Concussion-Associated Polygenic Profiles of Elite Male Rugby Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. Sample Collection
2.2.2. Genotyping Assays
2.3. Calculation of TGS
2.4. Data Analysis
3. Results
3.1. TGS
3.2. SNP Epistasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCrory, P.; Meeuwisse, W.; Dvorak, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, B.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J.; et al. Consensus Statement on Concussion in Sport—the 5th International Conference on Concussion in Sport Held in Berlin, October 2016. Br. J. Sports Med. 2018, 51, 838–847. [Google Scholar]
- Hind, K.; Konerth, N.; Entwistle, I.; Theadom, A.; Lewis, G.; King, D.; Chazot, P.; Hume, P. Cumulative Sport-Related Injuries and Longer Term Impact in Retired Male Elite- and Amateur-Level Rugby Code Athletes and Non-Contact Athletes: A Retrospective Study. Sport. Med. 2020, 50, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- West, S.W.; Starling, L.; Kemp, S.; Williams, S.; Cross, M.; Taylor, A.; Brooks, J.H.M.; Stokes, K.A. Trends in Match Injury Risk in Professional Male Rugby Union: A 16-Season Review of 10,851 Match Injuries in the English Premiership (2002–2019): The Rofessional Ugby Njury Urveillance Roject. Br. J. Sports Med. 2020, 55, 676–682. [Google Scholar] [CrossRef]
- Gardner, A.; Iverson, G.L.; Levi, C.R.; Schofield, P.W.; Kay-Lambkin, F.; Kohler, R.M.N.; Stanwell, P. A Systematic Review of Concussion in Rugby League. Br. J. Sports Med. 2015, 49, 495–498. [Google Scholar] [CrossRef]
- Tierney, R.; Mansell, J.; Higgins, M.; McDevitt, J.; Toone, N.; Gaughan, J.; Mishra, A.; Krynetskiy, E. Apolipoprotein E Genotype and Concussion in College Athletes. Clin. J. Sport Med. 2010, 20, 464–468. [Google Scholar] [CrossRef]
- Terrell, T.; Bostick, R.; Barth, J.; McKeag, D.; Cantu, R.; Sloane, R.; Galloway, L.; Erlanger, D.; Valentine, V.; Bielak, K. Genetic Polymorphisms, Concussion Risk, and Post Concussion Neurocognitive Deficits in College and High School Athletes. Br. J. Sports Med. 2013, 47, e1. [Google Scholar] [CrossRef]
- Terrell, T.; Bostick, R.; Abramson, R.; Xie, D.; Barfield, W.; Cantu, R.; Stanek, M.; Ewing, T. APOE, APOE Promoter, and Tau Genotypes and Risk for Concussion in College Athletes. Clin. J. Sport Med. 2008, 18, 10–17. [Google Scholar] [CrossRef]
- Cross, M.; Kemp, S.; Smith, A.; Trewartha, G.; Stokes, K. Professional Rugby Union Players Have a 60% Greater Risk of Time Loss Injury after Concussion: A 2-Season Prospective Study of Clinical Outcomes. Br. J. Sports Med. 2016, 50, 926–931. [Google Scholar] [CrossRef]
- Manley, G.; Gardner, A.; Schneider, K.; Guskiewicz, K.; Bailes, J.; Cantu, R.; Castellani, R.; Turner, M.; Jordan, B.; Randolph, C.; et al. A Systematic Review of Potential Long-Term Effects of Sport-Related Concussion. Br. J. Sports Med. 2017, 51, 969–977. [Google Scholar] [CrossRef]
- Cunningham, J.; Broglio, S.; Wilson, F. Influence of Playing Rugby on Long-Term Brain Health Following Retirement: A Systematic Review and Narrative Synthesis. BMJ Open Sport Exerc. Med. 2018, 4, e000356. [Google Scholar] [CrossRef]
