Genetic and Phenotypic Factors Affecting Glycemic Response to Metformin Therapy in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohorts and Participants
2.2. DNA Isolation and Genotyping
2.3. Statistical Analysis
2.3.1. Association of Independent Variables with Response to Metformin Therapy
2.3.2. Prediction of Response to Metformin Therapy Using Machine Learning
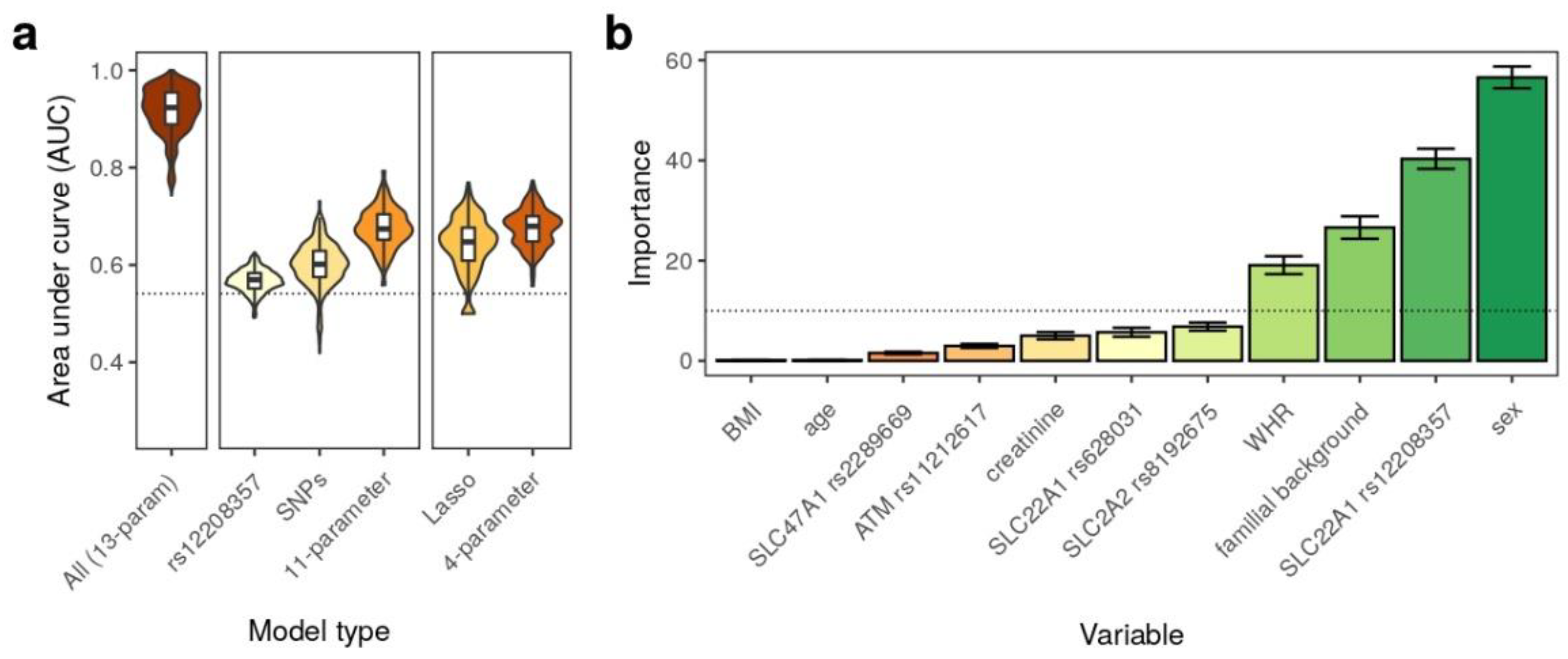
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rich, S.S. Mapping genes in diabetes. Genetic epidemiological perspective. Diabetes 1990, 39, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef]
- Wang, Y.; An, H.; Liu, T.; Qin, C.; Sesaki, H.; Guo, S.; Radovick, S.; Hussain, M.; Maheshwari, A.; Wondisford, F.E.; et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019, 29, 1511–1523. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- He, L. Metformin and Systemic Metabolism. Trends Pharm. Sci. 2020, 41, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Jonker, J.W.; Kato, Y.; Kusuhara, H.; Schinkel, A.H.; Sugiyama, Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002, 302, 510–515. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Pharmacologic therapy for type 2 diabetes mellitus. Ann. Intern. Med. 1999, 131, 281–303. [Google Scholar] [CrossRef]
- Florez, J.C. Does metformin work for everyone? A genome-wide association study for metformin response. Curr. Diab. Rep. 2011, 11, 467–469. [Google Scholar] [CrossRef]
- Haupt, E.; Knick, B.; Koschinsky, T.; Liebermeister, H.; Schneider, J.; Hirche, H. Oral antidiabetic combination therapy with sulfonylureas and metformin. Diabete. Metab. 1991, 17, 224–231. [Google Scholar]
- GoDARTS and UKPDS Diabetes Pharmacogenetics Study Group; Wellcome Trust Case Control Consortium 2; Zhou, K.; Bellenguez, C.; Spencer, C.C.; Bennett, A.J.; Coleman, R.L.; Tavendale, R.; Hawley, S.A.; Donnelly, L.A.; et al. Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat. Genet. 2011, 43, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef]
- Liang, X.; Giacomini, K.M. Transporters involved in metformin pharmacokinetics and treatment response. J. Pharm. Sci. 2017, 106, 2245–2250. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsumoto, T.; Morimoto, R.; Arioka, S.; Omote, H.; Moriyama, Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. 2005, 102, 17923–17928. [Google Scholar] [CrossRef] [PubMed]
- Mato, E.P.M.; Guewo-Fokeng, M.; Essop, M.F.; Owira, P.M.O. Genetic polymorphisms of organic cation transporter 1 (OCT1) and responses to metformin therapy in individuals with type 2 diabetes. Medicine 2018, 97, e11349. [Google Scholar] [CrossRef]
- Dedov, I.I.; Shestakova, M.V.; Vikulova, O.K.; Zheleznyakova, A.V.; Isakov, M.A. Diabetes mellitus in Russian Federation: Prevalence, morbidity, mortality, parameters of glycaemic control and structure of glucose lowering therapy according to the Federal Diabetes Register, status 2017. Diabetes Mellit. 2018, 21, 144–159. [Google Scholar] [CrossRef]
