Nonalcoholic Fatty Liver Disease in Patients with Inherited and Sporadic Motor Neuron Degeneration
Abstract
:1. Introduction
2. Materials and Methods
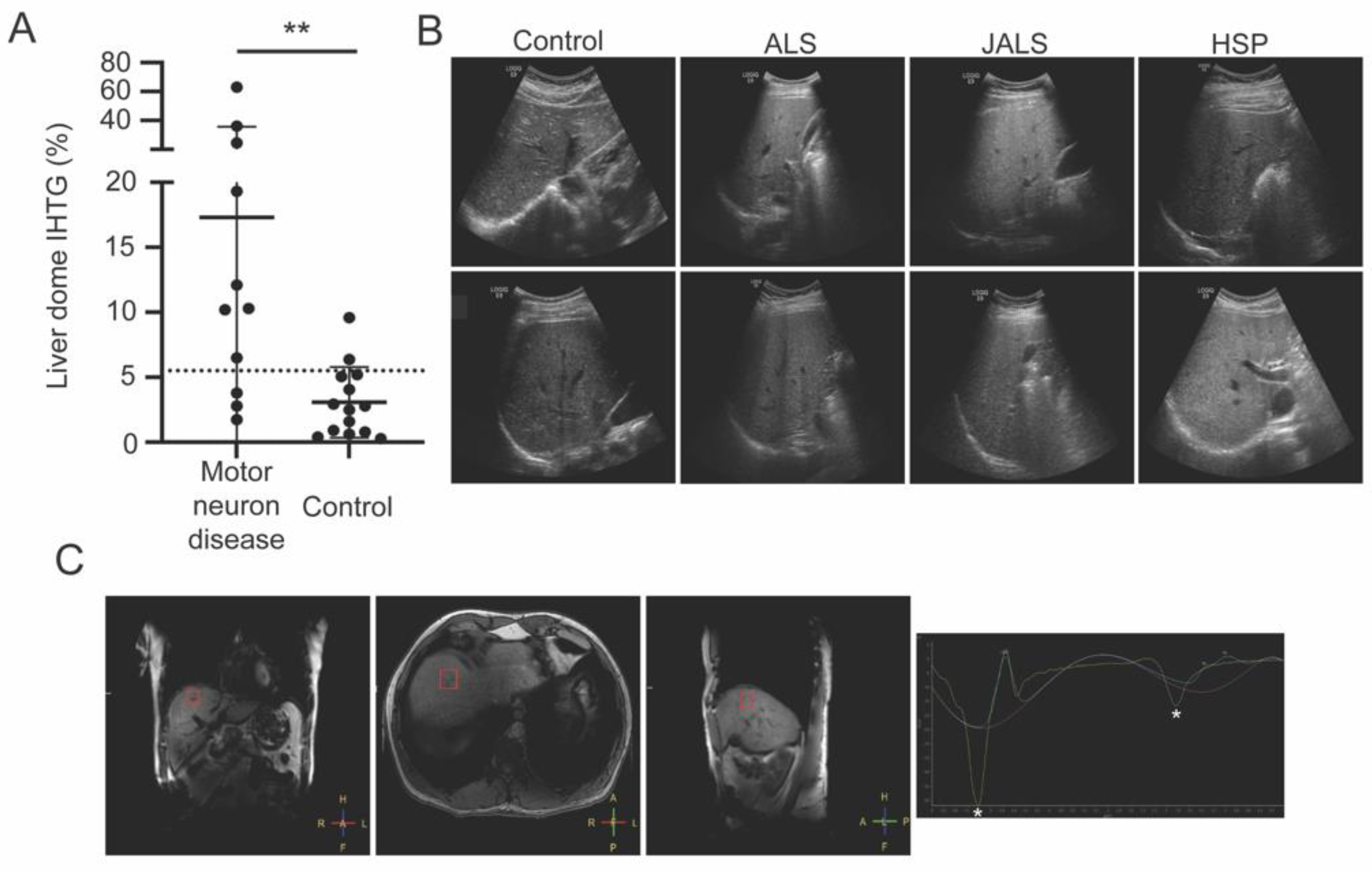
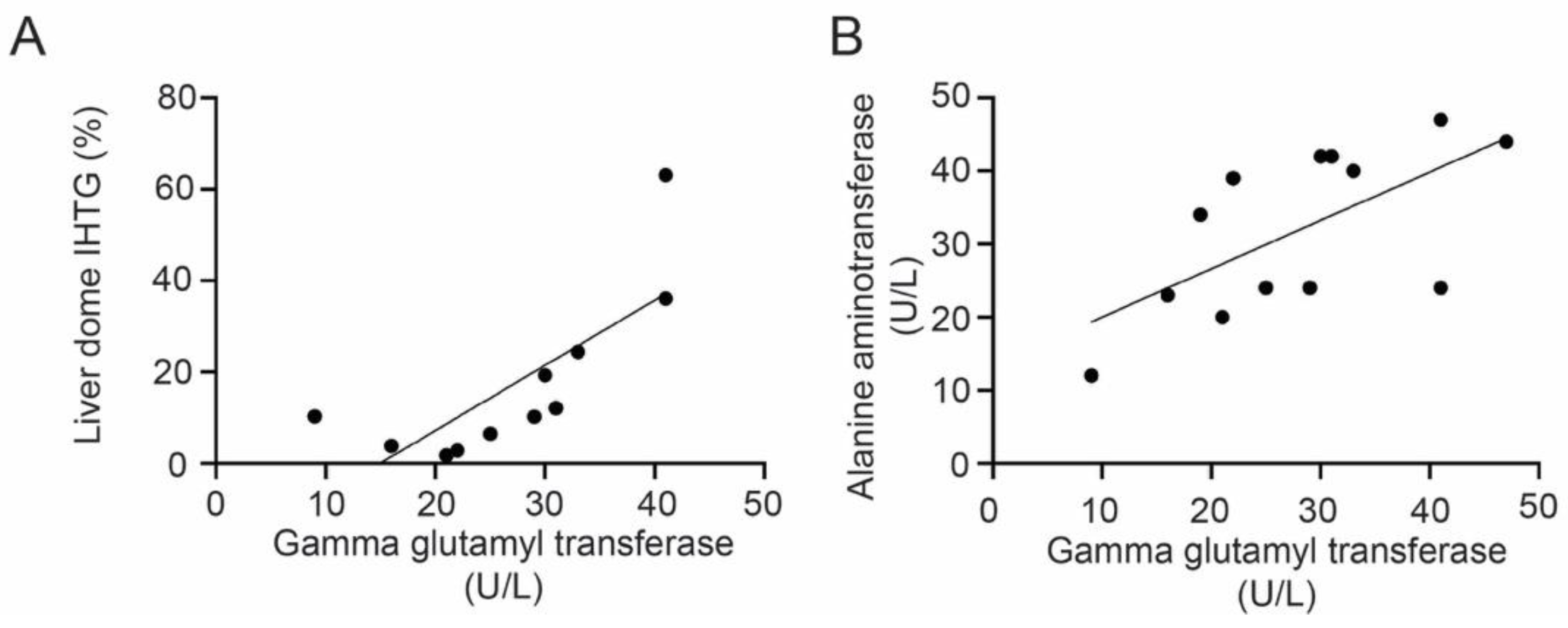
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanning, K.C.; Kaplan, A.; Henderson, C.E. Motor Neuron Diversity in Development and Disease. Annu. Rev. Neurosci. 2010, 33, 409–440. [Google Scholar] [CrossRef] [PubMed]
- Nodera, H.; Takamatsu, N.; Muguruma, N.; Ukimoto, K.; Nishio, S.; Oda, M.; Izumi, Y.; Kaji, R. Frequent Hepatic Steatosis in Amyotrophic Lateral Sclerosis: Implication for Systemic Involvement. Neurol. Clin. Neurosci. 2015, 3, 58–62. [Google Scholar] [CrossRef]
- Deguise, M.O.; Baranello, G.; Mastella, C.; Beauvais, A.; Michaud, J.; Leone, A.; de Amicis, R.; Battezzati, A.; Dunham, C.; Selby, K.; et al. Abnormal Fatty Acid Metabolism Is a Core Component of Spinal Muscular Atrophy. Ann. Clin. Transl. Neurol. 2019, 6, 1519–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Guber, R.D.; Takyar, V.; Kokkinis, A.; Fox, D.A.; Alao, H.; Kats, I.; Bakar, D.; Remaley, A.T.; Hewitt, S.M.; Kleiner, D.E.; et al. Nonalcoholic Fatty Liver Disease in Spinal and Bulbar Muscular Atrophy. Neurology 2017, 89, 2481–2490. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.; Kim, R.; Jung, Y.J.; Han, K.; Shin, C.M.; Lee, J.-Y. Serum Gamma-Glutamyltransferase Activity and Parkinson’s Disease Risk in Men and Women. Sci. Rep. 2020, 10, 1258. [Google Scholar] [CrossRef] [Green Version]
