Genetic and Epigenetic Association of Hepatocyte Nuclear Factor-1α with Glycosylation in Post-Traumatic Stress Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Blood Processing
2.3. N-glycan Determination in Plasma and IgG
2.4. IgG Isolation
2.5. N-glycan Release and Labelling
2.6. Hydrophilic Interaction High-Performance Liquid Chromatography (HILIC)
2.7. Ultraperformance Liquid Chromatographic (UPLC) Analysis of IgG N-glycans
2.8. Genotyping
2.9. Analysis of CpG Methylation in the HNF1A Gene
2.10. Statistical Analysis
3. Results
3.1. Association of HNF1A Polymorphisms with PTSD
3.2. Association of HNF1A Gene Methylation and HNF1A Polymorphisms
3.3. Correlation of HNF1A Gene Methylation and N-glycome
3.4. Association of HNF1A Polymorphisms and N-glycome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tudor, L.; Nedic Erjavec, G.; Nikolac Perkovic, M.; Konjevod, M.; Svob Strac, D.; Uzun, S.; Kozumplik, O.; Jovanovic, T.; Lauc, G.; Pivac, N. N-glycomic Profile in Combat Related Post-Traumatic Stress Disorder. Biomolecules 2019, 9, 834. [Google Scholar] [CrossRef] [PubMed]
- Konjevod, M.; Tudor, L.; Svob Strac, D.; Nedic Erjavec, G.; Barbas, C.; Zarkovic, N.; Nikolac Perkovic, M.; Uzun, S.; Kozumplik, O.; Lauc, G.; et al. Metabolomic and glycomic findings in posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Hoge, C.W.; McFarlane, A.C.; Vermetten, E.; Lanius, R.A.; Nievergelt, C.M.; Hobfoll, S.E.; Koenen, K.C.; Neylan, T.C.; Hyman, S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Primers 2015, 1, 15057. [Google Scholar] [CrossRef] [PubMed]
- Britvic, D.; Anticevic, V.; Kaliterna, M.; Lusic, L.; Beg, A.; Brajevic-Gizdic, I.; Kudric, M.; Cuk, Z.; Krolo, V.; Pivac, N. Comorbidities with PTSD among combat veterans; 15 years postwar analysis. Int. J. Clin. Health Psychol. 2015, 15, 81–92. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Morath, J.; Vanhooren, V.; Elbert, T.; Kolassa, S.; Libert, C.; Bürkle, A.; Kolassa, I.T. N-glycosylation profiling of plasma provides evidence for accelerated physiological aging in post-traumatic stress disorder. Transl. Psychiatry 2013, 3, e320. [Google Scholar] [CrossRef]
- Miao, X.R.; Chen, Q.B.; Wei, K.; Tao, K.M.; Lu, Z.J. Posttraumatic stress disorder: From diagnosis to prevention. Mil. Med. Res. 2018, 5, 32. [Google Scholar] [CrossRef]
- Zhang, K.; Qu, S.; Chang, S.; Li, G.; Cao, C.; Fang, K.; Olff, M.; Wang, L.; Wang, J. An overview of posttraumatic stress disorder genetic studies by analyzing and integrating genetic data into genetic database PTSDgene. Neurosci. Biobehav. Rev. 2017, 83, 647–656. [Google Scholar] [CrossRef]
- Lebois, L.A.M.; Wolff, J.D.; Ressler, K.J. Neuroimaging genetic approaches to Posttraumatic Stress Disorder. Exp. Neurol. 2016, 284, 141–152. [Google Scholar] [CrossRef]
- Lauc, G.; Zoldos, V. Protein glycosylation—An evolutionary crossroad between genes and environment. Mol. Biosyst. 2010, 6, 2373–2379. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Freeze, H.H.; Ng, B.G. Golgi glycosylation and human inherited diseases. Cold Spring Harb. Perspect. Biol. 2011, 3, a005371. [Google Scholar] [CrossRef] [PubMed]
