Association of TNF–α rs1800629 with Adult Acute B-Cell Lymphoblastic Leukemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. DNA Extraction
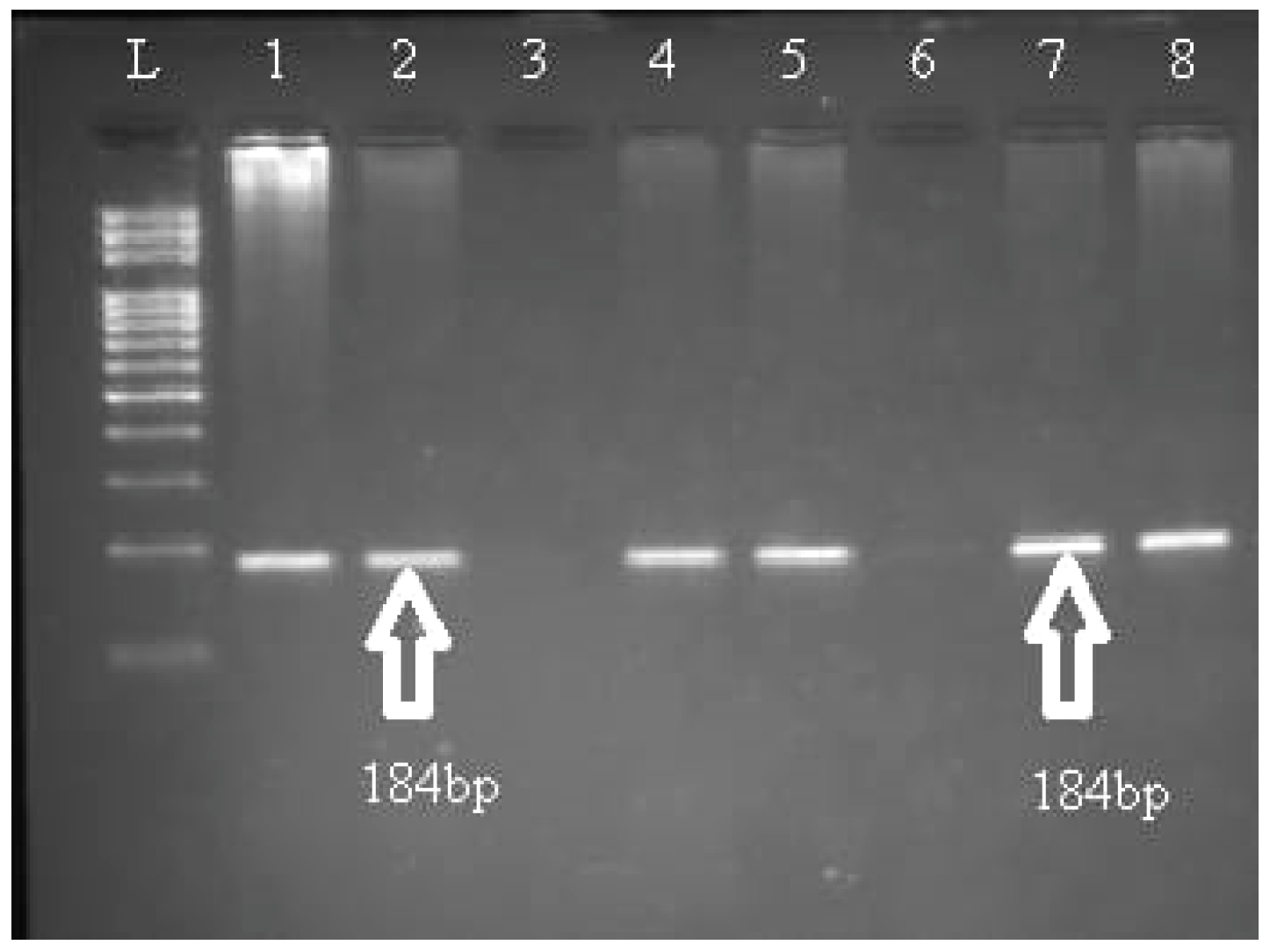
2.2. Molecular Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef]
- Paul, S.; Kantarjian, H.; Jabbour, E.J. Adult Acute Lymphoblastic Leukemia. Mayo Clin. Proc. 2016, 91, 1645–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, W.J.; Chang, W.S.; Hsu, H.F.; Ji, H.X.; Hsiao, C.L.; Tsai, C.W.; Yeh, S.P.; Chen, C.M.; Bau, D.T. Significant Association of Interleukin-10 Polymorphisms with Childhood Leukemia Susceptibility in Taiwan. In Vivo 2016, 30, 265–269. [Google Scholar] [PubMed]
- Gong, L.L.; Han, F.F.; Lv, Y.L.; Liu, H.; Wan, Z.R.; Zhang, W.; Shi, M.B.; Pei, L.X.; Liu, L.H. TNF-α and LT-α polymorphisms and the risk of Leukemia: A meta-analysis. Tumori J. 2017, 103, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Sinaga, B.Y.M.; Amir, Z. Tumor necrosis factor-alpha-308g/a polymorphism associated with increased risk for pulmonary tuberculosis in Medan City, Indonesia. Open Access Maced. J. Med. Sci. 2021, 9, 7–11. [Google Scholar] [CrossRef]
- Elahi, M.M.; Asotra, K.; Matata, B.M.; Mastana, S.S. Tumor necrosis factor alpha-308 gene locus promoter polymorphism: An analysis of association with health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Wang, J.; Brand, D.D.; Zheng, S.G. Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front. Immunol. 2018, 9, 784. [Google Scholar] [CrossRef] [Green Version]
- Purdue, M.P.; Lan, Q.; Kricker, A.; Grulich, A.E.; Vajdic, C.M.; Turner, J.; Whitby, D.; Chanock, S.; Rothman, N.; Armstrong, B.K. Polymorphisms in immune function genes and risk of non-Hodgkin lymphoma: Findings from the New South Wales non-Hodgkin Lymphoma Study. Carcinogenesis 2007, 28, 704–712. [Google Scholar] [CrossRef]
- Wang, S.S.; Purdue, M.P.; Cerhan, J.R.; Zheng, T.; Menashe, I.; Armstrong, B.K.; Lan, Q.; Hartge, P.; Kricker, A.; Zhang, Y.; et al. Common gene variants in the Tumor Necrosis Factor (TNF) and TNF receptor superfamilies and NF-kB transcription factors and non-hodgkin lymphoma risk. PLoS ONE 2009, 4, e5360. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.; Rahman, H.A.; Khorshied, M.; Sami, R.; Nasr, N.; Khorshid, O. Tumor necrosis factor alpha-308 and Lymphotoxin alpha+252 genetic polymorphisms and the susceptibility to non-Hodgkin lymphoma in Egypt. Leuk. Res. 2012, 36, 694–698. [Google Scholar] [CrossRef]
- Hajeer, A.H.; Hutchinson, I.V. TNF-α gene polymorphism: Clinical and biological implications. Microsc. Res. Tech. 2000, 50, 216–228. [Google Scholar] [CrossRef]
