RETRACTED: RADseq Data Suggest Occasional Hybridization between Microcebus murinus and M. ravelobensis in Northwestern Madagascar
Abstract
:1. Introduction
2. Materials and Methods
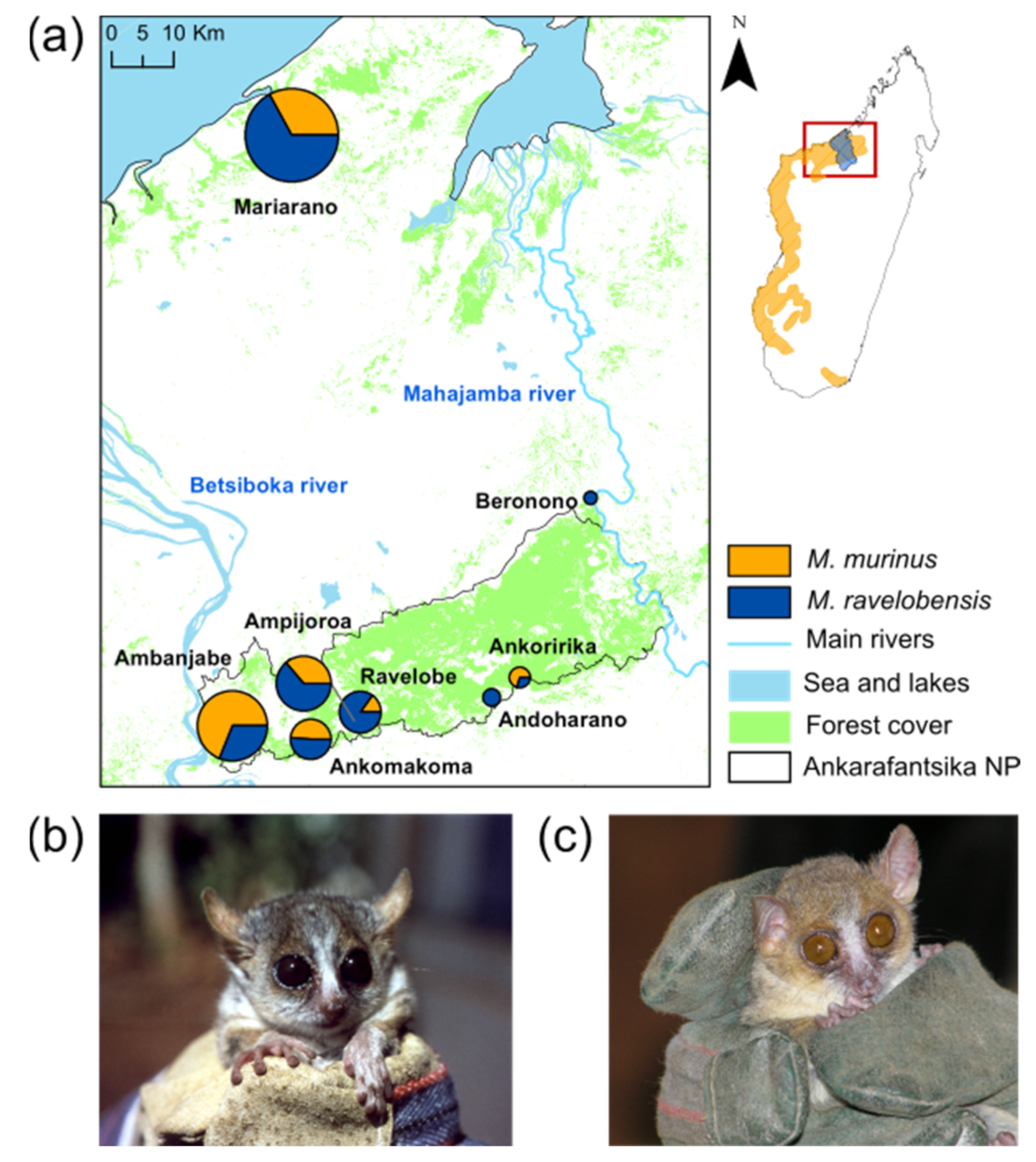
2.1. Study Area and Sample Collection
2.2. DNA Extraction, RAD Sequencing, and Genotyping
2.3. Hybrid Identification
2.4. Test for Introgression between M. murinus and M. ravelobensis
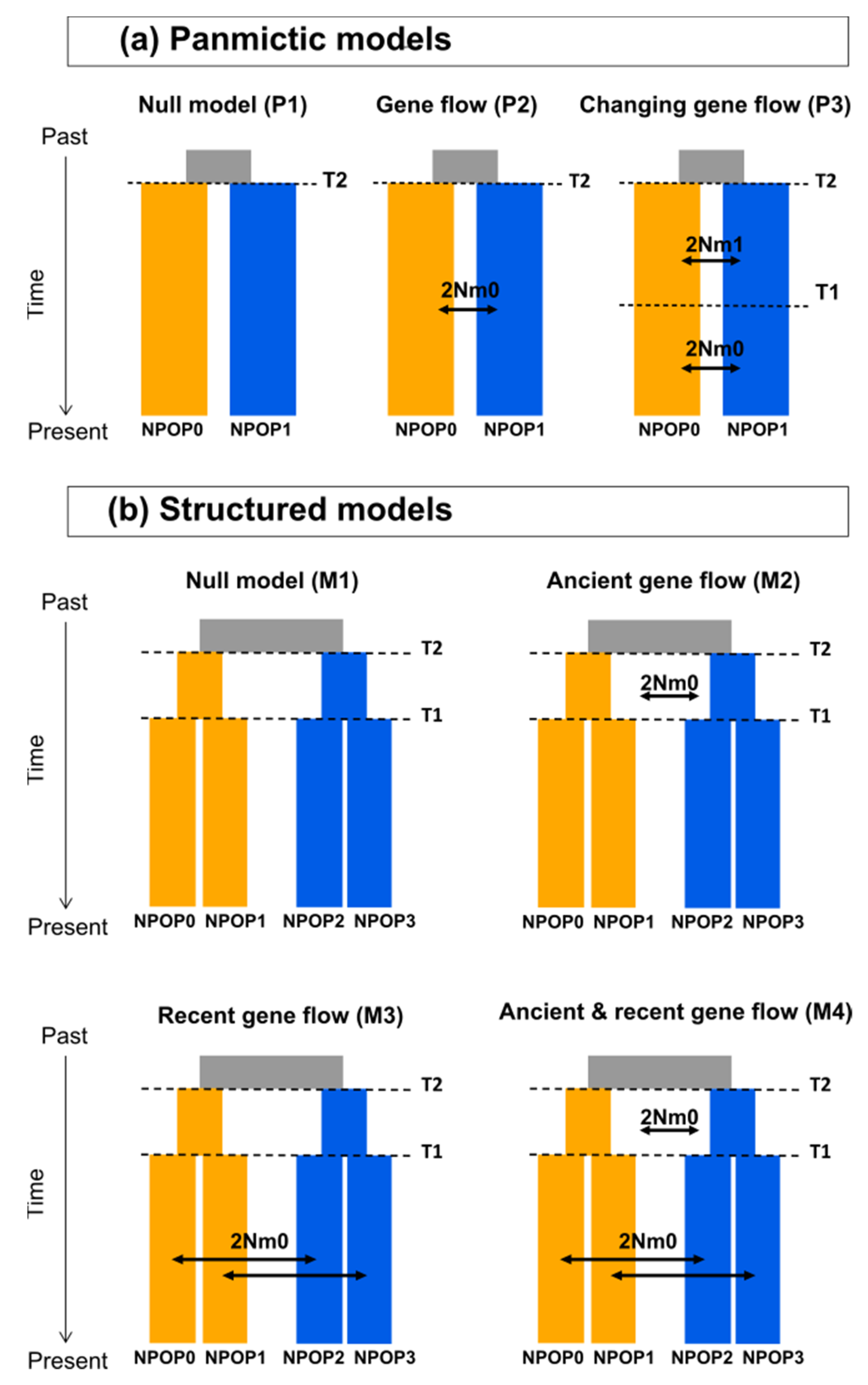
2.5. Demographic Modelling with Fastsimcoal2
3. Results
3.1. RADseq Data Statistics
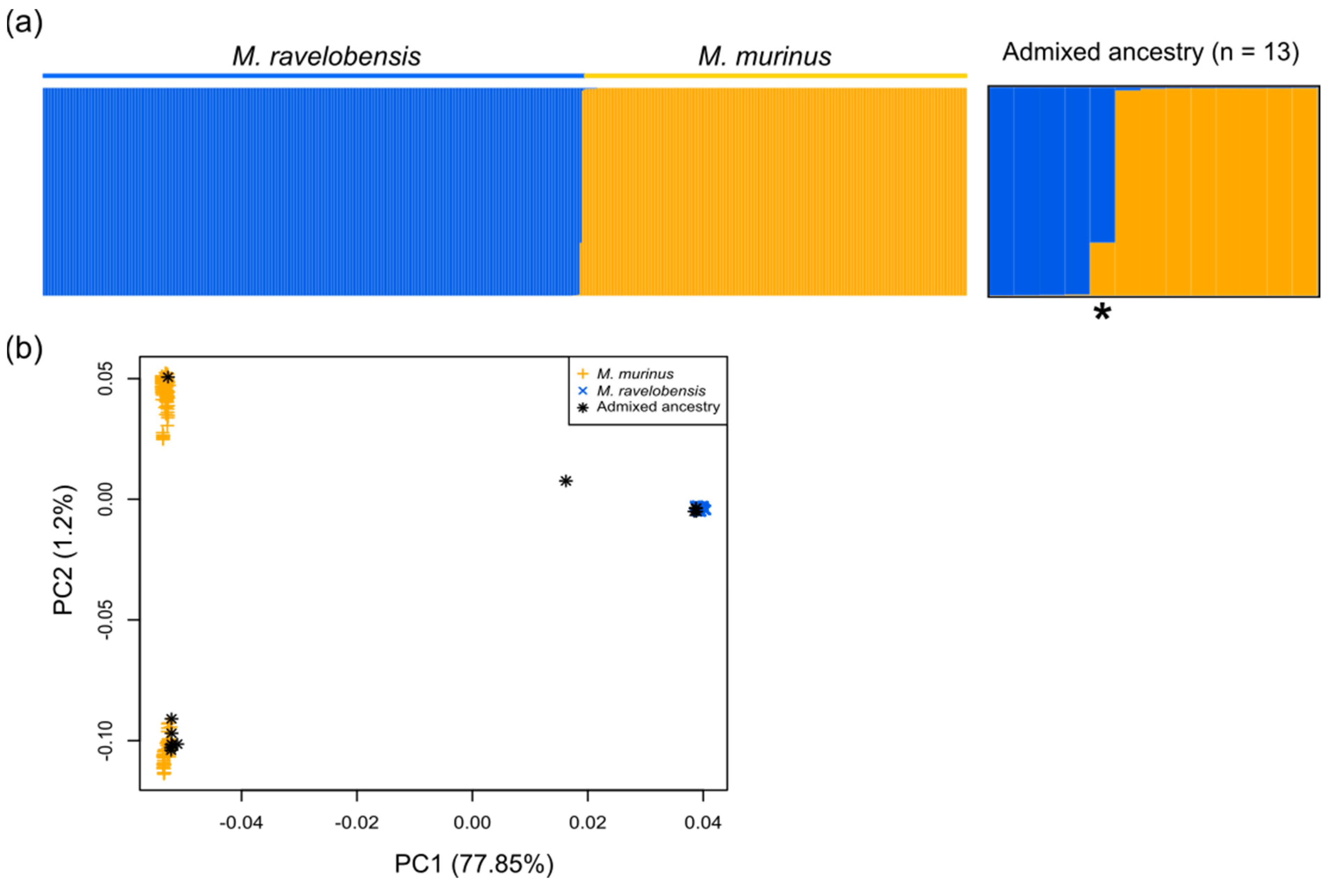
3.2. Identification of Individuals with Admixed Ancestry
3.3. Test for Introgression between M. murinus and M. ravelobensis
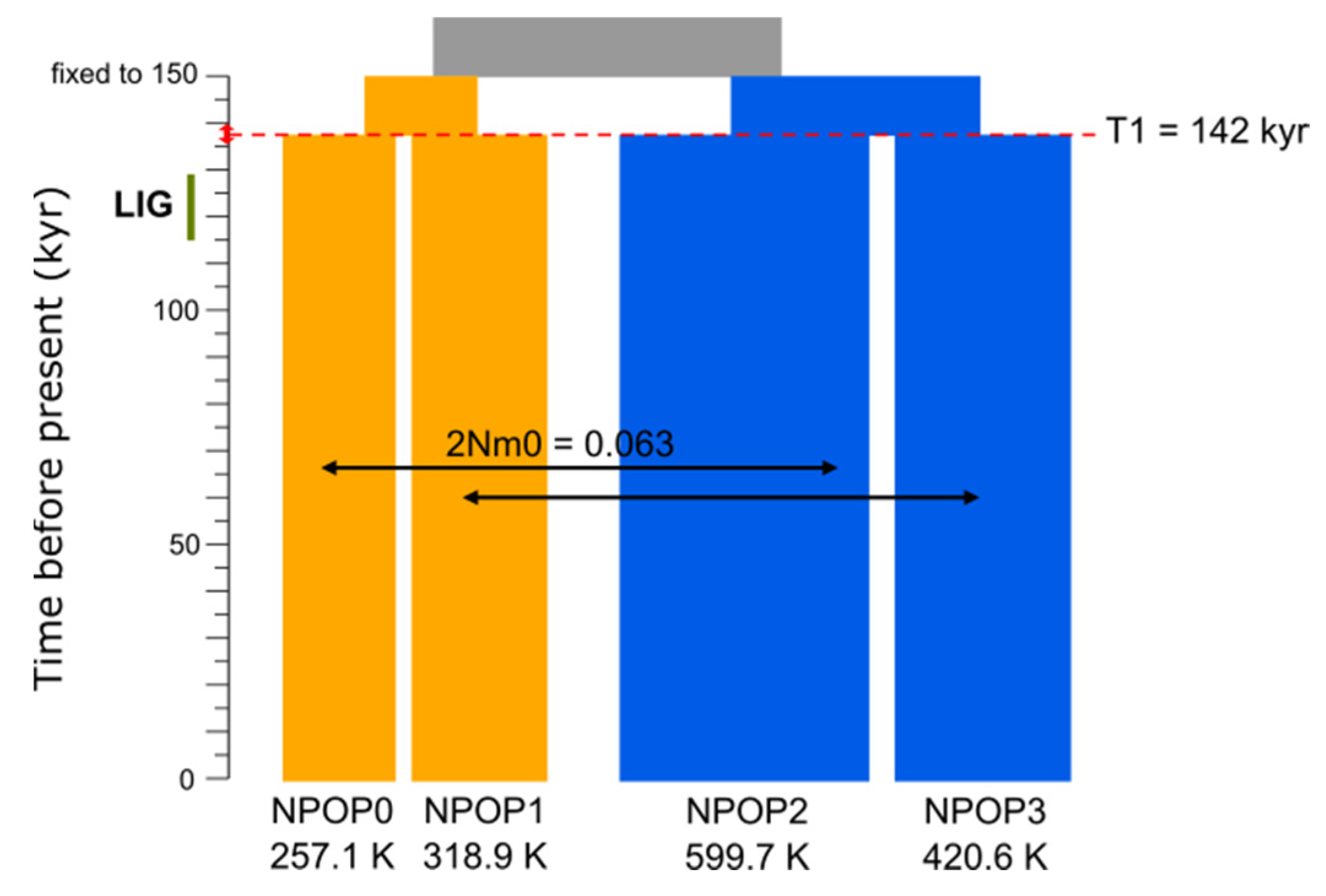
3.4. Demographic Modelling with Fastsimcoal2
4. Discussion
4.1. Occasional Hybridization between M. murinus and M. ravelobensis
4.2. Hybridization between M. murinus and M. ravelobensis: A Recent Event
4.3. Under Which Circumstances May Hybridization Occur?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adavoudi, R.; Pilot, M. Consequences of Hybridization in Mammals. Genes 2022, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Zinner, D.; Arnold, M.L.; Roos, C. The Strange Blood: Natural Hybridization in Primates. Evol. Anthropol. 2011, 20, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.; Godinho, R.; Randi, E.; Ferrand, N.; Alves, P.C. Molecular Analysis of Hybridisation between Wild and Domestic Cats (Felis Silvestris) in Portugal: Implications for Conservation. Conserv. Genet. 2008, 9, 1–11. [Google Scholar] [CrossRef]
- Zinner, D.; Groeneveld, L.F.; Keller, C.; Roos, C. Mitochondrial Phylogeography of Baboons (Papio spp.): Indication for Introgressive Hybridization? BMC Evol. Biol. 2009, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Godinho, R. Real-Time Assessment of Hybridization between Wolves and Dogs: Combining Noninvasive Samples with Ancestry Informative Markers. Mol. Ecol. Resour. 2015, 15, 317–328. [Google Scholar] [CrossRef]
- Combosch, D.J.; Vollmer, S.V. Trans-Pacific RAD-Seq Population Genomics Confirms Introgressive Hybridization in Eastern Pacific Pocillopora Corals. Mol. Phylogenet. Evol. 2015, 88, 154–162. [Google Scholar] [CrossRef]
- Meier, J.I.; Sousa, V.C.; Marques, D.A.; Selz, O.M.; Wagner, C.E.; Excoffier, L.; Seehausen, O. Demographic Modelling with Whole-Genome Data Reveals Parallel Origin of Similar Pundamilia Cichlid Species after Hybridization. Mol. Ecol. 2017, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Pöschel, J.; Heltai, B.; Graciá, E.; Quintana, M.F.; Velo-antón, G.; Arribas, O.; Valdeón, A.; Wink, M.; Fritz, U.; Vamberger, M. Complex Hybridization Patterns in European Pond Turtles (Emys Orbicularis) in the Pyrenean Region. Sci. Rep. 2018, 8, 15925. [Google Scholar] [CrossRef]