- Lauc, G.; Rudan, I.; Campbell, H.; Rudd, P.M. Complex genetic regulation of protein glycosylation. Mol. Biosyst. 2010, 6, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Stanta, J.L.; Saldova, R.; Struwe, W.B.; Byrne, J.C.; Leweke, F.M.; Rothermund, M.; Rahmoune, H.; Levin, Y.; Guest, P.C.; Bahn, S.; et al. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. J. Proteome Res. 2010, 9, 4476–4489. [Google Scholar] [CrossRef]
- Boeck, C.; Pfister, S.; Bürkle, A.; Vanhooren, V.; Libert, C.; Salinas-Manrique, J.; Dietrich, D.E.; Kolassa, I.T.; Karabatsiakis, A. Alterations of the serum N-glycan profile in female patients with Major Depressive Disorder. J. Affect. Disord. 2018, 234, 139–147. [Google Scholar] [CrossRef]
- Park, D.I.; Stambuk, J.; Razdorov, G.; Pucic-Bakovic, M.; Martins-de-Souza, D.; Lauc, G.; Turck, C.W. Blood plasma/IgG N-glycome biosignatures associated with major depressive disorder symptom severity and the antidepressant response. Sci. Rep. 2018, 8, 179. [Google Scholar] [CrossRef]
- Gudelj, I.; Baciarello, M.; Ugrina, I.; De Gregori, M.; Napolioni, V.; Ingelmo, P.M.; Bugada, D.; De Gregori, S.; Derek, L.; Pucic-Bakovic, M.; et al. Changes in total plasma and serum N-glycome composition and patient-controlled analgesia after major abdominal surgery. Sci. Rep. 2016, 6, 31234. [Google Scholar] [CrossRef]
- Zoldos, V.; Horvat, T.; Novokmet, M.; Cuenin, C.; Muzinic, A.; Pucic, M.; Huffman, J.E.; Gornik, O.; Polasek, O.; Campbell, H.; et al. Epigenetic silencing of HNF1A associates with changes in the composition of the human plasma N-glycome. Epigenetics 2012, 7, 164–172. [Google Scholar] [CrossRef]
- Lauc, G.; Essafi, A.; Huffman, J.E.; Hayward, C.; Knezevic, A.; Kattla, J.J.; Polasek, O.; Gornik, O.; Vitart, V.; Abrahams, J.L.; et al. Genomics meets glycomics-the first GWAS study of human N-Glycome identifies HNF1α as a master regulator of plasma protein fucosylation. PLoS Genet. 2010, 6, e1001256. [Google Scholar] [CrossRef]
- Josipovic, G.; Tadic, V.; Klasic, M.; Zanki, V.; Beceheli, I.; Chung, F.; Ghantous, A.; Keser, T.; Madunic, J.; Boskovic, M.; et al. Antagonistic and synergistic epigenetic modulation using orthologous CRISPR/dCas9-based modular system. Nucleic Acids Res. 2019, 47, 9637–9657. [Google Scholar] [CrossRef]
- Stambuk, T.; Klasic, M.; Zoldos, V.; Lauc, G. N-glycans as functional effectors of genetic and epigenetic disease risk. Mol. Asp. Med. 2021, 79, 100891. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Weathers, F.W.; Keane, T.M.; Davidson, J.R. Clinician-administered PTSD scale: A review of the first ten years of research. Depress. Anxiety 2001, 13, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Akmacic, I.T.; Ugrina, I.; Stambuk, J.; Gudelj, I.; Vuckovic, F.; Lauc, G.; Pucic-Bakovic, M. High-throughput glycomics: Optimization of sample preparation. Biochem. Mosc. 2015, 80, 934–942. [Google Scholar] [CrossRef]
- Pucic, M.; Knezevic, A.; Vidic, J.; Adamczyk, B.; Novokmet, M.; Polasek, O.; Gornik, O.; Supraha-Goreta, S.; Wormald, M.R.; Redzic, I.; et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteom. 2011, 10, M111010090. [Google Scholar] [CrossRef] [PubMed]