- Hume, P.; Theadom, A.; Lewis, G.; Quarrie, K.; Brown, S.; Hill, R.; Marshall, S. A Comparison of Cognitive Function in Former Rugby Union Players Compared with Former Non-Contact-Sport Players and the Impact of Concussion History. Sport. Med. 2017, 47, 1209–1220. [Google Scholar] [CrossRef]
- Gallo, V.; Motley, K.; Kemp, S.P.T.; Mian, S.; Patel, T.; James, L.; Pearce, N.; Mcelvenny, D.; Sciences, H.; Mary, Q. Concussion and Long-Term Cognitive Impairment among Professional or Elite Sport-Persons: A Systematic Review. J. Neurol. Neurosurg. Psychiatry 2020, 91, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.J.; Rist, B.; Fraser, C.L.; Cohen, A.; Maller, J.J. Neurophysiological and Cognitive Impairment Following Repeated Sports Concussion Injuries in Retired Professional Rugby League Players. Brain Inj. 2018, 32, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Antrobus, M.R.; Brazier, J.; Stebbings, G.K.; Day, S.H.; Heffernan, S.M.; Kilduff, L.P.; Erskine, R.M.; Williams, A.G. Genetic Factors That Could Affect Concussion Risk in Elite Rugby. Sports 2021, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Fehily, B.; Fitzgerald, M. Traumatic Brain Injury-Review Repeated Mild Traumatic Brain Injury: Potential Mechanisms of Damage. Cell Transplant. 2017, 26, 1131–1155. [Google Scholar] [CrossRef] [PubMed]
- England Professional Rugby Injury Surveillance Project Steering Group. England Professional Rugby Injury Surveillance Project 2017–2018 Season Report; England Professional Rugby Injury Surveillance Project Steering Group: London, UK, 2019; p. 15. [Google Scholar]
- Cosgrave, M.; Williams, S. The Epidemiology of Concussion in Professional Rugby Union in Ireland. Phys. Ther. Sport 2019, 35, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, J.; Ranson, C.; Oatley, G.; Mostafa, M.; Mathema, P.; Crick, T.; Moore, I.S. On Average, a Professional Rugby Union Player Is More Likely than Not to Sustain a Concussion after 25 Matches. Br. J. Sports Med. 2019, 53, 969–973. [Google Scholar] [CrossRef]
- Panenka, W.J.; Gardner, A.J.; Dretsch, M.N.; Crynen, G.C.; Crawford, F.C.; Iverson, G.L. Systematic Review of Genetic Risk Factors for Sustaining a Mild Traumatic Brain Injury. J. Neurotrauma. 2017, 37, 2093–2099. [Google Scholar] [CrossRef]
- Carmelli, D.; DeCarli, C.; Swan, G.E.; Jack, L.M.; Reed, T.; Wolf, P.A.; Miller, B.L. Evidence For Genetic Variance in White Matter Hyperintensity Volume in Normal Elderly Male Twins. Stroke 1998, 29, 1177–1181. [Google Scholar] [CrossRef]
- Geschwind, D.H.; Miller, B.L.; DeCarli, C.; Carmelli, D. Heritability of Lobar Brain Volumes in Twins Supports Genetic Models of Cerebral Laterality and Handedness. Proc. Natl. Acad. Sci. USA 2002, 99, 3176–3181. [Google Scholar] [CrossRef]
- Carmelli, D.; Swan, G.E.; DeCarli, C.; Reed, T. Quantitative Genetic Modeling of Regional Brain Volumes and Cognitive Performance in Older Male Twins. Biol. Psychol. 2002, 61, 139–155. [Google Scholar] [CrossRef]