- Bondar, I.A.; Shabelnikova, O.Y.; Sokolova, E.A.; Pyankova, O.V.; Filipenko, M.L. Phenotypic and genetic characteristics of patients with type 2 diabetes with different responses to metformin therapy in Novosibirsk region. Diabetes Mellit. 2016, 19, 125–131. [Google Scholar] [CrossRef]
- Müllenbach, R.; Lagoda, P.J.; Welter, C. An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet. 1989, 5, 391. [Google Scholar]
- Van Leeuwen, N.; Nijpels, G.; Becker, M.L.; Deshmukh, H.; Zhou, K.; Stricker, B.H.C.; Uitterlinden, A.G.; Hofman, A.; Van ’T Riet, E.; Palmer, C.N.A.; et al. A gene variant near ATM is significantly associated with metformin treatment response In type 2 diabetes: A replication and meta-analysis of five cohorts. Diabetologia 2012, 55, 1971–1977. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, Y.; Ye, W.; Wang, Y.; Li, X.; Tian, Y.; Liu, Z.; Li, S.; Yan, J. RS11212617 is associated with metformin treatment response in type 2 diabetes in Shanghai local Chinese population. Int. J. Clin. Pract. 2014, 68, 1462–1466. [Google Scholar] [CrossRef]
- Altall, R.M.; Qusti, S.Y.; Filimban, N.; Alhozali, A.M.; Alotaibi, N.A.; Dallol, A.; Chaudhary, A.G.; Bakhashab, S. SLC22A1 and ATM genes polymorphisms are associated with the risk of type 2 diabetes mellitus in Western Saudi Arabia: A case-control study. Appl. Clin. Genet. 2019, 12, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ye, W.; Wang, Y.; Jiang, Z.; Meng, X.; Xiao, Q.; Zhao, Q.; Yan, J. Genetic variants of OCT1 influence glycemic response to metformin in Han Chinese patients with type-2 diabetes mellitus in Shanghai. Int. J. Clin. Exp. Pathol. 2015, 8, 9533–9542. [Google Scholar] [PubMed]
- Tarasova, L.; Kalnina, I.; Geldnere, K.; Bumbure, A.; Ritenberga, R.; Nikitina-Zake, L.; Fridmanis, D.; Vaivade, I.; Pirags, V.; Klovins, J. Association of genetic variation in the organic cation transporters OCT1, OCT2 and multidrug and toxin extrusion 1 transporter protein genes with the gastrointestinal side effects and lower BMI in metformin-treated type 2 diabetes patients. Pharm. Genom. 2012, 22, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.A.; McAteer, J.B.; De Bakker, P.I.W.; Franks, P.W.; Pollin, T.I.; Hanson, R.L.; Saxena, R.; Fowler, S.; Shuldiner, A.R.; Knowler, W.C.; et al. Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. Diabetes 2010, 59, 2672–2681. [Google Scholar] [CrossRef]
- Xiao, D.; Guo, Y.; Li, X.; Yin, J.Y.; Zheng, W.; Qiu, X.W.; Xiao, L.; Liu, R.R.; Wang, S.Y.; Gong, W.J.; et al. The Impacts of SLC22A1 rs594709 and SLC47A1 rs2289669 Polymorphisms on Metformin Therapeutic Efficacy in Chinese Type 2 Diabetes Patients. Int. J. Endocrinol. 2016, 2016, 4350712. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yee, S.W.; Seiser, E.L.; Van Leeuwen, N.; Tavendale, R.; Bennett, A.J.; Groves, C.J.; Coleman, R.L.; Van Der Heijden, A.A.; Beulens, J.W.; et al. Variation in the glucose transporter gene SLC2A2 is associated with glycemic response to metformin. Nat. Genet. 2016, 48, 1055–1059. [Google Scholar] [CrossRef]