- Knipler, M.L.; Dowton, M.; Mikac, K.M. Genome-Wide SNPs Detect Hybridisation of Marsupial Gliders (Petaurus Breviceps Breviceps × Petaurus Norfolcensis) in the Wild. Genes 2021, 12, 1327. [Google Scholar] [CrossRef]
- Kays, R.; Curtis, A.; Kirchman, J.J. Rapid Adaptive Evolution of Northeastern Coyotes via Hybridization with Wolves. Biol. Lett. 2009, 6, 89–93. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Luikart, G.; Aitken, S.N. Conservation and the Genetics of Populations, 2nd ed.; USA, Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Quilodrán, C.S.; Nussberger, B.; Montoya-Burgos, J.I.; Currat, M. Hybridization and Introgression during Density-Dependent Range Expansion: European Wildcats as a Case Study. Evolution 2019, 73, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Rohfritsch, A.; Borsa, P. Genetic Structure of Indian Scad Mackerel Decapterus Russelli: Pleistocene Vicariance and Secondary Contact in the Central Indo-West Pacific Seas. Heredity 2005, 95, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Veríssimo, J.; Znari, M.; Stuckas, H.; Fritz, U.; Pereira, P.; Teixeira, J.; Arculeo, M.; Marrone, F.; Sacco, F.; Naimi, M.; et al. Pleistocene Diversification in Morocco and Recent Demographic Expansion in the Mediterranean Pond Turtle Mauremys Leprosa. Biol. J. Linn. Soc. 2016, 119, 943–959. [Google Scholar] [CrossRef]
- Harrington, S.M.; Hollingsworth, B.D.; Higham, T.E.; Reeder, T.W. Pleistocene Climatic Fluctuations Drive Isolation and Secondary Contact in the Red Diamond Rattlesnake (Crotalus Ruber) in Baja California. J. Biogeogr. 2018, 45, 64–75. [Google Scholar] [CrossRef]
- Dilyté, J.; Sabatino, S.; Godinho, R.; Brito, J.C. Diversification and Gene Flow of Tilapia Species Driven by Ecological Changes in Lowland and Mountain Areas of Southern Mauritania. Evol. Ecol. 2020, 34, 133–146. [Google Scholar] [CrossRef]
- Poux, C.; Madsen, O.; Marquard, E.; Vieites, D.R.; de Jong, W.W.; Vences, M. Asynchronous Colonization of Madagascar by the Four Endemic Clades of Primates, Tenrecs, Carnivores, and Rodents as Inferred from Nuclear Genes. Syst. Biol. 2005, 54, 719–730. [Google Scholar] [CrossRef]
- MacPhee, R.D.E.; Burney, D.A. Dating of Modified Femora of Extinct Dwarf Hippopotamus from Southern Madagascar: Implications for Constraining Human Colonization and Vertebrate Extinction Events. J. Archaeol. Sci. 1991, 18, 695–706. [Google Scholar] [CrossRef]
- Dewar, R.E.; Radimilahy, C.; Wright, H.T.; Jacobs, Z.; Kelly, G.O.; Berna, F. Stone Tools and Foraging in Northern Madagascar Challenge Holocene Extinction Models. Proc. Natl. Acad. Sci. USA USA 2013, 110, 12583–12588. [Google Scholar] [CrossRef]
