, X.; Cheng, J. Comparative Mitogenomic Analyses and New Insights into the Phylogeny of Thamnocephalidae (Branchiopoda: Anostraca). Genes 2022, 13, 1765. [Google Scholar] [CrossRef]
- Boore, J.L. Animal Mitochondrial Genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.; Qu, S.; Jia, P.; Wang, B.; Gao, S.; Xu, T.; Zhang, W.; Huang, J.; Ye, K. Haplotype-Resolved Chinese Male Genome Assembly Based on High-Fidelity Sequencing. Fundam. Res. 2022, 2, 946–953. [Google Scholar] [CrossRef]
- Li, X.; Ellis, E.; Plotkin, D.; Imada, Y.; Yago, M.; Heckenhauer, J.; Cleland, T.P.; Dikow, R.B.; Dikow, T.; Storer, C.G.; et al. First Annotated Genome of a Mandibulate Moth, Neomicropteryx Cornuta, Generated Using PacBio HiFi Sequencing. Genome Biol. Evol. 2021, 13, evab229. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Ahn, S.J.; Seo, J.S.; Jeon, E.J.; Cho, M.Y.; Choi, H.S. Characterization of the Complete Mitochondrial Genome of Miamiensis Avidus Causing Flatfish Scuticociliatosis. Genetica 2022, 150, 407–420. [Google Scholar] [CrossRef] [PubMed]
- FastQC. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics: Cambridge, UK, 2018. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Allio, R.; Schomaker-Bastos, A.; Romiguier, J.; Prosdocimi, F.; Nabholz, B.; Delsuc, F. MitoFinder: Efficient Automated Large-Scale Extraction of Mitogenomic Data in Target Enrichment Phylogenomics. Mol. Ecol. Resour. 2020, 20, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Yang, C.; Liu, S. MitoZ: A Toolkit for Animal Mitochondrial Genome Assembly, Annotation and Visualization. Nucleic Acids Res. 2019, 47, e63. [Google Scholar] [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) Version 1.3.1: Expanded Toolkit for the Graphical Visualization of Organellar Genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef]
- Gogoi, B.; Bhau, B.S. DNA Barcoding of the Genus Nepenthes (Pitcher Plant): A Preliminary Assessment towards Its Identification. BMC Plant Biol. 2018, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Ma, X.; Boyko, A.R.; Bustamante, C.D.; Oleksiak, M.F. SNP Identification, Verification, and Utility for Population Genetics in a Non-Model Genus. Bmc Genet. 2010, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Carraretto, D.; Aketarawong, N.; Di Cosimo, A.; Manni, M.; Scolari, F.; Valerio, F.; Malacrida, A.R.; Gomulski, L.M.; Gasperi, G. Transcribed Sex-Specific Markers on the Y Chromosome of the Oriental Fruit Fly, Bactrocera Dorsalis. BMC Genet. 2020, 21, 125. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, S.; Wang, Q.; Li, Z.; Li, Z.; He, A.; Chen, J.; Liu, L.; Zou, K. Novel Evolutionary Insights into Nemacheilid Cavefish: Evidence from Comparative Analysis of Mitochondrial Genomes. J. Oceanol. Limnol. 2022, 40, 1640–1653. [Google Scholar] [CrossRef]
- Iriarte, A.; Lamolle, G.; Musto, H. Codon Usage Bias: An Endless Tale. J. Mol. Evol. 2021, 89, 589–593. [Google Scholar] [CrossRef]
- Hurst, L.D. The Ka/Ks Ratio: Diagnosing the Form of Sequence Evolution. Trends Genet. TIG 2002, 18, 486. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) Database at NCBI: Current Status, Taxonomic Expansion, and Functional Annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]

| Genus | Species | Size (bp) | Accession No | Resource |
|---|---|---|---|---|
| Triplophysa | T. bombifrons | 16,568 | OP499856 | this study |
| T. bombifrons | 16,569 | NC_027189 | [17] | |