- Saldova, R.; Shehni, A.A.; Haakensen, V.D.; Steinfeld, I.; Hilliard, M.; Kifer, I.; Helland, A.; Yakhini, Z.; Borresen-Dale, A.L.; Rudd, P.M. Association of N-Glycosylation with Breast Carcinoma and Systemic Features Using High-Resolution Quantitative UPLC. J. Proteome Res. 2014, 13, 2314–2327. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Pucic Bakovic, M.; Kristic, J.; Novokmet, M.; Huffman, J.E.; Vitart, V.; Hayward, C.; Rudand, I.; Wilson, J.F.; Campbell, H.; et al. The association between galactosylation of immunoglobulin G and body mass index. Prog. Neuropsychopharmacol. Biol. Psych. 2014, 48, 20–25. [Google Scholar] [CrossRef]
- Pucic, M.; Muzinic, A.; Skledar, M.; Pivac, N.; Lauc, G.; Gornik, O. Changes in plasma and IgG N-glycome during childhood and adolescence. Glycobiology 2012, 22, 975–982. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Harries, L.W.; Ellard, S.; Stride, A.; Morgan, N.G.; Hattersley, A.T. Isomers of the TCF1 gene encoding hepatocyte nuclear factor-1 α show differential expression in the pancreas and define the relationship between mutation position and clinical phenotype in monogenic diabetes. Hum. Mol. Genet. 2006, 15, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.P.; Barber, M.J.; Guan, Y.; Ridker, P.M.; Lange, L.A.; Chasman, D.I.; Walston, J.D.; Cooper, G.M.; Jenny, N.S.; Rieder, M.J.; et al. Polymorphisms of the HNF1A gene encoding hepatocyte nuclear factor-1 α are associated with C-reactive protein. Am. J. Hum. Genet. 2008, 82, 1193–1201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Y.; McDade, T.W.; Kuzawa, C.W.; Borja, J.; Li, Y.; Adair, L.S.; Mohlke, K.L.; Lange, L.A. Genome-wide association with C-reactive protein levels in CLHNS: Evidence for the CRP and HNF1A loci and their interaction with exposure to a pathogenic environment. Inflammation 2012, 35, 574–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Real Hernandez, L.M.; Fan, J.; Johnson, M.H.; Gonzalez de Mejia, E. Berry Phenolic Compounds Increase Expression of Hepatocyte Nuclear Factor-1α (HNF-1α) in Caco-2 and Normal Colon Cells Due to High Affinities with Transcription and Dimerization Domains of HNF-1α. PLoS ONE 2015, 10, e0138768. [Google Scholar] [CrossRef]
- Yamagata, K.; Furuta, H.; Oda, N.; Kaisaki, P.J.; Menzel, S.; Cox, N.J.; Fajans, S.S.; Signorini, S.; Stoffel, M.; Bell, G.I. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1). Nature 1996, 384, 458–460. [Google Scholar] [CrossRef]
- Yamagata, K.; Oda, N.; Kaisaki, P.J.; Menzel, S.; Furuta, H.; Vaxillaire, M.; Southam, L.; Cox, R.D.; Lathrop, G.M.; Boriraj, V.V.; et al. Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature 1996, 384, 455–458. [Google Scholar] [CrossRef]
- Voight, B.F.; Scott, L.J.; Steinthorsdottir, V.; Morris, A.P.; Dina, C.; Welch, R.P.; Zeggini, E.; Huth, C.; Aulchenko, Y.S.; Thorleifsson, G.; et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010, 42, 579–589. [Google Scholar] [CrossRef]
- Ridker, P.M.; Pare, G.M.; Parker, A.; Zee, R.Y.L.; Danik, J.S.; Buring, J.E.; Kwiatkowski, D.; Cook, N.R.; Miletich, J.P.; Chasman, D.I. Loci related to metabolic-syndrome pathways including LEPR, HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: The women’s genome health study. Am. J. Hum. Genet. 2008, 82, 1185–1192. [Google Scholar] [CrossRef]