- Burney, D.A.; Robinson, G.S.; Burney, L.P. Sporormiella and the Late Holocene Extinctions in Madagascar. Proc. Natl. Acad. Sci. 2003, 100, 10800–10805. [Google Scholar] [CrossRef]
- Perez, V.R.; Godfrey, L.R.; Nowak-kemp, M.; Burney, D.A.; Ratsimbazafy, J.; Vasey, N. Evidence of Early Butchery of Giant Lemurs in Madagascar. J. Hum. Evol. 2005, 2005, 722–742. [Google Scholar] [CrossRef]
- Douglass, K.; Hixon, S.; Wright, H.T.; Godfrey, L.R.; Crowley, B.E. A Critical Review of Radiocarbon Dates Clarifies the Human Settlement of Madagascar. Quat. Sci. Rev. 2019, 221. [Google Scholar] [CrossRef]
- Wilmé, L.; Goodman, S.M.; Ganzhorn, J.U. Biogeographic Evolution of Madagascar’s Microendemic Biota. Science 2006, 312, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.; Montade, V.; Salmona, J.; Metzger, J.; Bremond, L.; Kasper, T.; Daut, G.; Rouland, S.; Ranarilalatiana, S.; Rakotondravony, R.; et al. Past Environmental Changes Affected Lemur Population Dynamics Prior to Human Impact in Madagascar. Commun. Biol. 2021, 4, 1084. [Google Scholar] [CrossRef] [PubMed]
- Rabarivola, C.; Meyers, D.; Rumpler, Y. Distribution and Morphological Characters of Intermediate Forms Between the Black Lemur (Eulemur macaco macaco) and the Sclater ’s Lemur (E. m. flavifrons). Primates 1991, 32, 269–273. [Google Scholar] [CrossRef]
- Wyner, Y.M.; Johnson, S.E.; Stumpf, R.M. Genetic Assessment of a White-Collared Red-Fronted Lemur Hybrid Zone at Andringitra, Madagascar. Am. J. Primatol. 2002, 66, 51–66. [Google Scholar] [CrossRef]
- Pastorini, J.; Zaramody, A.; Curtis, D.J.; Nievergelt, C.M.; Mundy, N.I. Genetic Analysis of Hybridization and Introgression between Wild Mongoose and Brown Lemurs. BMC Evol. Biol. 2009, 13, 32. [Google Scholar] [CrossRef]
- Vasey, N.; Tattersall, I.A.N. Do Ruffed Lemurs Form a Hybrid Zone? Distribution and Discovery of Varecia, with Systematic and Conservation Implications. Am. Mus. Novitates 2002, 3376, 1–26. [Google Scholar] [CrossRef]
- Fausser, J.-L.; Prosper, P.; Donati, G.; Ramanamanjato, J.-B.; Rumpler, Y. Phylogenetic Relationships between Hapalemur Species and Subspecies Based on Mitochondrial DNA Sequences. BMC Evol. Biol. 2002, 2, 4. [Google Scholar] [CrossRef]
- Williams, R.C.; Blanco, M.B.; Poelstra, J.W.; Hunnicutt, K.E.; Comeault, A.A.; Yoder, A.D. Conservation Genomic Analysis Reveals Ancient Introgression and Declining Levels of Genetic Diversity in Madagascar’s Hibernating Dwarf Lemurs. Heredity 2020, 124, 236–251. [Google Scholar] [CrossRef]
- Hotaling, S.; Foley, M.E.; Lawrence, N.M.; Bocanegra, J.; Blanco, M.B.; Rasoloarison, R.; Kappeler, P.M.; Barrett, M.A.; Yoder, A.D.; Weisrock, D.W. Species Discovery and Validation in a Cryptic Radiation of Endangered Primates: Coalescent-Based Species Delimitation in Madagascar’s Mouse Lemurs. Mol. Ecol. 2016, 25, 2029–2045. [Google Scholar] [CrossRef]