- Rathmann, W.; Strassburger, K.; Bongaerts, B.; Kuss, O.; Müssig, K.; Burkart, V.; Szendroedi, J.; Kotzka, J.; Knebel, B.; Al-Hasani, H.; et al. A variant of the glucose transporter gene SLC2A2 modifies the glycaemic response to metformin therapy in recently diagnosed type 2 diabetes. Diabetologia 2019, 62, 286–291. [Google Scholar] [CrossRef]
- IGSR: The International Genome Sample Resource. Available online: https://www.internationalgenome.org (accessed on 27 June 2022).
- Genome Aggregation Database. Available online: https://gnomad.broadinstitute.org (accessed on 27 June 2022).
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 5, 1–26. [Google Scholar]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Singh, S.; Usman, K.; Banerjee, M. Pharmacogenetic studies update in type 2 diabetes mellitus. World J. Diabetes 2016, 7, 302–315. [Google Scholar] [CrossRef]
- Becker, M.L.; Visser, L.E.; van Schaik, R.H.N.; Hofman, A.; Uitterlinden, A.G.; Stricker, B.H.C. Genetic variation in the organic cation transporter 1 is associated with metformin response in patients with diabetes mellitus. Pharm. J. 2009, 9, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Nasykhova, Y.A.; Tonyan, Z.N.; Mikhailova, A.A.; Danilova, M.M.; Glotov, A.S. Pharmacogenetics of type 2 diabetes-progress and prospects. Int. J. Mol. Sci. 2020, 21, 6842. [Google Scholar] [CrossRef] [PubMed]
- Florez, J.C.; Barrett-Connor, E.; Jablonski, K.A.; Knowler, W.C.; Taylor, A.; Shuldiner, A.R.; Mather, K.; Pollin, T.I.; Horton, E.; White, N.H. The C allele of ATM rs11212617 does not associate with metformin response in the diabetes prevention program. Diabetes Care 2012, 35, 1864–1867. [Google Scholar] [CrossRef] [PubMed]
- Vilvanathan, S.; Gurusamy, U.; Mukta, V.; Das, A.K.; Chandrasekaran, A. Allele and genotype frequency of a genetic variant in ataxia telangiectasia mutated gene affecting glycemic response to metformin in South Indian population. Indian J. Endocrinol. Metab. 2014, 18, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Rotroff, D.M.; Yee, S.W.; Zhou, K.; Marvel, S.W.; Shah, H.S.; Jack, J.R.; Havener, T.M.; Hedderson, M.M.; Kubo, M.; Herman, M.A.; et al. Genetic variants in CPA6 and PRPF31 are associated with variation in response to metformin in individuals with type 2 diabetes. Diabetes 2018, 67, 1428–1440. [Google Scholar] [CrossRef]
- Shokri, F.; Ghaedi, H.; Ghafouri Fard, S.; Movafagh, A.; Abediankenari, S.; Mahrooz, A.; Kashi, Z.; Omrani, M.D. Impact of ATM and SLC22A1 polymorphisms on therapeutic response to metformin in Iranian diabetic patients. Int. J. Mol. Cell Med. 2016, 5, 1–7. [Google Scholar]