- Takeuchi, S.; Takeuchi, N.; Tsukasaki, K.; Bartram, C.R.; Zimmermann, M.; Schrappe, M.; Taguchi, H.; Koeffler, H.P. Genetic polymorphisms in the tumour necrosis factor locus in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 2002, 119, 985–987. [Google Scholar] [CrossRef]
- Šedý, J.; Bekiaris, V.; Ware, C.F. Tumor necrosis factor superfamily in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2015, 7, a016279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jevtovic-Stoimenov, T.; Kocic, G.; Pavlovic, D.; Macukanovic-Golubovic, L.; Marjanovic, G.; Djordjevic, V.; Tošić, N.; Pavlović, S. Polymorphisms of tumor-necrosis factor-alpha-308 and lymphotoxin-alpha + 250: Possible modulation of susceptibility to apoptosis in chronic lymphocytic leukemia and non-Hodgkin lymphoma mononuclear cells. Leuk. Lymphoma 2008, 49, 2163–2172. [Google Scholar] [CrossRef]
- Georgescu, A.M.; Banescu, C.; Azamfirei, R.; Hutanu, A.; Moldovan, V.; Badea, I.; Voidazan, S.; Dobreanu, M.; Chirtes, I.R.; Azamfirei, L. Evaluation of TNF- α genetic polymorphisms as predictors for sepsis susceptibility and progression. BMC Infect. Dis. 2020, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Demeter, J.; Porzsolt, F.; Rämisch, S.; Schmidt, D.; Schmid, M.; Messer, G. Polymorphism of the tumour necrosis factor-alpha and lymphotoxin-alpha genes in chronic lymphocytic leukaemia. Br. J. Haematol. 1997, 97, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, H.; Farajnia, S.; Rezamand, A.; Movassaghpour, A.A.; Esmaeili, H.A.; Monfaredan, A.; Mobarra, N.; Rahimfar, N.; Sahebi, L.; Farshdousti, M.; et al. Genetic variations of tumor necrosis factor -α-308 and lymphtoxin-α+252 in non-hodgkin lymphoma and acute lymphoblastic leukemia patients. Iran. J. Basic Med. Sci. 2013, 16, 990–995. [Google Scholar]
- Bogunia-Kubik, K.; Mazur, G.; Urbanowicz, I.; Wróbel, T.; Kuliczkowski, K.; Woźniak, M.; Lange, A. Lack of association between the TNF-α promoter gene polymorphism and susceptibility to B-cell chronic lymphocytic leukaemia. Int. J. Immunogenet. 2006, 33, 21–24. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhu, J.H.; Huang, S.Y.; Cui, Z.; He, J.; Jia, W.H. The association between the polymorphisms of TNF-α and non-Hodgkin lymphoma: A meta-analysis. Tumor Biol. 2014, 35, 12509–12517. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Chen, Y.-X.; Lin, X.-B.; Zhong, X.-Y.; Zhong, L.-Y.; Ou, R.-M.; Jiang, W.Q.; Guan, Z.Z. Relationship between tumor necrosis factor genetic polymorphisms and acute lymphocytic leukemia. Ai Zheng= Aizheng= Chin. J. Cancer 2003, 22, 861–866. [Google Scholar]