- Poelstra, J.W.; Salmona, J.; Tiley, G.P.; Schüßler, D.; Blanco, M.B.; Andriambeloson, J.B.; Bouchez, O.; Campbell, C.R.; Etter, P.D.; Hohenlohe, P.A.; et al. Cryptic Patterns of Speciation in Cryptic Primates: Microendemic Mouse Lemurs and the Multispecies Coalescent. Syst. Biol. 2021, 70, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Schüßler, D.; Blanco, M.B.; Salmona, J.; Poelstra, J.; Andriambeloson, J.B.; Miller, A.; Randrianambinina, B.; Rasolofoson, D.W.; Mantilla-Contreras, J.; Chikhi, L.; et al. Ecology and Morphology of Mouse Lemurs (Microcebus spp.) in a Hotspot of Microendemism in Northeastern Madagascar, with the Description of a New Species. Am. J. Primatol. 2020, 82, e23180. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, R.A.; Louis, E.E.J.; Richardson, M.; Schwitzer, C.; Langrand, O.; Rylands, A.B. Lemurs of Madagascar; Conservation International: Crystal City, VA, USA, 2010. [Google Scholar]
- Radespiel, U. Can Behavioral Ecology Help to Understand the Divergent Geographic Range Sizes of Mouse Lemurs. In Gremlins of the Night: Biology, Behaviorm and Conservation Biogeography of the Cheirogaleidae; Lehman, S.M., Radespiel, U., Zimmermann, E., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 498–519. [Google Scholar]
- Sgarlata, G.M.; Salmona, J.; Le Pors, B.; Rasolondraibe, E.; Jan, F.; Ralantoharijaona, T.; Rakotonanahary, A.; Randriamaroson, J.; Marques, A.J.; Aleixo-Pais, I.; et al. Genetic and Morphological Diversity of Mouse Lemurs (Microcebus spp.) in Northern Madagascar: The Discovery of a Putative New Species? Am. J. Primatol. 2019, 81, e23070. [Google Scholar] [CrossRef] [PubMed]
- Gligor, M.; Ganzhorn, J.U.; Rakotondravony, D.; Ramilijaona, O.R.; Razafimahatratra, E.; Zischler, H.; Hapke, A. Hybridization between Mouse Lemurs in an Ecological Transition Zone in Southern Madagascar. Mol. Ecol. 2009, 18, 520–533. [Google Scholar] [CrossRef]
- Hapke, A.; Gligor, M.; Rakotondranary, J.; Rosenkranz, D.; Zupke, O. Hybridization of Mouse Lemurs: Different Patterns under Different Ecological Conditions. BMC Evol. Biol. 2011, 11, e297. [Google Scholar] [CrossRef] [PubMed]
- Poelstra, J.W.; Montero, B.K.; Lüdemann, J.; Yang, Z.; Rakotondranary, S.J.; Hohenlohe, P.; Stetter, N.; Ganzhorn, J.U.; Yoder, A.D. RADseq Data Reveal a Lack of Admixture in a Mouse Lemur Contact Zone Contrary to Previous Microsatellite Results. bioRxiv 2021. [Google Scholar] [CrossRef]
- Blair, C.; Heckman, K.L.; Russell, A.L.; Yoder, A.D. Multilocus Coalescent Analyses Reveal the Demographic History and Speciation Patterns of Mouse Lemur Sister Species. BMC Evol. Biol. 2014, 14, 57. [Google Scholar] [CrossRef]