- Bartley, A.J.; Jones, D.W.; Weinberger, D.R. Genetic Variability of Human Brain Size and Cortical Gyral Patterns. Brain 1997, 120, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.W.; Comper, P.; Hutchison, M.G.; Sharma, B. The Role of Apolipoprotein E Episilon (ε)-4 Allele on Outcome Following Traumatic Brain Injury: A Systematic Review. Brain Inj. 2015, 29, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Merritt, V.; Arnett, P. Apolipoprotein E (APOE) Ε4 Allele Is Associated with Increased Symptom Reporting Following Sports Concussion. J. Int. Neuropsychol. Soc. 2016, 22, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lendon, C.L.; Harris, J.M.; Pritchard, A.L.; Nicoll, J.A.R.; Teasdale, G.M.; Murray, G. Genetic Variation of the APOE Promoter and Outcome after Head Injury. Neurology 2003, 61, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.S.; Gopinath, S.P.; Valadka, A.B.; Van, M.; Swank, P.R.; Goodman, J.C. Variants of the Endothelial Nitric Oxide Gene and Cerebral Blood Flow after Severe Traumatic Brain Injury. J. Neurotrauma 2011, 28, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.K.; Pronger, A.M.; Ferguson, A.R.; Temkin, N.R.; Sharma, S.; Rosand, J.; Sorani, M.D.; McAllister, T.W.; Barber, J.; Winkler, E.A.; et al. TRACK-TBI Investigators. Association of a Common Genetic Variant within ANKK1 with Six-Month Cognitive Performance after Traumatic Brain Injury. Neurogenetics 2015, 16, 169–180. [Google Scholar] [CrossRef]
- McAllister, T.W.; Flashman, L.A.; Harker Rhodes, C.; Tyler, A.L.; Moore, J.H.; Saykin, A.J.; McDonald, B.C.; Tosteson, T.D.; Tsongalis, G.J. Single Nucleotide Polymorphisms in ANKK1 and the Dopamine D2 Receptor Gene Affect Cognitive Outcome Shortly after Traumatic Brain Injury: A Replication and Extension Study. Brain Inj. 2008, 22, 705–714. [Google Scholar] [CrossRef]
- McAllister, T.W.; Rhodes, C.H.; Flashman, L.A.; McDonald, B.C.; Belloni, D.; Saykin, A.J. Effect of the Dopamine D2 Receptor T Allele on Response Latency after Mild Traumatic Brain Injury. Am. J. Psychiatry 2005, 162, 1749–1751. [Google Scholar] [CrossRef]
- Dretsch, M.N.; Williams, K.; Emmerich, T.; Crynen, G.; Ait-Ghezala, G.; Chaytow, H.; Mathura, V.; Crawford, F.C.; Iverson, G.L. Brain-Derived Neurotropic Factor Polymorphisms, Traumatic Stress, Mild Traumatic Brain Injury, and Combat Exposure Contribute to Postdeployment Traumatic Stress. Brain Behav. 2016, 6, e00392. [Google Scholar] [CrossRef]
- Lipsky, R.H.; Sparling, M.B.; Ryan, L.M.; Xu, K.; Salazar, A.M.; Goldman, D.; Warden, D.L. Association of COMT Val158Met Genotype with Executive Functioning Following Traumatic Brain Injury. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Mc Fie, S.; Abrahams, S.; Patricios, J.; Suter, J.; Posthumus, M.; September, A.V. The Association between COMT Rs4680 and 5-HTTLPR Genotypes and Concussion History in South African Rugby Union Players. J. Sports Sci. 2018, 36, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Antrobus, M.R.; Brazier, J.; Callus, P.; Herbert, A.J.; Stebbings, G.K.; Day, S.H.; Kilduff, L.P.; Bennett, M.A.; Erskine, R.M.; Raleigh, S.M.; et al. Concussion-Associated Gene Variant COMT Rs4680 Is Associated with Elite Rugby Athlete Status. Clin. J. Sport Med. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, S.M.; Kilduff, L.P.; Erskine, R.M.; Day, S.H.; Stebbings, G.K.; Cook, C.J.; Raleigh, S.M.; Bennett, M.A.; Wang, G.; Collins, M.; et al. COL5A1 Gene Variants Previously Associated with Reduced Soft Tissue Injury Risk Are Associated with Elite Athlete Status in Rugby. BMC Genom. 2017, 18 (Suppl. 8), 820. [Google Scholar] [CrossRef]