- Christensen, M.M.; Pedersen, R.S.; Stage, T.B.; Brasch-Andersen, C.; Nielsen, F.; Damkier, P.; Beck-Nielsen, H.; Brøsen, K. A gene-gene interaction between polymorphisms in the OCT2 and MATE1 genes influences the renal clearance of metformin. Pharm. Genom. 2013, 23, 526–534. [Google Scholar] [CrossRef]
- Christensen, M.M.H.; Højlund, K.; Hother-Nielsen, O.; Stage, T.B.; Damkier, P.; Beck-Nielsen, H.; Brøsen, K. Steady-state pharmacokinetics of metformin is independent of the OCT1 genotype in healthy volunteers. Eur. J. Clin. Pharm. 2015, 71, 691–697. [Google Scholar] [CrossRef]
- Dujic, T.; Zhou, K.; Yee, S.W.; van Leeuwen, N.; de Keyser, C.E.; Javorský, M.; Goswami, S.; Zaharenko, L.; Hougaard Christensen, M.M.; Out, M.; et al. Variants in pharmacokinetic transporters and glycemic response to metformin: A metgen meta-analysis. Clin. Pharmacol. Ther. 2017, 101, 763–772. [Google Scholar] [CrossRef]
- Raj, G.M.; Mathaiyan, J.; Wyawahare, M.; Priyadarshini, R. Lack of effect of the SLC47A1 and SLC47A2 gene polymorphisms on the glycemic response to metformin in type 2 diabetes mellitus patients. Drug Metab. Pers. Ther. 2018, 33, 175–185. [Google Scholar] [CrossRef]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef] [PubMed]
- Zazuli, Z.; Duin, N.J.C.B.; Jansen, K.; Vijverberg, S.J.H.; Maitland-van der Zee, A.H.; Masereeuw, R. The Impact of Genetic Polymorphisms in Organic Cation Transporters on Renal Drug Disposition. Int. J. Mol. Sci. 2020, 21, 6627. [Google Scholar] [CrossRef] [PubMed]
- Pryor, R.; Cabreiro, F. Repurposing metformin: An old drug with new tricks in its binding pockets. Biochem. J. 2015, 471, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Sundelin, E.; Gormsen, L.C.; Jensen, J.B.; Vendelbo, M.H.; Jakobsen, S.; Munk, O.L.; Christensen, M.; Brøsen, K.; Frøkiaer, J.; Jessen, N. Genetic Polymorphisms in Organic Cation Transporter 1 Attenuates Hepatic Metformin Exposure in Humans. Clin. Pharmacol. Ther. 2017, 102, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Shikata, E.; Yamamoto, R.; Takane, H.; Shigemasa, C.; Ikeda, T.; Otsubo, K.; Ieiri, I. Human organic cation transporter (OCT1 and OCT2) gene polymorphisms and therapeutic effects of metformin. J. Hum. Genet. 2007, 52, 117–122. [Google Scholar] [CrossRef]
- Goswami, S.; Gong, L.; Giacomini, K.; Altman, R.B.; Klein, T.E. PharmGKB summary: Very important pharmacogene information for SLC22A1. Pharm. Genom. 2014, 24, 324–328. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Li, J.; Shan, Z.; Yang, W.; Liu, J.; Tian, H.; Zhou, Z.; Ji, Q.; Weng, J.; Jia, W.; et al. Gender-differential effects on blood glucose levels between acarbose and metformin in Chinese patients with newly diagnosed type 2 diabetes: A sub-analysis of the MARCH trial. Endocr. J. 2021, 68, 69–79. [Google Scholar] [CrossRef]