- Schneider, N.; Chikhi, L.; Currat, M.; Radespiel, U. Signals of Recent Spatial Expansions in the Grey Mouse Lemur (Microcebus murinus). BMC Evol. Biol. 2010, 10, 105. [Google Scholar] [CrossRef]
- Teixeira, H.; Salmona, J.; Arredondo, A.; Mourato, B.; Manzi, S.; Rakotondravony, R.; Mazet, O.; Chikhi, L.; Metzger, J.; Radespiel, U. Impact of Model Assumptions on Demographic Inferences – the Study Case of Two Sympatric Mouse Lemurs in Northwestern Madagascar. BMC Ecol. Evol. 2021, 21, 197. [Google Scholar] [CrossRef]
- Zimmermann, E.; Cepok, S.; Rakotoarison, N.; Zietemann, V.; Radespiel, U. Sympatric Mouse Lemurs in North-West Madagascar: A New Rufous Mouse Lemur Species (Microcebus ravelobensis). Folia Primatol. 1998, 69, 106–114. [Google Scholar] [CrossRef]
- Olivieri, G.; Zimmermann, E.; Randrianambinina, B.; Rasoloharijaona, S.; Rakotondravony, D.; Guschanski, K.; Radespiel, U. The Ever-Increasing Diversity in Mouse Lemurs: Three New Species in North and Northwestern Madagascar. Mol. Phylogenet. Evol. 2007, 43, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Schüßler, D.; Andriamalala, Y.R.; van der Bach, R.; Katzur, C.; Kolbe, C.; Maheritafika, M.H.R.; Rasolozaka, M.; Razafitsalama, M.; Renz, M.; Steffens, T.S.; et al. Thirty Years of Deforestation within the Entire Ranges of Nine Endangered Lemur Species (3 CR, 4 EN, 2 VU) in Northwestern Madagascar. Ecotropica 2022. under revision. [Google Scholar]
- Twyford, A.D.; Ennos, R.A. Next-Generation Hybridization and Introgression. Heredity 2011, 108, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.W.; Hohenlohe, P.A.; Etter, P.D.; Boone, J.Q.; Catchen, J.M.; Blaxter, M.L. Genome-Wide Genetic Marker Discovery and Genotyping Using next-Generation Sequencing. Nat. Rev. Genet. 2011, 12, 499–510. [Google Scholar] [CrossRef]
- Davey, J.L.; Blaxter, M.W. RADseq: Next-Generation Population Genetics. Brief. Funct. Genomics 2010, 9, 416–423. [Google Scholar] [CrossRef]
- Andriatsitohaina, B.; Ramsay, M.S.; Kiene, F.; Lehman, S.M.; Rasoloharijaona, S.; Rakotondravony, R.; Radespiel, U. Ecological Fragmentation Effects in Mouse Lemurs and Small Mammals in Northwestern Madagascar. Am. J. Primatol. 2020, 82, e23059. [Google Scholar] [CrossRef]
- Rina Evasoa, M.; Zimmermann, E.; Hasiniaina, A.F.; Rasoloharijaona, S.; Randrianambinina, B.; Radespiel, U. Sources of Variation in Social Tolerance in Mouse Lemurs (Microcebus spp.). BMC Ecol. 2019, 19, 20. [Google Scholar] [CrossRef]
- Seutin, G.; White, B.N.; Boag, P.T. Preservation of Avian Blood and Tissue Samples for DNA Analyses. Can. J. Zool. 1991, 69, 82–90. [Google Scholar] [CrossRef]