- Kunutsor, S.K.; Laukkanen, J.A. Gamma Glutamyltransferase and Risk of Future Dementia in Middle-aged to Older Finnish Men: A New Prospective Cohort Study. Alzheimers Dement. 2016, 12, 931–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, D.; Finck, B.N. Emerging Therapeutic Approaches for the Treatment of NAFLD and Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef]
- Ferramosca, A.; di Giacomo, M.; Zara, V. Antioxidant Dietary Approach in Treatment of Fatty Liver: New Insights and Updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Veldink, J.H.; Kalmijn, S.; Groeneveld, G.-J.; Wunderink, W.; Koster, A.; de Vries, J.H.M.; van der Luyt, J.; Wokke, J.H.J.; van den Berg, L.H. Intake of Polyunsaturated Fatty Acids and Vitamin E Reduces the Risk of Developing Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2007, 78, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Carrera-Juliá, S.; Moreno, M.L.; Barrios, C.; de la Rubia Ortí, J.E.; Drehmer, E. Antioxidant Alternatives in the Treatment of Amyotrophic Lateral Sclerosis: A Comprehensive Review. Front. Physiol. 2020, 11, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallsworth, K.; Thoma, C.; Moore, S.; Ploetz, T.; Anstee, Q.M.; Taylor, R.; Day, C.P.; Trenell, M.I. Non-Alcoholic Fatty Liver Disease Is Associated with Higher Levels of Objectively Measured Sedentary Behaviour and Lower Levels of Physical Activity than Matched Healthy Controls. Frontline Gastroenterol. 2015, 6, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, J.H.; Kim, H.J.; Park, E.C.; Jang, S.I. Association between Sitting Time and Non-Alcoholic Fatty Live Disease in South Korean Population: A Cross-Sectional Study. Lipids Health Dis. 2020, 19, 212. [Google Scholar] [CrossRef] [PubMed]