- Morjane, I.; Kefi, R.; Charoute, H.; Lakbakbi, E.; Yaagoubi, F.; Hechmi, M.; Saile, R.; Abdelhak, S.; Barakat, A. Association study of HNF1A polymorphisms with metabolic syndrome in the Moroccan population. Diabetes Metab. Syndr. 2017, 11, S853–S857. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Chambers, J.C.; Zhang, W.; Clarke, R.; Hopewell, J.C.; Peden, J.F.; Erdmann, J.; Braund, P.; Engert, J.C.; Bennett, D. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. J. Am. Med. Assoc. 2009, 302, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.P.; Gross, M.D.; Carlson, C.S.; Bielinski, S.J.; Lange, L.A.; Fornage, M.; Jenny, N.S.; Walston, J.; Tracy, R.P.; Williams, O.D.; et al. Common coding variants of the HNF1A gene are associated with multiple cardiovascular risk phenotypes in community-based samples of younger and older European-American adults: The Coronary Artery Risk Development in Young Adults Study and The Cardiovascular Health Study. Circ. Cardiovasc. Genet. 2009, 2, 244–254. [Google Scholar] [PubMed]
- Pierce, B.L.; Ahsan, H. Genome-wide “pleiotropy scan” identifies HNF1A region as a novel pancreatic cancer susceptibility locus. Cancer Res. 2011, 71, 4352–4358. [Google Scholar] [CrossRef]
- Jiang, M.M.; Gu, X.; Yang, J.; Wang, M.M.; Li, H.M.; Fang, M.; Gao, C.F. Association of a functional intronic polymorphism rs735396 in HNF1A gene with the susceptibility to hepatocellular carcinoma in Han Chinese population. Int. J. Clin. Exp. Pathol. 2017, 10, 671–679. [Google Scholar]
- Barrett, J.C.; Lee, J.C.; Lees, C.W.; Prescott, N.J.; Anderson, C.A.; Phillips, A.; Wesley, E.; Parnell, K.; Zhang, H.; Drummond, H.; et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat. Genet. 2009, 41, 1330–1334. [Google Scholar]
- Kathiresan, S.; Willer, C.J.; Peloso, G.M.; Demissie, S.; Musunuru, K.; Schadt, E.E.; Kaplan, L.; Bennett, D.; Li, Y.; Tanaka, T.; et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 2009, 41, 56–65. [Google Scholar] [CrossRef]
- Yuan, X.; Waterworth, D.; Perry, J.R.; Lim, N.; Song, K.; Chambers, J.C.; Zhang, W.; Vollenweider, P.; Stirnadel, H.; Johnson, T.; et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am. J. Hum. Genet. 2008, 83, 520–528. [Google Scholar] [CrossRef]
- Michopoulos, V.; Rothbaum, A.O.; Jovanovic, T.; Almli, L.M.; Bradley, B.; Rothbaum, B.O.; Gillespie, C.F.; Ressler, K.J. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatry 2015, 172, 353–362. [Google Scholar] [CrossRef]
- Mellon, S.H.; Gautam, A.; Hammamieh, R.; Jett, M.; Wolkowitz, O.M. Metabolism, Metabolomics, and Inflammation in Posttraumatic Stress Disorder. Biol. Psychiatry 2018, 83, 866–875. [Google Scholar] [CrossRef]
- Kim, T.D.; Lee, S.; Yoon, S. Inflammation in Post-Traumatic Stress Disorder (PTSD): A Review of Potential Correlates of PTSD with a Neurological Perspective. Antioxidants 2020, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, A.; Pavic, T.; Vuckovic, F.; Bennett, A.J.; Shah, N.; Medvidovic, E.P.; Groves, C.J.; Sekerija, M.; Chandler, K.; Burrows, C.; et al. Plasma fucosylated glycans and C-reactive protein as biomarkers of HNF1A-MODY in young adult–onset nonautoimmune diabetes. Diabetes Care 2019, 42, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Keser, T.; Gornik, I.; Vuckovic, F.; Selak, N.; Pavic, T.; Lukic, E.; Gudelj, I.; Gasparovic, H.; Biocina, B.; Tilin, T.; et al. Increased plasma N-glycome complexity is associated with higher risk of type 2 diabetes. Diabetologia 2018, 61, 506. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.E.; Knezevic, A.; Vitart, V.; Kattla, J.; Adamczyk, B.; Novokmet, M.; Igl, W.; Pucic, M.; Zgaga, L.; Johannson, A.; et al. Polymorphisms in B3GAT1, SLC9A9 and MGAT5 are associated with variation within the human plasma N-glycome of 3533 European adults. Hum. Mol. Genet. 2011, 20, 5000–5011. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, S.Z.; Tsepilov, Y.A.; Klaric, L.; Mangino, M.; Thareja, G.; Shadrina, A.S.; Simurina, M.; Dagostino, C.; Dmitrieva, J.; Vilaj, M.; et al. Defining the genetic control of human blood plasma N-glycome using genome-wide association study. Hum. Mol. Genet. 2019, 28, 2062–2077. [Google Scholar]
- Nemansky, M.; Schiphorst, W.E.; Van den Eijnden, D.H. Branching and elongation with lactosaminoglycan chains of N-linked oligosaccharides result in a shift toward termination with α 2->3-linked rather than with α 2->6-linked sialic acid residues. FEBS Lett. 1995, 363, 280–284. [Google Scholar] [CrossRef]
- Dotz, V.; Lemmers, R.F.H.; Reiding, K.R.; Hipgrave Ederveen, A.L.; Lieverse, A.G.; Mulder, M.T.; Sijbrands, E.J.G.; Wuhrer, M.; van Hoek, M. Plasma protein N-glycan signatures of type 2 diabetes. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2613–2622. [Google Scholar] [CrossRef]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef]
- Catera, M.; Borelli, V.; Malagolini, N.; Chiricolo, M.; Venturi, G.; Reis, C.A.; Osorio, H.; Abruzzo, P.M.; Capri, M.; Monti, D.; et al. Identification of novel plasma glycosylationassociated markers of aging. Oncotarget 2016, 7, 7455–7468. [Google Scholar] [CrossRef]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G glycosylation in aging and diseases. Cell. Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef]
- Shi, H.; Leng, S.; Liang, H.; Zheng, Y.; Chen, L. Association study of C-reactive protein associated gene HNF1A with ischemic stroke in Chinese population. BMC Med. Genet. 2016, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Dallali, H.; Hechmi, M.; Morjane, I.; Elouej, S.; Jmel, H.; Ben Halima, Y.; Abid, A.; Bahlous, A.; Barakat, A.; Jamoussi, H.; et al. Association of HNF1A gene variants and haplotypes with metabolic syndrome: A case-control study in the Tunisian population and a meta-analysis. Diabetol. Metab. Syndr. 2022, 14, 25. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Chen, M.Z.; Olefski, J.M.; Marth, J.D. Pathway to diabetes through attenuation of pancreatic β cell glycosylation and glucose transport. Nat. Med. 2011, 17, 1067–1075. [Google Scholar] [CrossRef]
- Klasic, M.; Markulin, D.; Vojta, A.; Samarzija, I.; Birus, I.; Dobrinic, P.; Ventham, N.T.; Trbojevic-Akmacic, I.; Simurina, M.; Stambuk, J.; et al. Promoter methylation of the MGAT3 and BACH2 genes correlates with the composition of the immunoglobulin G glycome in inflammatory bowel disease. Clin. Epigenet. 2018, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.M.; Siegmund, K.D.; Pan, F.; Weisenberger, D.J.; Kanel, G.; Laird, P.W.; Yang, A.S. Epigenetic profiling of somatic tissues from human autopsy specimens identifies tissue- and individual-specific DNA methylation patterns. Hum. Mol. Genet. 2009, 18, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, A.; Gornik, O.; Polasek, O.; Pucic, M.; Redzic, I.; Novokmet, M.; Rudd, P.M.; Wright, A.F.; Campbell, H.; Rudan, I.; et al. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology 2010, 20, 959–969. [Google Scholar] [CrossRef]