- Williams, A.G.; Folland, J.P. Similarity of Polygenic Profiles Limits the Potential for Elite Human Physical Performance. J. Physiol. 2008, 586, 113–121. [Google Scholar] [CrossRef]
- Gómez-Gallego, F.; Ruiz, J.R.; Buxens, A.; Altmäe, S.; Artieda, M.; Santiago, C.; González-Freire, M.; Verde, Z.; Arteta, D.; Martínez, A.; et al. Are Elite Endurance Athletes Genetically Predisposed to Lower Disease Risk? Physiol. Genom. 2010, 41, 82–90. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Gómez-Gallego, F.; Santiago, C.; González-Freire, M.; Verde, Z.; Foster, C.; Lucia, A. Is There an Optimum Endurance Polygenic Profile? J. Physiol. 2009, 587, 1527–1534. [Google Scholar] [CrossRef]
- Banting, L.K.; Pushkarev, V.P.; Cieszczyk, P.; Zarebska, A.; Maciejewska-Karlowska, A.; Sawczuk, M.; Leonska-Duniec, A.; Dyatlov, D.A.; Orekhov, E.F.; Degtyarev, A.V.; et al. Elite Athletes’ Genetic Predisposition for Altered Risk of Complex Metabolic Traits. BMC Genom. 2015, 16, 25. [Google Scholar] [CrossRef]
- Eynon, N.; Ruiz, J.R.; Meckel, Y.; Morán, M.; Lucia, A. Mitochondrial Biogenesis Related Endurance Genotype Score and Sports Performance in Athletes. Mitochondrion 2011, 11, 64–69. [Google Scholar] [CrossRef]
- Santiago, C.; Ruiz, J.R.; Muniesa, C.A.; González-Freire, M.; Gómez-Gallego, F.; Lucia, A. Does the Polygenic Profile Determine the Potential for Becoming a World-Class Athlete? Insights from the Sport of Rowing. Scand. J. Med. Sci. Sports 2010, 20, e188–e194. [Google Scholar] [CrossRef]
- Del Coso, J.; Salinero, J.J.; Lara, B.; Gallo-Salazar, C.; Areces, F.; Herrero, D.; Puente, C. Polygenic Profile and Exercise-Induced Muscle Damage by a Competitive Half-Ironman. J. Strength Cond. Res. 2020, 34, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zaken, S.; Meckel, Y.; Lidor, R.; Nemet, D.; Eliakim, A. Genetic Profiles and Prediction of the Success of Young Athletes’ Transition from Middle- to Long-Distance Runs: An Exploratory Study. Pediatr. Exerc. Sci. 2013, 25, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zaken, S.; Meckel, Y.; Nemet, D.; Eliakim, A. Genetic Score of Power-Speed and Endurance Track and Field Athletes. Scand. J. Med. Sci. Sport. 2015, 25, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Drozdovska, S.B.; Dosenko, V.E.; Ahmetov, I.I.; Ilyin, V.N. The Association of Gene Polymorphisms with Athlete Status in Ukrainians. Biol. Sport 2013, 30, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, S.M.; Kilduff, L.P.; Day, S.H.; Pitsiladis, Y.P.; Williams, A.G. Genomics in Rugby Union: A Review and Future Prospects. Eur. J. Sport Sci. 2015, 15, 460–468. [Google Scholar] [CrossRef]
- Heffernan, S.M.; Kilduff, L.P.; Erskine, R.M.; Day, S.H.; McPhee, J.S.; McMahon, G.E.; Stebbings, G.K.; Neale, J.P.H.; Lockey, S.J.; Ribbans, W.J.; et al. Association of ACTN3 R577X but Not ACE I/D Gene Variants with Elite Rugby Union Player Status and Playing Position. Physiol. Genom. 2016, 48, 196–201. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA-J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Heffernan, S.M.; Stebbings, G.K.; Kilduff, L.P.; Erskine, R.M.; Day, S.H.; Morse, C.I.; McPhee, J.S.; Cook, C.J.; Vance, B.; Ribbans, W.J.; et al. Fat Mass and Obesity Associated (FTO) Gene Influences Skeletal Muscle Phenotypes in Non-Resistance Trained Males and Elite Rugby Playing Position. BMC Genet. 2017, 18, 4. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Numberger, J.I. A Rapid Non-Enzymatic Method for the Preparation of HMW DNA from Blood for RFLP Studies. Nucleic Acids Res. 1991, 19, 5444. [Google Scholar] [CrossRef]