- Schütt, M.; Zimmermann, A.; Hood, R.; Hummel, M.; Seufert, J.; Siegel, E.; Tytko, A.; Holl, R.W.; DPV Initiative; German BMBF Competence Network Diabetes Mellitus. Gender-specific Effects of Treatment with Lifestyle, Metformin or Sulfonylurea on Glycemic Control and Body Weight: A German Multicenter Analysis on 9 108 Patients. Exp. Clin. Endocrinol. Diabetes. 2015, 123, 622–626. [Google Scholar] [CrossRef]
- Wu, K.; Li, X.; Xu, Y.; Zhang, X.; Guan, Z.; Zhang, S.; Li, Y. SLC22A1 rs622342 Polymorphism Predicts Insulin Resistance Improvement in Patients with Type 2 Diabetes Mellitus Treated with Metformin: A Cross-Sectional Study. Int. J. Endocrinol. 2020, 8, 2975898. [Google Scholar] [CrossRef]
| Characteristics | All T2DM Patients (n = 464) | T2DM Patients Taking Metformin (n = 299) | Controls (n = 129) |
|---|---|---|---|
| Male (n) | 155 | 93 | 91 |
| Female (n) | 309 | 206 | 38 |
| Age (years) | 61.11 ± 13.65 | 60.92 ± 13.00 | 40 ± 14.28 |
| BMI (kg/m2) | 31.96 ± 8.2 | 32.58 ± 6.51 | 24.43 ± 2.79 |
| FBG (mmol/L) | 7.88 ± 2.4 | 7.87 ± 2.36 | 4.66 ± 0.37 |
| Family history of diabetes (n) | 158 | 108 | 0 |
| Creatinine (mmol/L) | 0.09 ± 0.03 | 0.09 ± 0.02 | NA |
| WHR | 0.98 ± 0.097 | 0.95 ± 0.090 | NA |
| HbA1c (%) | 7.53 ± 1.14 | 7.44 ± 1.17 | NA |
| # | Gene Symbol | Region | dbSNP ID | Nucleotide Change | Amino Acid Change | Function | References |
|---|---|---|---|---|---|---|---|
| 1 | ATM | 11q22.3 | rs11212617 | intron C/A | - | ↑ | [11,19,20,21] |
| 2 | SLC22A1 | 6q25.3 | rs628031 | c.1222A > G | Met408Val | ↑ SE | [22,23] |
| 3 | SLC22A1 | 6q25.3 | rs12208357 | c.181C > T | Arg61Cys | ↓ | [12] |
| 4 | SLC47A1 | 17p11.2 | rs2289669 | intron G/A | - | ↑↓ | [24,25] |
| 5 | SLC2A2 | 3q26.2 | rs8192675 | intron A/G | - | ↑ | [26,27] |
| Genotype/ Allele | Patients with Glycemic Response | Non-Responder Patients | p-Value | ||
|---|---|---|---|---|---|
| (Monotherapy/Combination Therapy) n (%) | (Monotherapy) n (%) | (Monotherapy/Combination Therapy) n (%) | (Monotherapy) n (%) | ||
| rs11212617 ATM | |||||
| AA | 66 (26) | 37 (31) | 11 (25) | 5 (42) | p1 = 1.0000 p2 = 0.5622 p3 = 0.3126 p4 = 0.5208 |
| AC | 133 (52) | 61 (51) | 25 (57) | 4 (33) | p1 = 0.6255 p2 = 0.5975 p3 = 0.2455 p4 = 0.3646 |
| CC | 56 (22) | 21 (18) | 8 (18) | 3 (25) | p1 = 0.6923 p2 = 1.0000 p3 = 0.7310 p4 = 0.4599 |
| A | 265 (52) | 135 (57) | 47 (53) | 14 (58) | p1 = 0.8182 p2 = 0.6168 p3 = 0.6768 p4 = 1.0000 |
| C | 245 (48) | 103 (43) | 41 (47) | 10 (42) | |
| rs628031 SLC22A1 | |||||
| AA | 28 (11) | 13 (11) | 8 (18) | 0 (0) | p1 = 0.2080 p2 = 0.2908 p3 = 0.6219 p4 = 0.6079 |
| AG | 128 (50) | 64 (54) | 22 (50) | 7 (58) | p1 = 0.1000 p2 = 07253 p3 = 0.7695 p4 = 1.0000 |
| GG | 98 (39) | 42 (35) | 14 (32) | 5 (42) | p1 = 0.5004 p2 = 0.7143 p3 = 1.0000 p4 = 0.7549 |
| A | 184 (36) | 90 (38) | 38 (43) | 7 (29) | p1 = 0.2329 p2 = 0.4435 p3 = 0.5236 p4 = 0.5081 |
| G | 324 (64) | 148 (62) | 50 (57) | 17 (71) | |
| rs12208357 SLC22A1 | |||||