- Haslam, S.M.; Julien, S.; Burchell, J.M.; Monk, C.R.; Ceroni, A.; Garden, O.A.; Dell, A. Characterizing the glycome of the mammalian immune system. Immunol. Cell. Biol. 2008, 86, 564–573. [Google Scholar] [CrossRef]
- Suhre, K.; Trbojević-Akmačić, I.; Ugrina, I.; Mook-Kanamori, D.O.; Spector, T.; Graumann, J.; Lauc, G.; Falchi, M. Fine-Mapping of the Human Blood Plasma N-Glycome onto Its Proteome. Metabolites 2019, 9, 122. [Google Scholar] [CrossRef]
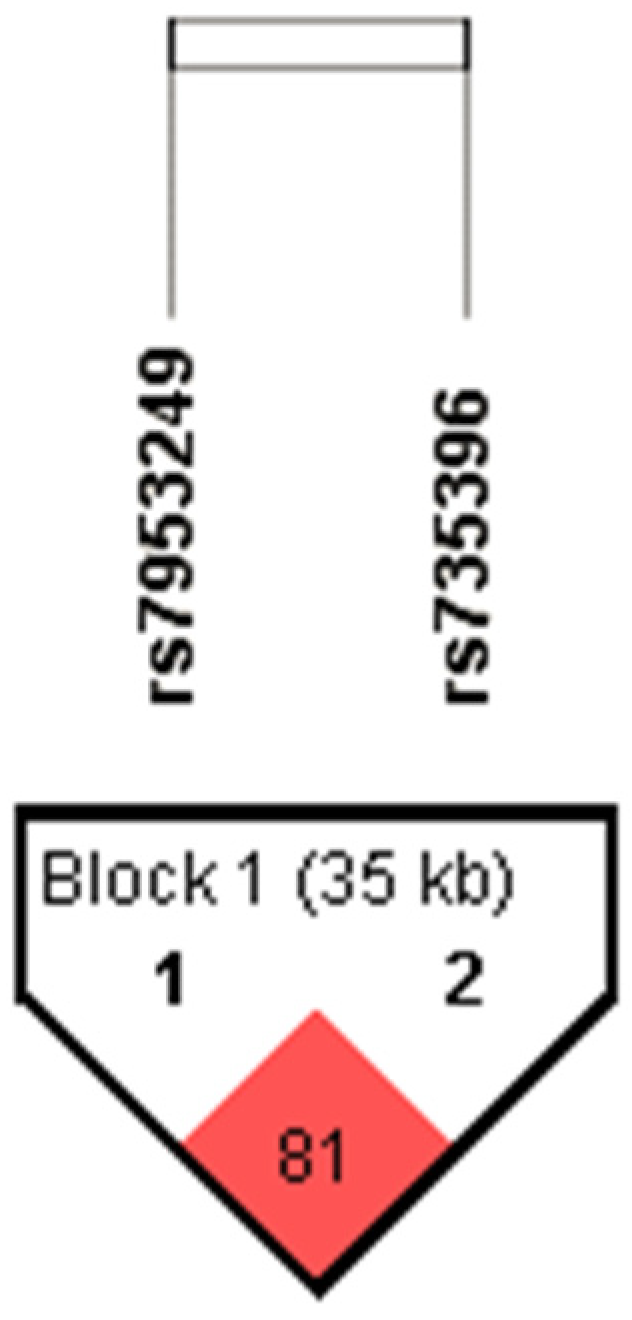

| SNP | Control Subjects | Subjects with PTSD | Statistics | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| HNF1A-AS1 rs7953249 | AA | 86 | 33.3 | 113 | 38.0 | χ2 = 9.263; df = 2; p = 0.010 |
| AG | 117 | 45.3 | 149 | 50.2 | ||
| GG | 55 | 21.3 | 35 | 11.8 | ||
| A | 289 | 56.0 | 375 | 63.1 | χ2 = 5.830; df = 1; p = 0.016 | |
| G | 227 | 44.0 | 219 | 36.9 | ||
| HNF1A rs735396 | TT | 110 | 42.6 | 130 | 43.8 | χ2 = 5.605; df = 2; p = 0.061 |
| TC | 108 | 41.9 | 140 | 47.1 | ||
| CC | 40 | 15.5 | 27 | 9.1 | ||
| T | 328 | 63.6 | 400 | 67.3 | χ2 = 1.589; df = 1; p = 0.208 | |
| C | 188 | 36.4 | 194 | 32.7 | ||
| Haplotype HNF1A rs7953249-rs735396 | AT | 274 | 53.1 | 351 | 59.1 | χ2 = 6.383; df = 3; p = 0.094 |
| GC | 173 | 33.5 | 170 | 28.6 | ||
| GT | 54 | 10.5 | 49 | 8.2 | ||
| AC | 15 | 2.9 | 24 | 4.0 | ||
| Methylation Site | Diagnostic Group | HNF1A-AS1 | HNF1A | Haplotype | ||
|---|---|---|---|---|---|---|
| Genetic Model | Allelic Model | Genetic Model | Allelic Model | |||
| CpG1 | Control | H = 0.248; df = 2; p = 0.883 | U = 4713.0; p = 0.650 | H = 0.006; df = 2; p = 0.997 | U = 4689.0; p = 0.938 | H = 0.556; df = 3; p = 0.906 |
| PTSD | H = 0.736; df = 2; p = 0.692 | U = 4538.5; p = 0.831 | H = 0.081; df = 2; p = 0.960 | U = 4129.5; p = 0.871 | H = 2.521; df = 3; p = 0.472 | |
| CpG2 | Control | H = 1.647; df = 2; p = 0.439 | U = 4405.0; p = 0.223 | H = 1.318; df = 2; p = 0.517 | U = 4372.0; p = 0.379 | H = 0.181; df = 3; p = 0.981 |
| PTSD | H = 0.007; df = 2; p = 0.997 | U = 4592.5; p = 0.941 | H = 0.467; df = 2; p = 0.792 | U = 4184.5; p = 0.989 | H = 4.866; df = 3; p = 0.182 | |
| CpG3 | Control | H = 2.156; df = 2; p = 0.340 | U = 4309.0; p = 0.145 | H = 3.198; df = 2; p = 0.202 | U = 4022.0; p = 0.078 | H = 3.437; df = 3; p = 0.329 |
| PTSD | H = 0.661; df = 2; p = 0.719 | U = 4357.5; p = 0.498 | H = 0.692; df = 2; p = 0.708 | U = 4105.5; p = 0.821 | H = 1.319; df = 3; p = 0.725 | |
| Glycan Peak | Diagnostic Group | Methylation Site | ||
|---|---|---|---|---|
| CpG1 | CpG2 | CpG3 | ||
| Spearman’s rho coefficient (p value) | Spearman’s rho coefficient (p value) | Spearman’s rho coefficient (p value) | ||
| GP10 (FA2G2) | Control | −0.134 (0.188) | −0.333 (0.001) | −0.187 (0.064) |
| PTSD | −0.132 (0.195) | −0.033 (0.748) | −0.028 (0.785) | |
| GP14 (A2G2S1) | Control | 0.259 (0.010) | 0.179 (0.076) | 0.093 (0.360) |
| PTSD | −0.211 (0.037) | −0.220 (0.030) | −0.174 (0.086) | |