- Hixson, J.E.; Vernier, D.T. Restriction Isotyping of Human Apolipoprotein E by Gene Amplification and Cleavage with HhaI. J. Lipid Res. 1990, 31, 545–548. [Google Scholar] [CrossRef]
- Zweig, M.H.; Campbell, G. Receiver-Operating Characteristic (ROC) Plots: A Fundamental Evaluation Tool in Clinical Medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.H.; Gilbert, J.C.; Tsai, C.T.; Chiang, F.T.; Holden, T.; Barney, N.; White, B.C. A Flexible Computational Framework for Detecting, Characterizing, and Interpreting Statistical Patterns of Epistasis in Genetic Studies of Human Disease Susceptibility. J. Theor. Biol. 2006, 241, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Egorova, E.S.; Borisova, A.V.; Mustafina, L.J.; Arkhipova, A.A.; Gabbasov, R.T.; Druzhevskaya, A.M.; Astratenkova, I.V.; Ahmetov, I.I. The Polygenic Profile of Russian Football Players. J. Sports Sci. 2014, 32, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.C.; Baumert, P.; Larruskain, J.; Gil, S.M.; Lekue, J.A.; Rienzi, E.; Moreno, S.; Tannure, M.; Murtagh, C.F.; Ade, J.D.; et al. The Genetic Association with Injury Risk in Male Academy Soccer Players Depends on Maturity Status. Scand. J. Med. Sci. Sports 2021, 31, 338–350. [Google Scholar] [CrossRef]
- Chen, J.; Lipska, B.; Halim, N.; Ma, Q.; Matsumoto, M.; Melhem, S.; Kolachana, B.; Hyde, T.; Herman, M.; Apud, J.; et al. Functional Analysis of Genetic Variation in Catechol-O-Methyltransferase (COMT): Effects on MRNA, Protein, and Enzyme Activity in Postmortem Human Brain. Am. J. Hum. Genet. 2004, 75, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Riba, J.; Krämer, U.; Heldmann, M.; Richter, S.; Münte, T. Dopamine Agonist Increases Risk Taking but Blunts Reward-Related Brain Activity. PLoS ONE 2008, 3, e2479. [Google Scholar] [CrossRef] [PubMed]
- Dalley, J.; Roiser, J. Dopamine, Serotonin and Impulsivity. Neuroscience 2012, 215, 42–58. [Google Scholar] [CrossRef]
- Poorkaj, P.; Bird, T.D.; Wijsman, E.; Nemens, E.; Garruto, R.M.; Anderson, L.; Andreadis, A.; Wiederholt, W.C.; Raskind, M.; Schellenberg, G.D. Tau Is a Candidate Gene for Chromosome 17 Frontotemporal Dementia. Ann. Neurol. 1998, 43, 815–825. [Google Scholar] [CrossRef]
- Xu, L.; Ryu, J.; Nguyen, J.V.; Arena, J.; Rha, E.; Vranis, P.; Hitt, D.; Marsh-Armstrong, N.; Koliatsos, V.E. Evidence for Accelerated Tauopathy in the Retina of Transgenic P301S Tau Mice Exposed to Repetitive Mild Traumatic Brain Injury. Exp. Neurol. 2015, 273, 168–176. [Google Scholar] [CrossRef]
- Charlier, R.; Caspers, M.; Knaeps, S.; Mertens, E.; Lambrechts, D.; Lefevre, J.; Thomis, M. Limited Potential of Genetic Predisposition Scores to Predict Muscle Mass and Strength Performance in Flemish Caucasians between 19 and 73 Years of Age. Physiol. Genom. 2017, 49, 160–166. [Google Scholar] [CrossRef]