- Kamali-Sarvestani, E.; Merat, A.; Talei, A.-R. Polymorphism in the genes of alpha and beta tumor necrosis factors (TNF-alpha and TNF-beta) and gamma interferon (IFN-gamma) among Iranian women with breast cancer. Cancer Lett. 2005, 223, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, S.M.; Alkhiary, W.; Mokhtar, N.; Talaab, M. Tumor necrosis factor-α (TNF-α) −308 G/A and lymphotoxin-α (LT-α) +252 A/G genetic polymorphisms in Egyptian acute lymphoblastic leukemia. Comp. Clin. Pathol. 2018, 27, 363–369. [Google Scholar] [CrossRef]
- Tayel, M.Y.; Nazir, A.; Abdelhamid, I.M.; Helmy, M.A.S.; Zaki, N.E.; Elsharkawy, N.S.; Fayad, A.I. TNF-α -308 G>A and IL10 -1082A>G polymorphisms as potential risk factors for lymphoproliferative disorders in autoimmune rheumatic diseases. Egypt J. Med. Hum. Genet. 2020, 21, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Figgett, W.A.; Vincent, F.B.; Saulep-Easton, D.; Mackay, F. Roles of ligands from the TNF superfamily in B cell development, function, and regulation. Semin. Immunol. 2014, 26, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Rickert, R.C.; Jellusova, J.; Miletic, A.V. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol. Rev. 2011, 244, 115–133. [Google Scholar] [CrossRef]
- Hughes, A.M.; Lightfoot, T.; Simpson, J.; Ansell, P.; McKinney, P.A.; Kinsey, S.E.; Mitchell, C.D.; Eden, T.O.; Greaves, M.; Roman, E. Allergy and risk of childhood leukaemia: Results from the UKCCS. Int. J. Cancer 2007, 121, 819–824. [Google Scholar] [CrossRef]
- Rudant, J.; Lightfoot, T.; Urayama, K.Y.; Petridou, E.; Dockerty, J.D.; Magnani, C.; Milne, E.; Spector, L.G.; Ashton, L.J.; Dessypris, N.; et al. Childhood acute lymphoblastic leukemia and indicators of early immune stimulation: A childhood leukemia international consortium study. Am. J. Epidemiol. 2015, 181, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Linabery, A.M.; Jurek, A.M.; Duval, S.; Ross, J.A. The association between atopy and childhood/adolescent leukemia: A meta-analysis. Am. J. Epidemiol. 2010, 171, 749–764. [Google Scholar] [CrossRef] [Green Version]
| Category | Cases (n = 165) | Controls (n = 165) | (p-Value) |
|---|---|---|---|
| Gender | |||
| Male n (%) | 101 (61.2%) | 107(64.8%) | 0.494 |
| Female n (%) | 64 (38.8%) | 58 (35.2%) | |
| Age | |||
| Mean ± SD (year) | 44.64 ± 14.28 | 46.72 ± 15.87 | 0.213 |
| Range (year) | 19–75 | 18–74 | |
| <mean age n (%) | 84 (50.9%) | 92 (55.8%) | 0.377 |
| >mean age n (%) | 81 (49.1%) | 73 (44.2%) | |
| Immunophenotype | |||
| B-ALL n (%) | 137 (83%) | - | - |
| T-ALL n (%) | 28 (17%) | - | - |
| SNP | Genotype | ALL (n = 165) | Controls (n = 165) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|
| rs1800629 | GG | 25 (15.2%) | 39 (23.6%) | 0.58 (0.33 to 1.01) | 0.053 |