| GP21 (A2BG2S2) | Control | 0.057 (0.574) | 0.079 (0.440) | 0.124 (0.220) |
| PTSD | −0.271 (0.007) | −0.239 (0.018) | −0.251 (0.013) | |
| GP24 (A3G3S2) | Control | −0.029 (0.778) | −0.009 (0.927) | 0.089 (0.381) |
| PTSD | −0.049 (0.632) | −0.234 (0.020) | −0.276 (0.006) | |
| GP26 (A3G3S2) | Control | −0.056 (0.582) | 0.051 (0.613) | 0.029 (0.779) |
| PTSD | −0.101 (0.321) | −0.254 (0.012) | −0.283 (0.005) | |
| GP30 (A3G3S3) | Control | −0.051 (0.618) | −0.050 (0.626) | 0.044 (0.663) |
| PTSD | −0.047 (0.648) | −0.238 (0.018) | −0.270 (0.007) | |
| GP31 (FA3G3S3) | Control | −0.017 (0.864) | 0.033 (0.746) | −0.034 (0.740) |
| PTSD | −0.119 (0.244) | −0.254 (0.012) | −0.258 (0.010) | |
| IgGP14 (FA2G2) | Control | −0.070 (0.489) | −0.264 (0.008) | −0.163 (0.108) |
| PTSD | −0.214 (0.034) | −0.105 (0.304) | −0.017 (0.869) | |
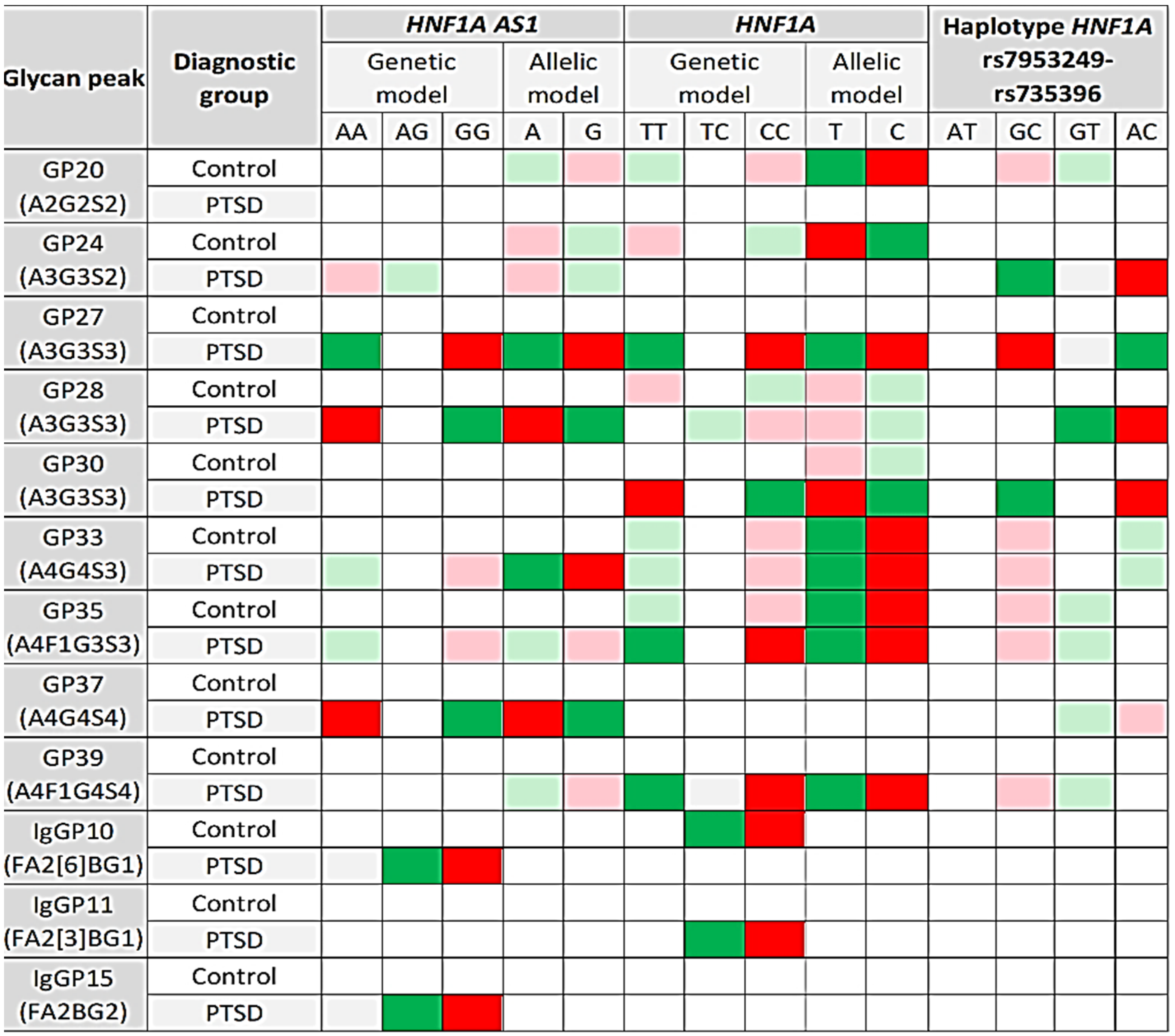
| Glycan Peak | Diagnostic Group | HNF1A AS1 | HNF1A | Haplotype | ||
|---|---|---|---|---|---|---|
| Genetic Model | Allelic Model | Genetic Model | Allelic Model | |||
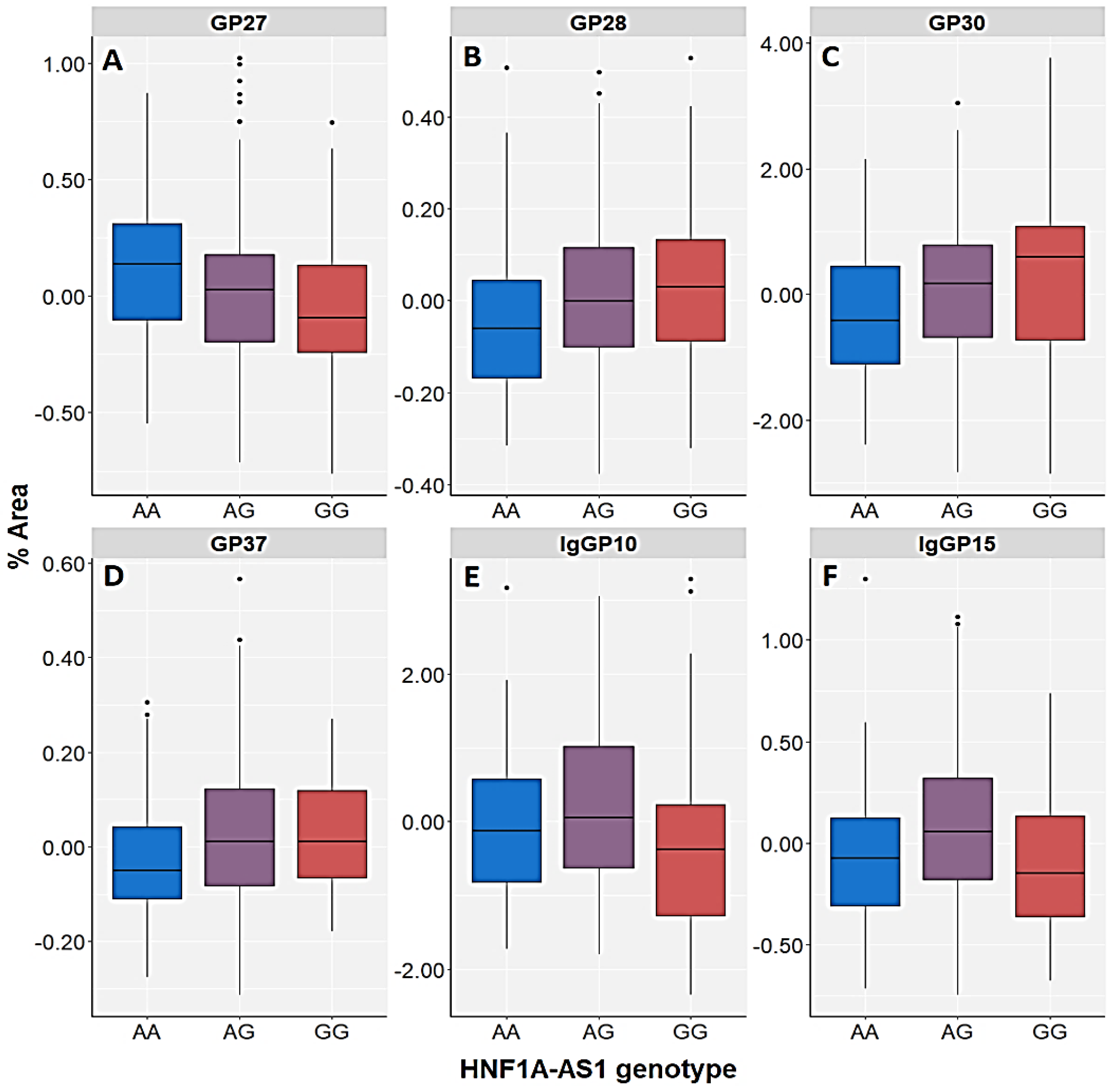
| GP20 (A2G2S2) | Control | H = 4.337; df = 2; p = 0.114 | U = 29,274.5; p = 0.036 | H = 7.421; df = 2; p = 0.024 | U = 26,244.0; p = 0.005 | H = 8.145; df = 3; p = 0.043 |
| PTSD | H = 2.353; df = 2; p = 0.308 | U = 39,466.5; p = 0.429 | H = 2.832; df = 2; p = 0.243 | U = 36,330.5; p = 0.190 | H = 3.366; df = 3; p = 0.339 | |
| GP24 (A3G3S2) | Control | H = 4.569; df = 2; p = 0.102 | U = 29,452.5; p = 0.046 | H = 6.657; df = 2; p = 0.036 | U = 26,502.0; p = 0.008 | H = 7.072; df = 3; p = 0.070 |
| PTSD | H = 6.779; df = 2; p = 0.034 | U = 36,322.5; p = 0.019 | H = 3.704; df = 2; p = 0.157 | U = 36,850.5; p = 0.296 | H = 11.942; df = 3; p = 0.008 | |
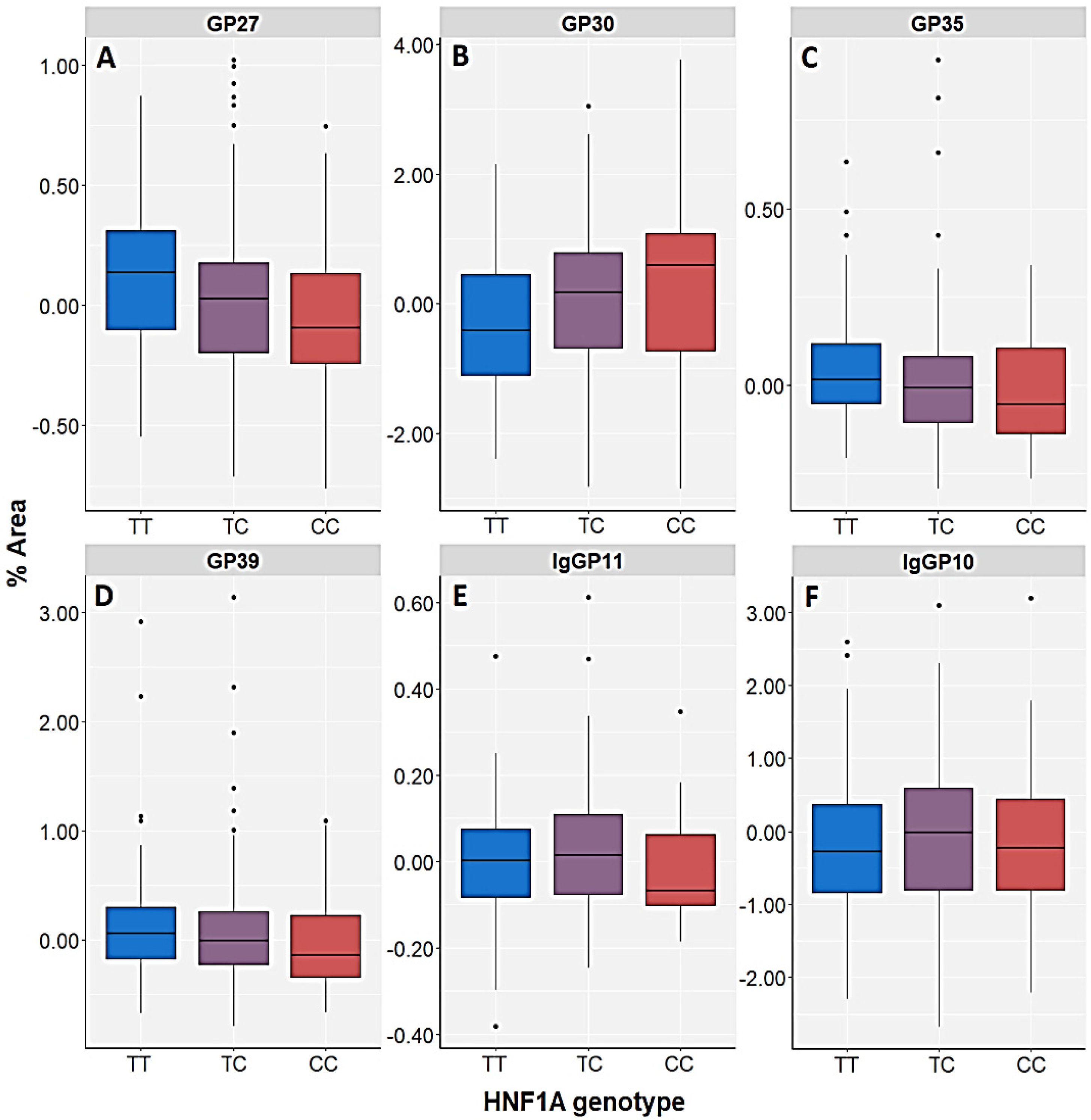
| GP27 (A3G3S3) | Control | H = 3.248; df = 2; p = 0.197 | U = 29,824.5; p = 0.077 | H = 4.628; df = 2; p = 0.099 | U = 27,360.0; p = 0.033 | H = 5.798; df = 3; p = 0.122 |
| PTSD | H = 12.838; df = 2; p = 0.002 | U = 34,190.5; p = 0.001 | H = 12.259; df = 2; p = 0.002 | U = 32,402.5; p = 0.001 | H = 13.673; df = 3; p = 0.003 | |
| GP28 (A3G3S3) | Control | H = 3.105; df = 2; p = 0.212 | U = 30,204.5; p = 0.122 | H = 6.085; df = 2; p = 0.048 | U = 26,972.0; p = 0.018 | H = 5.716; df = 3; p = 0.126 |
| PTSD | H = 12.309; df = 2; p = 0.002 | U = 34,460.5; p = 0.001 | H = 7.080; df = 2; p = 0.029 | U = 34,596.5; p = 0.028 | H = 12.275; df = 3; p = 0.006 | |
| GP30 (A3G3S3) | Control | H = 3.247; df = 2; p = 0.197 | U = 30,194.5; p = 0.121 | H = 3.798; df = 2; p = 0.150 | U = 27,584.0; p = 0.046 | H = 4.169; df = 3; p = 0.244 |
| PTSD | H = 14.029; df = 2; p = 0.001 | U = 34,054.5; p = 0.001 | H = 9.678; df = 2; p = 0.008 | U = 33,452.5; p = 0.006 | H = 16.311; df = 3; p = 0.001 | |
| GP33 (A4G4S3) | Control | H = 3.702; df = 2; p = 0.157 | U = 29,734.5; p = 0.068 | H = 6.904; df = 2; p = 0.032 | U = 26,502.0; p = 0.008 | H = 8.216; df = 3; p = 0.042 |
| PTSD | H = 8.399; df = 2; p = 0.015 | U = 35,444.5; p = 0.005 | H = 8.925; df = 2; p = 0.012 | U = 33,438.5; p = 0.005 | H = 9.770; df = 3; p = 0.021 | |
| GP35 (A4F1G3S3) | Control | H = 2.617; df = 2; p = 0.270 | U = 30,150.5; p = 0.115 | H = 7.175; df = 2; p = 0.028 | U = 26,310.0; p = 0.006 | H = 8.900; df = 3; p = 0.031 |