| CC | 219 (87) | 100 (84) | 32 (73) | 11 (92) | p1 = 0.0250 p2 = 0.1179 p3 = 1.0000 p4 = 0.6913 |
| CT | 32 (13) | 18 (15) | 9 (20) | 1 (8) | p1 = 0.1627 p2 = 0.4776 p3 = 1.0000 p4 = 1.0000 |
| TT | 2 (1) | 1 (1) | 3 (7) | 0 (0) | p1 = 0.0246 p2 = 0.0604 p3 = 1.0000 p4 = 1.0000 |
| C | 470 (93) | 218 (92) | 73 (83) | 23 (96) | p1 = 0.0059 p2 = 0.0418 p3 = 1.0000 p4 = 0.7036 |
| T | 36 (7) | 20 (8) | 15 (17) | 1 (4) | |
| rs2289669 SLC47A1 | |||||
| AA | 37 (14.5) | 20 (17) | 11 (25) | 4 (33) | p1 = 0.1163 p2 = 0.2638 p3 = 0.0940 p4 = 0.2312 |
| AG | 88 (34.5) | 36 (30) | 10 (23) | 1 (8) | p1 = 0.1637 p2 = 0.4340 p3 = 0.0667 p4 = 0.1773 |
| GG | 130 (51) | 63 (53) | 23 (52) | 7 (59) | p1 = 1.0000 p2 = 0.8623 p3 = 0.7702 p4 = 0.7705 |
| A | 162 (32) | 76 (32) | 32 (36) | 9 (37.5) | p1 = 0.3911 p2 = 0.5078 p3 = 0.6547 p4 = 0.6485 |
| G | 348 (68) | 162 (68) | 56 (64) | 15 (62.5) | |
| rs8192675 SLC2A2 | |||||
| AA | 147 (58) | 71 (60) | 20 (45) | 5 (42) | p1 = 0.1404 p2 = 0.1134 p3 = 0.3720 p4 = 0.3578 |
| AG | 90 (35) | 39 (33) | 21 (48) | 7 (58) | p1 = 0.1306 p2 = 0.0998 p3 = 0.1293 p4 = 0.1110 |
| GG | 17 (7) | 9 (7) | 3 (7) | 0 (0) | p1 = 1.0000 p2 = 1.0000 p3 = 1.0000 p4 = 1.0000 |
| A | 384 (76) | 181 (76) | 61 (69) | 17 (71) | p1 = 0.2323 p2 = 0.2538 p3 = 0.6287 p4 = 0.6190 |
| G | 124 (24) | 57 (24) | 27 (31) | 7 (29) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasykhova, Y.A.; Barbitoff, Y.A.; Tonyan, Z.N.; Danilova, M.M.; Nevzorov, I.A.; Komandresova, T.M.; Mikhailova, A.A.; Vasilieva, T.V.; Glavnova, O.B.; Yarmolinskaya, M.I.; et al. Genetic and Phenotypic Factors Affecting Glycemic Response to Metformin Therapy in Patients with Type 2 Diabetes Mellitus. Genes 2022, 13, 1310. https://doi.org/10.3390/genes13081310
Nasykhova YA, Barbitoff YA, Tonyan ZN, Danilova MM, Nevzorov IA, Komandresova TM, Mikhailova AA, Vasilieva TV, Glavnova OB, Yarmolinskaya MI, et al. Genetic and Phenotypic Factors Affecting Glycemic Response to Metformin Therapy in Patients with Type 2 Diabetes Mellitus. Genes. 2022; 13(8):1310. https://doi.org/10.3390/genes13081310
Chicago/Turabian StyleNasykhova, Yulia A., Yury A. Barbitoff, Ziravard N. Tonyan, Maria M. Danilova, Ivan A. Nevzorov, Tatiana M. Komandresova, Anastasiia A. Mikhailova, Tatiana V. Vasilieva, Olga B. Glavnova, Maria I. Yarmolinskaya, and et al. 2022. "Genetic and Phenotypic Factors Affecting Glycemic Response to Metformin Therapy in Patients with Type 2 Diabetes Mellitus" Genes 13, no. 8: 1310. https://doi.org/10.3390/genes13081310
APA StyleNasykhova, Y. A., Barbitoff, Y. A., Tonyan, Z. N., Danilova, M. M., Nevzorov, I. A., Komandresova, T. M., Mikhailova, A. A., Vasilieva, T. V., Glavnova, O. B., Yarmolinskaya, M. I., Sluchanko, E. I., & Glotov, A. S. (2022). Genetic and Phenotypic Factors Affecting Glycemic Response to Metformin Therapy in Patients with Type 2 Diabetes Mellitus. Genes, 13(8), 1310. https://doi.org/10.3390/genes13081310