| T. tenuis | 16,571 | NC_030511 | ||
| T. dorsalis | 16,572 | NC_029423 | [15] | |
| T. strauchii | 16,590 | NC_026714 | [17] | |
| T. stoliczkai | 16,571 | NC_017890 | ||
| T. xichangensis | 16,570 | NC_030513 | ||
| T. dalaica | 16,569 | NC_037925 | ||
| T. wuweiensis | 16,681 | NC_030512 | ||
| T. venusta | 16,574 | NC_029330 | [16] | |
| T. angeli | 16,569 | NC_065113 | ||
| T. bleekeri | 16,568 | NC_018774 | [11] | |
| T. anterodorsalis | 16,567 | NC_024597 | [10] | |
| T. orientalis | 16,562 | NC_030505 | ||
| T. stenura | 16,569 | NC_032692 | [12] | |
| T. stewarti | 16,567 | NC_030506 | ||
| T. lixianensis | 16,570 | NC_030521 | [7] | |
| T. tibetana | 16,574 | NC_030483 | [9] | |
| T. pappenheimi | 16,571 | NC_037924 | [6] | |
| T. pappenheimi | 16,572 | NC_033972 | ||
| T. robusta | 16,570 | NC_025632 | [19] | |
| T. siluroides | 16,574 | NC_024611 | [5] | |
| T. wangmoensis | 16,569 | NC_037704 | [8] | |
| T. xiangxiensis | 16,598 | NC_029492 | [13] | |
| T. rosa | 16,585 | NC_019587 | [14] | |
| T. nasobarbatula | 16,605 | NC_058005 | [18] | |
| T. baotianensis | 16,576 | NC_056365 | [4] | |
| T. zhenfengensis | 16,564 | NC_063617 | [38] | |
| T. yarkandensis | 16,574 | NC_027517 | [3] | |
| Aborichthys | A. elongatus | 16,544 | NC_031582 |
| Locus | Start | Stop | Size (bp) | Start Coding | Stop Coding | Strand |
|---|---|---|---|---|---|---|
| tRNAPhe | 1 | 69 | 69 | H | ||
| 12S rRNA | 70 | 1017 | 948 | H | ||
| tRNAVal | 1020 | 1091 | 72 | H | ||
| 16S rRNA | 1092 | 2768 | 1677 | H | ||
| tRNALeu | 2769 | 2843 | 75 | H | ||
| nad1 | 2844 | 3818 | 975 | ATG | TAG | H |
| tRNAIle | 3826 | 3896 | 71 | H | ||
| tRNAGln | 3895 | 3965 | 71 | L | ||
| tRNAMet | 3967 | 4035 | 69 | H | ||
| nad2 | 4036 | 5082 | 1047 | ATG | TAG | H |
| tRNATrp | 5081 | 5150 | 70 | H | ||
| tRNAAla | 5153 | 5221 | 69 | L | ||
| tRNAAsn | 5223 | 5295 | 73 | L | ||
| tRNACys | 5327 | 5392 | 66 | L | ||
| tRNATyr | 5393 | 5460 | 68 | L | ||
| cox1 | 5462 | 7012 | 1551 | GTG | TAA | H |
| tRNASer | 7013 | 7083 | 71 | L | ||
| tRNAAsp | 7086 | 7158 | 73 | H | ||
| cox2 | 7172 | 7876 | 705 | ATG | TAA | H |
| tRNALys | 7863 | 7938 | 76 | H | ||
| atp8 | 7940 | 8107 | 168 | ATG | TAA | H |
| atp6 | 8098 | 8781 | 684 | ATG | TAA | H |
| cox3 | 8781 | 9581 | 801 | ATG | TAA | H |
| tRNAGly | 9565 | 9637 | 73 | H | ||
| nad3 | 9638 | 9988 | 351 | ATG | TAG | H |
| tRNAArg | 9987 | 10,056 | 70 | H | ||
| nad4l | 10,057 | 10,353 | 297 | ATG | TAA | H |
| nad4 | 10,347 | 11,729 | 1383 | ATG | TAG | H |
| tRNAHis | 11,729 | 11,798 | 70 | H | ||
| tRNASer | 11,799 | 11,866 | 68 | H | ||
| tRNALeu | 11,868 | 11,940 | 73 | H | ||
| nad5 | 11,941 | 13,779 | 1839 | ATG | TAA | H |
| nad6 | 13,776 | 14,297 | 522 | ATG | TAG | L |
| tRNAGlu | 14,298 | 14,366 | 69 | L | ||
| cob | 14,372 | 15,532 | 1161 | ATG | TAA | H |
| tRNAThr | 15,513 | 15,583 | 71 | H | ||
| tRNAPro | 15,582 | 15,651 | 70 | L |
| AminoAcid | Symbol | Codon | No. | Percent | RSCU |
|---|---|---|---|---|---|
| * | Ter | UAA | 132 | 2.39% | 1.3573 |
| * | Ter | AGA | 89 | 1.61% | 0.9152 |
| * | Ter | UAG | 89 | 1.61% | 0.9152 |
| * | Ter | AGG | 79 | 1.43% | 0.8123 |
| A | Ala | GCC | 139 | 2.52% | 1.8533 |
| A | Ala | GCA | 75 | 1.36% | 1 |
| A | Ala | GCU | 63 | 1.14% | 0.84 |
| A | Ala | GCG | 23 | 0.42% | 0.3067 |
| C | Cys | UGC | 70 | 1.27% | 1.0526 |
| C | Cys | UGU | 63 | 1.14% | 0.9474 |