| Subject | Age/ Onset (years) | BMI (kg/m2) | Disease | Gene | Medications * | CK (U/L) | Total Bilirubin (mg/dL) | ALT (U/L) | HOMA-IR | GGT (U/L) | Cholesterol (mg/dL) | Triglyc-erides (mg/dL) | Free Fatty Acid (mEq/L) | Liver Dome IHTG (%) | Right Lobe IHTG (%) | Left Lobe IHTG (%) | Ultrasound |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MND01 | 59/49 | N/A | PLS | N/A | Tizanidine | 109 | 0.5 | 24 | N/A | 25 | 187 | 80 | 0.5 | 6.5 | 8.2 | 4.3 | Normal |
| MND02 | 68/13 | N/A | ALS4 | SETX | Atorvastatin | 314 | 0.4 | 24 | 7.7 | 29 | 154 | 116 | 1.0 | 10.2 | 4.8 | 5.8 | Normal |
| MND03 | 67/67 | 33 | ALS | N/A | Atorvastatin, (AMX0035 research compound) | 386 | 0.6 | 39 | 3.1 | 22 | 134 | 76 | 0.5 | 2.8 | 6.3 | 4.0 | Normal |
| MND04 | 74/49 | 24 | HSP | SPG31 | Atorvastatin, solifenacin succinate | 285 | 0.7 | 23 | 1.2 | 26 | 127 | 55 | 0.3 | 3.8 | 3.3 | 10.6 | Normal |
| MND05 | 56/30 | N/A | HSP | SPG4 | Diltiazem | 107 | 0.9 | 20 | 1.4 | 21 | 269 | 64 | 0.3 | 1.8 | 3.0 | 5.1 | Normal |
| MND06 | 56/54 | 24 | ALS | N/A | Riluzole | 430 | 0.4 | 42 | 19.1 | 30 | 130 | 220 | 0.3 | 19.3 | 12.9 | 5.9 | Moderate hepatic steatosis |
| MND07 | 29/19 | N/A | HSP | KIF1A | None | 148 | 0.6 | 47 | 4.6 | 41 | 197 | 197 | 0.8 | 63.1 | 8.6 | 10.3 | Minimal hepatic steatosis |
| MND08 | 40/13 | 26 | ALS4 | SETX | None | 200 | 0.5 | 40 | 2.8 | 33 | 137 | 80 | 0.3 | 24.4 | 17.6 | 3.6 | Moderate hepatic steatosis |
| MND09 | 54/50 | 27 | HSP | SPG4 | None | 189 | 1.6 | 24 | 2.7 | 41 | 229 | 133 | 0.5 | 36.1 | 9.3 | 38.4 | Mild hepatic steatosis |
| MND10 | 67/30 | 21 | HSP | SPG4 | Duloxetine | 250 | 0.5 | 12 | 1.5 | 9 | 155 | 55 | 0.5 | 10.3 | 0.0 | 3.3 | Normal |