- Aleixo-Pais, I.; Salmona, J.; Sgarlata, G.M.; Rakotonanahary, A.; Sousa, A.P.; Parreira, B.; Kun-Rodrigues, C.; Ralantoharijaona, T.; Jan, F.; Rasolondraibe, E.; et al. The Genetic Structure of a Mouse Lemur Living in a Fragmented Habitat in Northern Madagascar. Conserv. Genet. 2019, 20, 229–243. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Korneliussen, T.S.; Albrechtsen, A.; Nielsen, R. Open Access ANGSD: Analysis of Next Generation Sequencing Data. BMC Bioinform. 2014, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Metzker, M.L. Sequencing Technologies—the next Generation. Nat. Rev. Genet. 2010, 11, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Skotte, L.; Korneliussen, T.S.; Albrechtsen, A. Estimating Individual Admixture Proportions from next Generation Sequencing Data. Genetics 2013, 195, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Korneliussen, T.S.; Moltke, I. Sequence Analysis NgsRelate: A Software Tool for Estimating Pairwise Relatedness from next-Generation Sequencing Data. Bioinformatics 2015, 31, 4009–4011. [Google Scholar] [CrossRef]
- Rochette, N.C.; Rivera-Colón, A.G.; Catchen, J.M. Stacks 2: Analytical Methods for Paired-end Sequencing Improve RADseq-based Population Genomics. Mol. Ecol. 2019, 28, 4737–4754. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing next-Generation DNA Sequencing Data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The Variant Call Format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Meisner, J.; Albrechtsen, A. Inferring Population Structure and Admixture Proportions in Low-Depth NGS Data. Genetics 2018, 210, 719–731. [Google Scholar] [CrossRef]
- Green, R.E.; Krause, J.; Briggs, A.W.; Maricic, T.; Stenzel, U.; Kircher, M.; Patterson, N.; Li, H.; Zhai, W.; Fritz, M.H.Y.; et al. A Draft Sequence of the Neandertal Genome. Science 2010, 328, 710–722. [Google Scholar] [CrossRef]
- Durand, E.Y.; Patterson, N.; Reich, D.; Slatkin, M. Testing for Ancient Admixture between Closely Related Populations. Mol. Biol. Evol. 2011, 28, 2239–2252. [Google Scholar] [CrossRef]
- Malinsky, M.; Matschiner, M.; Svardal, H. Dsuite - Fast D-statistics and Related Admixture Evidence from VCF Files. Mol. Ecol. Resour. 2020, 21, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Dupanloup, I.; Huerta-Sánchez, E.; Sousa, V.C.; Foll, M. Robust Demographic Inference from Genomic and SNP Data. PLoS Genet. 2013, 9, e1003905. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E.L. Arlequin Suite Ver 3.5: A New Series of Programs to Perform Population Genetics Analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Gutenkunst, R.N.; Hernandez, R.D.; Williamson, S.H.; Bustamante, C.D. Inferring the Joint Demographic History of Multiple Populations from Multidimensional SNP Frequency Data. PLoS Genet. 2009, 5, e1000695. [Google Scholar] [CrossRef] [PubMed]