| PTSD | H = 6.810; df = 2; p = 0.033 | U = 36,044.5; p = 0.013 | H = 11.234; df = 2; p = 0.004 | U = 33,032.5; p = 0.003 | H = 10.520; df = 3; p = 0.015 | |
| GP37 (A4G4S4) | Control | H = 2.004; df = 2; p = 0.367 | U = 30,952.5; p = 0.271 | H = 5.357; df = 2; p = 0.069 | U = 27,982.0; p = 0.080 | H = 3.184; df = 3; p = 0.364 |
| PTSD | H = 11.885; df = 2; p = 0.003 | U = 34,992.5; p = 0.003 | H = 3.600; df = 2; p = 0.165 | U = 35,862.5; p = 0.122 | H = 10.956; df = 3; p = 0.012 | |
| GP39 (A4F1G4S4) | Control | H = 1.513; df = 2; p = 0.469 | U = 30,694.5; p = 0.210 | H = 3.400; df = 2; p = 0.183 | U = 27,696.0; p = 0.054 | H = 3.873; df = 3; p = 0.276 |
| PTSD | H = 4.786; df = 2; p = 0.091 | U = 36,894.5; p = 0.039 | H = 10.322; df = 2; p = 0.006 | U = 33,270.5; p = 0.004 | H = 9.531; df = 3; p = 0.023 | |
| IgGP10 (FA2[6]BG1) | Control | H = 1.815; df = 2; p = 0.403 | U = 32,528.5; p = 0.871 | H = 10.827; df = 2; p =0.004 | U = 28,494.0; p = 0.151 | H = 5.306; df = 3; p = 0.151 |
| PTSD | H = 10.327; df = 2; p = 0.006 | U = 40,734.5; p = 0.871 | H = 5.342; df = 2; p = 0.069 | U = 36,724.5; p = 0.267 | H = 3.074; df = 3; p = 0.380 | |
| IgGP11 (FA2[3]BG1) | Control | H = 3.903; df = 2; p = 0.142 | U = 32,194.5; p = 0.718 | H = 5.492; df = 2; p = 0.064 | U = 29,614.0; p = 0.455 | H = 3.665; df = 3; p = 0.300 |
| PTSD | H = 5.028; df = 2; p = 0.081 | U = 39,974.5; p = 0.590 | H = 12.236; df = 2; p = 0.002 | U = 36,528.5; p = 0.227 | H = 2.229; df = 3; p = 0.526 | |
| IgGP15 (FA2BG2) | Control | H = 1.505; df = 2; p = 0.471 | U = 30,952.5; p = 0.271 | H = 3.232; df = 2; p = 0.199 | U = 28,584.0; p = 0.168 | H = 2.695; df = 3; p = 0.441 |
| PTSD | H = 11.692; df = 2; p = 0.003 | U = 39,186.5; p = 0.353 | H = 5.153; df = 2; p = 0.076 | U = 38,668.5; p = 0.905 | H = 2.187; df = 3; p = 0.535 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudor, L.; Konjevod, M.; Nedic Erjavec, G.; Nikolac Perkovic, M.; Uzun, S.; Kozumplik, O.; Zoldos, V.; Lauc, G.; Svob Strac, D.; Pivac, N. Genetic and Epigenetic Association of Hepatocyte Nuclear Factor-1α with Glycosylation in Post-Traumatic Stress Disorder. Genes 2022, 13, 1063. https://doi.org/10.3390/genes13061063
Tudor L, Konjevod M, Nedic Erjavec G, Nikolac Perkovic M, Uzun S, Kozumplik O, Zoldos V, Lauc G, Svob Strac D, Pivac N. Genetic and Epigenetic Association of Hepatocyte Nuclear Factor-1α with Glycosylation in Post-Traumatic Stress Disorder. Genes. 2022; 13(6):1063. https://doi.org/10.3390/genes13061063
Chicago/Turabian StyleTudor, Lucija, Marcela Konjevod, Gordana Nedic Erjavec, Matea Nikolac Perkovic, Suzana Uzun, Oliver Kozumplik, Vlatka Zoldos, Gordan Lauc, Dubravka Svob Strac, and Nela Pivac. 2022. "Genetic and Epigenetic Association of Hepatocyte Nuclear Factor-1α with Glycosylation in Post-Traumatic Stress Disorder" Genes 13, no. 6: 1063. https://doi.org/10.3390/genes13061063
APA StyleTudor, L., Konjevod, M., Nedic Erjavec, G., Nikolac Perkovic, M., Uzun, S., Kozumplik, O., Zoldos, V., Lauc, G., Svob Strac, D., & Pivac, N. (2022). Genetic and Epigenetic Association of Hepatocyte Nuclear Factor-1α with Glycosylation in Post-Traumatic Stress Disorder. Genes, 13(6), 1063. https://doi.org/10.3390/genes13061063