| MND11 | 59/58 | 24 | ALS | N/A | Riluzole | 1275 | 0.9 | 42 | 0.9 | 31 | 217 | 79 | 0.5 | 12.1 | 7.6 | 8.7 | Normal |
| MND12 | 37/13 | 33 | ALS4 | SETX | None | 177 | 0.5 | 44 | 4.9 | 47 | 175 | 100 | 0.4 | # Diffuse hepatic steatosis | # Diffuse hepatic steatosis | # Diffuse hepatic steatosis | Moderate hepatic steatosis |
| MND13 | 57/54 | N/A | ALS | C9orf72 | None | 234 | 0.4 | 34 | 4.0 | 19 | 204 | 86 | 0.8 | N/A | N/A | N/A | Moderate hepatic steatosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, B.; Kokkinis, A.; Gai, N.; Shamim, E.A.; Blackstone, C.; Fischbeck, K.H.; Grunseich, C. Nonalcoholic Fatty Liver Disease in Patients with Inherited and Sporadic Motor Neuron Degeneration. Genes 2022, 13, 936. https://doi.org/10.3390/genes13060936
Johnson B, Kokkinis A, Gai N, Shamim EA, Blackstone C, Fischbeck KH, Grunseich C. Nonalcoholic Fatty Liver Disease in Patients with Inherited and Sporadic Motor Neuron Degeneration. Genes. 2022; 13(6):936. https://doi.org/10.3390/genes13060936
Chicago/Turabian StyleJohnson, Brian, Angela Kokkinis, Neville Gai, Ejaz A. Shamim, Craig Blackstone, Kenneth H. Fischbeck, and Christopher Grunseich. 2022. "Nonalcoholic Fatty Liver Disease in Patients with Inherited and Sporadic Motor Neuron Degeneration" Genes 13, no. 6: 936. https://doi.org/10.3390/genes13060936
APA StyleJohnson, B., Kokkinis, A., Gai, N., Shamim, E. A., Blackstone, C., Fischbeck, K. H., & Grunseich, C. (2022). Nonalcoholic Fatty Liver Disease in Patients with Inherited and Sporadic Motor Neuron Degeneration. Genes, 13(6), 936. https://doi.org/10.3390/genes13060936