| GA | 117 (70.9%) | 94 (60%) | 1.84 (1.17 to 2.90) | 0.009 | |
| AA | 23 (13.9%) | 32 (19.4%) | 0.67 (0.37 to 1.21) | 0.185 | |
| GA +AA vs. GG | 140 (84.8%) | 126 (76.4%) | 1.73 (0.99 to 3.03) | 0.053 | |
| GA +GG vs. AA | 142 (86.1%) | 134 (81.2%) | 1.47 (0.82 to 2.65) | 0.194 | |
| Allele frequency | |||||
| G | 167 (50.6%) | 172 (52.1%) | 0.94 (0.69 to 1.28) | 0.697 | |
| A | 163 (49.4%) | 158 (47.9%) | |||
| SNP | Genotype | B-ALL (n = 137) | Controls (n = 165) | Odds Ratio (95% CI) | p-Value | T-ALL (n = 28) | Controls (n = 165) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| rs1800629 | GG | 17 (12.4%) | 39 (23.6%) | 0.46 (0.25 to 0.85) | 0.014 | 8 (28.6%) | 39 (23.6%) | 1.29 (0.53 to 3.16) | 0.575 |
| GA | 101 (73.7%) | 94 (60%) | 2.12 (1.30 to 3.46) | 0.003 | 16 (57.1%) | 94 (60%) | 1.01 (0.45 to 2.26) | 0.986 | |
| AA | 19 (13.9%) | 32 (19.4%) | 0.67 (0.36 to 1.24) | 0.204 | 4 (14.3%) | 32 (19.4%) | 0.69 (0.22 to 2.14) | 0.523 |
| Parameter | Category | rs1800629 | ||
|---|---|---|---|---|
| GG | GA | AA | ||
| Age group | <mean years | 11 | 63 | 10 |
| >mean years | 14 | 54 | 13 | |
| Chi square (p-value) | 1.39 (0.499) | |||
| Gender | Male | 16 | 69 | 16 |
| Female | 9 | 48 | 7 | |
| Chi square (p-value) | 1.0 (0.605) | |||
| Origin | B–ALL | 17 | 101 | 19 |
| T–ALL | 8 | 16 | 4 | |
| Chi square (p-value) | 4.91 (0.086) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalhabib, E.K.; Algarni, A.; Saboor, M.; Alanazi, F.; Ibrahim, I.K.; Alfeel, A.H.; Alanazi, A.M.; Alanazi, A.M.; Alruwaili, A.M.; Alanazi, M.H.; et al. Association of TNF–α rs1800629 with Adult Acute B-Cell Lymphoblastic Leukemia. Genes 2022, 13, 1237. https://doi.org/10.3390/genes13071237
Abdalhabib EK, Algarni A, Saboor M, Alanazi F, Ibrahim IK, Alfeel AH, Alanazi AM, Alanazi AM, Alruwaili AM, Alanazi MH, et al. Association of TNF–α rs1800629 with Adult Acute B-Cell Lymphoblastic Leukemia. Genes. 2022; 13(7):1237. https://doi.org/10.3390/genes13071237
Chicago/Turabian StyleAbdalhabib, Ezeldine K., Abdulrahman Algarni, Muhammad Saboor, Fehaid Alanazi, Ibrahim K. Ibrahim, Ayman H. Alfeel, Abdullah M. Alanazi, Abdulmajeed M. Alanazi, Abdulaziz M. Alruwaili, Muath H. Alanazi, and et al. 2022. "Association of TNF–α rs1800629 with Adult Acute B-Cell Lymphoblastic Leukemia" Genes 13, no. 7: 1237. https://doi.org/10.3390/genes13071237
APA StyleAbdalhabib, E. K., Algarni, A., Saboor, M., Alanazi, F., Ibrahim, I. K., Alfeel, A. H., Alanazi, A. M., Alanazi, A. M., Alruwaili, A. M., Alanazi, M. H., & Alshaikh, N. A. (2022). Association of TNF–α rs1800629 with Adult Acute B-Cell Lymphoblastic Leukemia. Genes, 13(7), 1237. https://doi.org/10.3390/genes13071237