| D | Asp | GAU | 61 | 1.10% | 1.0702 |
| D | Asp | GAC | 53 | 0.96% | 0.9298 |
| E | Glu | GAA | 60 | 1.09% | 1.1009 |
| E | Glu | GAG | 49 | 0.89% | 0.8991 |
| F | Phe | UUU | 146 | 2.64% | 1.2586 |
| F | Phe | UUC | 86 | 1.56% | 0.7414 |
| G | Gly | GGG | 64 | 1.16% | 1.0364 |
| G | Gly | GGA | 65 | 1.18% | 1.0526 |
| G | Gly | GGC | 70 | 1.27% | 1.1336 |
| G | Gly | GGU | 48 | 0.87% | 0.7773 |
| H | His | CAU | 108 | 1.96% | 1.1309 |
| H | His | CAC | 83 | 1.50% | 0.8691 |
| I | Ile | AUU | 173 | 3.13% | 1.3569 |
| I | Ile | AUC | 82 | 1.48% | 0.6431 |
| K | Lys | AAA | 110 | 1.99% | 1.0945 |
| K | Lys | AAG | 91 | 1.65% | 0.9055 |
| L | Leu | UUA | 149 | 2.70% | 1.3484 |
| L | Leu | CUU | 159 | 2.88% | 1.4389 |
| L | Leu | CUA | 104 | 1.88% | 0.9412 |
| L | Leu | UUG | 88 | 1.59% | 0.7964 |
| L | Leu | CUC | 84 | 1.52% | 0.7602 |
| L | Leu | CUG | 79 | 1.43% | 0.7149 |
| M | Met | AUG | 85 | 1.54% | 2.3224 |
| M | Met | AUA | 98 | 1.77% | 2.6776 |
| M | Met | AUC | 0 | 0.00% | 0 |
| M | Met | AUU | 0 | 0.00% | 0 |
| M | Met | GUG | 0 | 0.00% | 0 |
| N | Asn | AAU | 137 | 2.48% | 1.1861 |
| N | Asn | AAC | 94 | 1.70% | 0.8139 |
| P | Pro | CCU | 138 | 2.50% | 1.2267 |
| P | Pro | CCC | 149 | 2.70% | 1.3244 |
| P | Pro | CCA | 101 | 1.83% | 0.8978 |
| P | Pro | CCG | 62 | 1.12% | 0.5511 |
| Q | Gln | CAA | 108 | 1.96% | 1.2632 |
| Q | Gln | CAG | 63 | 1.14% | 0.7368 |
| R | Arg | CGC | 46 | 0.83% | 1.0395 |
| R | Arg | CGG | 55 | 1.00% | 1.2429 |
| R | Arg | CGA | 44 | 0.80% | 0.9944 |
| R | Arg | CGU | 32 | 0.58% | 0.7232 |
| S | Ser | UCA | 88 | 1.59% | 1.0539 |
| S | Ser | AGU | 92 | 1.67% | 1.1018 |
| S | Ser | UCU | 100 | 1.81% | 1.1976 |
| S | Ser | AGC | 113 | 2.05% | 1.3533 |
| S | Ser | UCC | 82 | 1.48% | 0.982 |
| S | Ser | UCG | 26 | 0.47% | 0.3114 |
| T | Thr | ACA | 103 | 1.87% | 1.0537 |
| T | Thr | ACU | 112 | 2.03% | 1.1458 |
| T | Thr | ACC | 135 | 2.44% | 1.3811 |
| T | Thr | ACG | 41 | 0.74% | 0.4194 |
| V | Val | GUA | 60 | 1.09% | 1.106 |
| V | Val | GUU | 73 | 1.32% | 1.3456 |
| V | Val | GUC | 51 | 0.92% | 0.9401 |
| V | Val | GUG | 33 | 0.60% | 0.6083 |
| W | Trp | UGA | 79 | 1.43% | 1.0064 |
| W | Trp | UGG | 78 | 1.41% | 0.9936 |
| Y | Tyr | UAU | 120 | 2.17% | 1.1429 |
| Y | Tyr | UAC | 90 | 1.63% | 0.8571 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Song, Y.; Xie, H.; Zi, F.; Chen, S.; Luo, S. Complete Mitogenome of the Triplophysa bombifrons: Comparative Analysis and Phylogenetic Relationships among the Members of Triplophysa. Genes 2023, 14, 128. https://doi.org/10.3390/genes14010128
Wang X, Song Y, Xie H, Zi F, Chen S, Luo S. Complete Mitogenome of the Triplophysa bombifrons: Comparative Analysis and Phylogenetic Relationships among the Members of Triplophysa. Genes. 2023; 14(1):128. https://doi.org/10.3390/genes14010128
Chicago/Turabian StyleWang, Xinyue, Yong Song, Haoyang Xie, Fangze Zi, Shengao Chen, and Site Luo. 2023. "Complete Mitogenome of the Triplophysa bombifrons: Comparative Analysis and Phylogenetic Relationships among the Members of Triplophysa" Genes 14, no. 1: 128. https://doi.org/10.3390/genes14010128
APA StyleWang, X., Song, Y., Xie, H., Zi, F., Chen, S., & Luo, S. (2023). Complete Mitogenome of the Triplophysa bombifrons: Comparative Analysis and Phylogenetic Relationships among the Members of Triplophysa. Genes, 14(1), 128. https://doi.org/10.3390/genes14010128