- Yoder, A.D.; Campbell, C.R.; Blanco, M.B.; Ganzhorn, J.U.; Goodman, S.M. Geogenetic Patterns in Mouse Lemurs (Genus Microcebus) Reveal the Ghosts of Madagascar’s Forests Past. Proc. Natl. Acad. Sci. USA 2016, 113, 8049–8056. [Google Scholar] [CrossRef]
- Campbell, C.R.; Tiley, G.P.; Poelstra, J.W.; Hunnicutt, K.E.; Larsen, P.A.; Lee, H.-J.; Thorne, J.L.; Dos Reis, M.; Yoder, A.D. Pedigree-Based and Phylogenetic Methods Support Surprising Patterns of Mutation Rate and Spectrum in the Gray Mouse Lemur. Heredity 2021, 127, 233–244. [Google Scholar] [CrossRef]
- Radespiel, U.; Lutermann, H.; Schmelting, B.; Zimmermann, E. An Empirical Estimate of the Generation Time of Mouse Lemurs. Am. J. Primatol. 2019, 81, e23062. [Google Scholar] [CrossRef]
- Rakotondravony, R.; Radespiel, U.T.E. Varying Patterns of Coexistence of Two Mouse Lemur Species (Microcebus ravelobensis and M. murinus) in a Heterogeneous Landscape. Am. J. Primatoll 2009, 938, 928–938. [Google Scholar] [CrossRef]
- Rendigs, A.; Radespiel, U.; Wrogemann, D.; Zimmermann, E. Relationship between Microhabitat Structure and Distribution of Mouse Lemurs (Microcebus spp.) in Northwestern Madagascar. Int. J. Primatol. 2003, 24, 47–64. [Google Scholar] [CrossRef]
- Radespiel, U.; Rakotondravony, R.; Rasoloharijaona, S.; Randrianambinina, B. A 24-Year Record of Female Reproductive Dynamics in Two Sympatric Mouse Lemur Species in Northwestern Madagascar. Int. J. Primatol. 2021, 1–25. [Google Scholar] [CrossRef]
- Rina Evasoa, M.; Radespiel, U.; Hasiniaina, A.F.; Rasoloharijaona, S.; Randrianambinina, B.; Rakotondravony, R.; Zimmermann, E. Variation in Reproduction of the Smallest-Bodied Primate Radiation, the Mouse Lemurs (Microcebus spp.): A Synopsis. Am. J. Primatol. 2018, 80, e22874. [Google Scholar] [CrossRef] [PubMed]
- Schmelting, B. Reproduction of Two Sympatric Mouse Lemur Species (Microcebus murinus and M. ravelobensis) in Northwest Madagascar: First Results of a Long Term Study. In Diversité et Endémisme à Madagascar; Lourenço, W.R., Goodman, S.M., Eds.; Societé de Biogeographie: Paris, France, 2000; pp. 165–175. [Google Scholar]
- Braune, P.; Schmidt, S.; Zimmermann, E. Acoustic Divergence in the Communication of Cryptic Species of Nocturnal Primates (Microcebus ssp.). BMC Biol. 2008, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Kollikowski, A.; Zimmermann, E.; Radespiel, U. First Experimental Evidence for Olfactory Species Discrimination in Two Nocturnal Primate Species (Microcebus lehilahytsara and M. murinus). Sci. Rep. 2019, 9, 20386. [Google Scholar] [CrossRef] [PubMed]
- Kollikowski, A.; Jeschke, S.; Radespiel, U. Experimental Evaluation of Spontaneous Olfactory Discrimination in Two Nocturnal Primates (Microcebus murinus and M. lehilahytsara). Chem. Senses 2020, 45, 581–592. [Google Scholar] [CrossRef]
- Yadav, A.; Jain, A.; Sahu, J.; Dubey, A. Interspecies Hybridization in Animals: An Overview. Ann. Anim. Resour. Sci. 2012, 23, 149–163. [Google Scholar] [CrossRef]
- Henkel, H.; Zimmermann, E.; Klein, A.; Randrianambinina, B.; Rasoloharijaona, S.; Rakotondravony, R.; Mester, S.; Radespiel, U. Indications of a Potential Alarming Population Decline in the Golden-Brown Mouse Lemur (Microcebus ravelobensis) in a Long-Term Study Site in the Ankarafantsika National Park. Lemur News 2020, 22, 51–53. [Google Scholar]
| Category | Model | Topology | Log10 (Lhood) | ΔLhood | # Parameters | AIC | ΔAIC/ Category | Rank |
|---|---|---|---|---|---|---|---|---|
| Panmictic models | P1 | Null model | −316,617.1 | 24,660.7 | 3 | 1,458,344.3 | 93,258.4 | 3° |
| P2 | Gene flow | −299,665.5 | 7709.1 | 4 | 1,380,267.3 | 15,181.3 | 2° | |
| P3 | Changing | −296,368.6 | 4412.3 | 6 | 1,365,085.9 | 0.0 | 1° | |
| Structured models | M1 | Null model | −368,272.5 | 25,697.0 | 6 | 1,696,275.0 | 77,170.0 | 4° |
| M2 | Ancient gene flow | −355,664.2 | 13,088.7 | 7 | 1,638,203.4 | 19,098.4 | 3° | |
| M3 | Recent gene flow | −351,517.8 | 8942.3 | 7 | 1,619,105.0 | 0.0 | 1° | |
| M4 | Ancient and recent gene flow | −354,384.8 | 11,809.4 | 7 | 1,632,310.6 | 13,205.6 | 2° | |
| M3 vs. M5 | M3 | Recent gene flow | −351,517.8 | 8942.3 | 7 | 1,619,105.0 | 154.8 | 2° |
| M5 | Recent gene flow and asymmetric structure | −351,483.8 | 8908.3 | 8 | 1,618,950.2 | 0.0 | 1° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, H.; van Elst, T.; Ramsay, M.S.; Rakotondravony, R.; Salmona, J.; Yoder, A.D.; Radespiel, U. RETRACTED: RADseq Data Suggest Occasional Hybridization between Microcebus murinus and M. ravelobensis in Northwestern Madagascar. Genes 2022, 13, 913. https://doi.org/10.3390/genes13050913
Teixeira H, van Elst T, Ramsay MS, Rakotondravony R, Salmona J, Yoder AD, Radespiel U. RETRACTED: RADseq Data Suggest Occasional Hybridization between Microcebus murinus and M. ravelobensis in Northwestern Madagascar. Genes. 2022; 13(5):913. https://doi.org/10.3390/genes13050913
Chicago/Turabian StyleTeixeira, Helena, Tobias van Elst, Malcolm S. Ramsay, Romule Rakotondravony, Jordi Salmona, Anne D. Yoder, and Ute Radespiel. 2022. "RETRACTED: RADseq Data Suggest Occasional Hybridization between Microcebus murinus and M. ravelobensis in Northwestern Madagascar" Genes 13, no. 5: 913. https://doi.org/10.3390/genes13050913
APA StyleTeixeira, H., van Elst, T., Ramsay, M. S., Rakotondravony, R., Salmona, J., Yoder, A. D., & Radespiel, U. (2022). RETRACTED: RADseq Data Suggest Occasional Hybridization between Microcebus murinus and M. ravelobensis in Northwestern Madagascar. Genes, 13(5), 913. https://doi.org/10.3390/genes13050913