- Thomaes, T.; Thomis, M.; Onkelinx, S.; Goetschalckx, K.; Fagard, R.; Lambrechts, D.; Vanhees, L. Genetic Predisposition Scores Associate with Muscular Strength, Size, and Trainability. Med. Sci. Sports Exerc. 2013, 45, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Yvert, T.; Miyamoto-Mikami, E.; Murakami, H.; Miyachi, M.; Kawahara, T.; Fuku, N. Lack of Replication of Associations between Multiple Genetic Polymorphisms and Endurance Athlete Status in Japanese Population. Physiol. Rep. 2016, 4, e13003. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Roche, M.D.; Fredericson, M.; Dragoo, J.L.; Horton, B.H.; Avins, A.L.; Belanger, H.G.; Ioannidis, J.P.A.; Abrams, G.D. A Genome-Wide Association Study for Concussion Risk. Med. Sci. Sport. Exerc. 2020, 53, 704–711. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Gene Abbreviation and Polymorphism Identifier | Alleles | Relevant Effects Associated with TBI |
|---|---|---|---|
| Ankyrin repeat and kinase domain containing 1 | ANKK1 rs1800497 | A/G | The A allele has been associated with altered cognitive behavioural capacity via modulation of expression of D2 receptors. |
| Apolipoprotein E | APOE rs429358, rs7412 | ε2, ε3, ε4 | Affects repair and plasticity of the brain. APOE isoforms have differing effects on neurite extension, which can influence ability to recover post-concussion. |
| rs405509 | G/T | Associated with functional regulation of APOE transcription. | |
| Brain-derived neurotrophic factor | BDNF rs6265 | Val/Met (C/T) | Affects repair and plasticity of the brain via strengthening existing synaptic connections and modulating the creation of new synapses. |
| Catechol-O-methyltransferase | COMT rs4680 | Met/ Val (A/G) | Affects cognitive behavioural capacity post-concussion and could increase impulsivity and risk taking. |
| Microtubule-associated protein tau | MAPT rs10445337 | C/T | Affects repair and plasticity of the brain via modulation of microtubule formation, structural stabilisation of the neuronal axons and drives growth of neurites. |
| Endothelial nitric oxide synthase | NOS3 rs2070744 | C/T | Could affect severity of concussion and cognitive behavioural capacity post-concussion via modulating cerebral blood. |
| Gene Name | Gene Abbreviation | Polymorphism | Alleles | Genotype Score | Frequency in Elite Rugby Athletes (%) | Frequency in Non-Athletes (%) |
|---|---|---|---|---|---|---|
| Ankyrin repeat and kinase domain containing 1 | ANKK1 | rs1800497 | A/G | GG = 2, GA = 1, AA = 0 | GG = 65.2, GA = 31.0, AA = 3.8 | GG = 65.2, GA = 30.6, AA = 4.2 |
| Apolipoprotein E | APOE | rs429358 and rs7412 rs405509 | ε4+/ε4− G/T | 0 = ε4+, 2 = ε4− GG = 2, GT = 1, TT = 0 | ε4+ = 28.9, ε4− = 71.1 GG = 25.8, GT = 48.7, TT = 25.5 | ε4+ = 28.2, ε4− = 71.8 GG = 26.2, GT = 47.3, TT = 26.5 |
| Brain-derived neurotrophic factor antisense RNA | BDNF-AS | rs6265 | C/T | CC = 2, CT = 1, TT = 0 | CC = 67.5, CT = 28.9, TT = 3.6 | CC = 66.3, CT = 30.1, TT = 3.6 |
| Catechol-O-methyltransferase | COMT | rs4680 | A/G | AA = 2, GA = 1, GG = 0 | AA = 24.8, GA = 49.8, GG = 25.4 | AA = 30.2, GA = 47.4, GG =22.4 |
| Microtubule-associated protein tau | MAPT | rs10445337 | C/T | CC = 2, TC = 1, TT = 0 | CC = 4.7, TC = 35.7, TT = 59.6 | CC = 4.7, TC = 31.4, TT = 63.9 |
| Endothelial nitric oxide synthase | NOS3 | rs2070744 | C/T | TT = 2, TC = 1, CC = 0 | TT = 37.6, TC = 47.6, CC = 14.8 | TT = 38.7, TC = 44.3, CC = 17.0 |
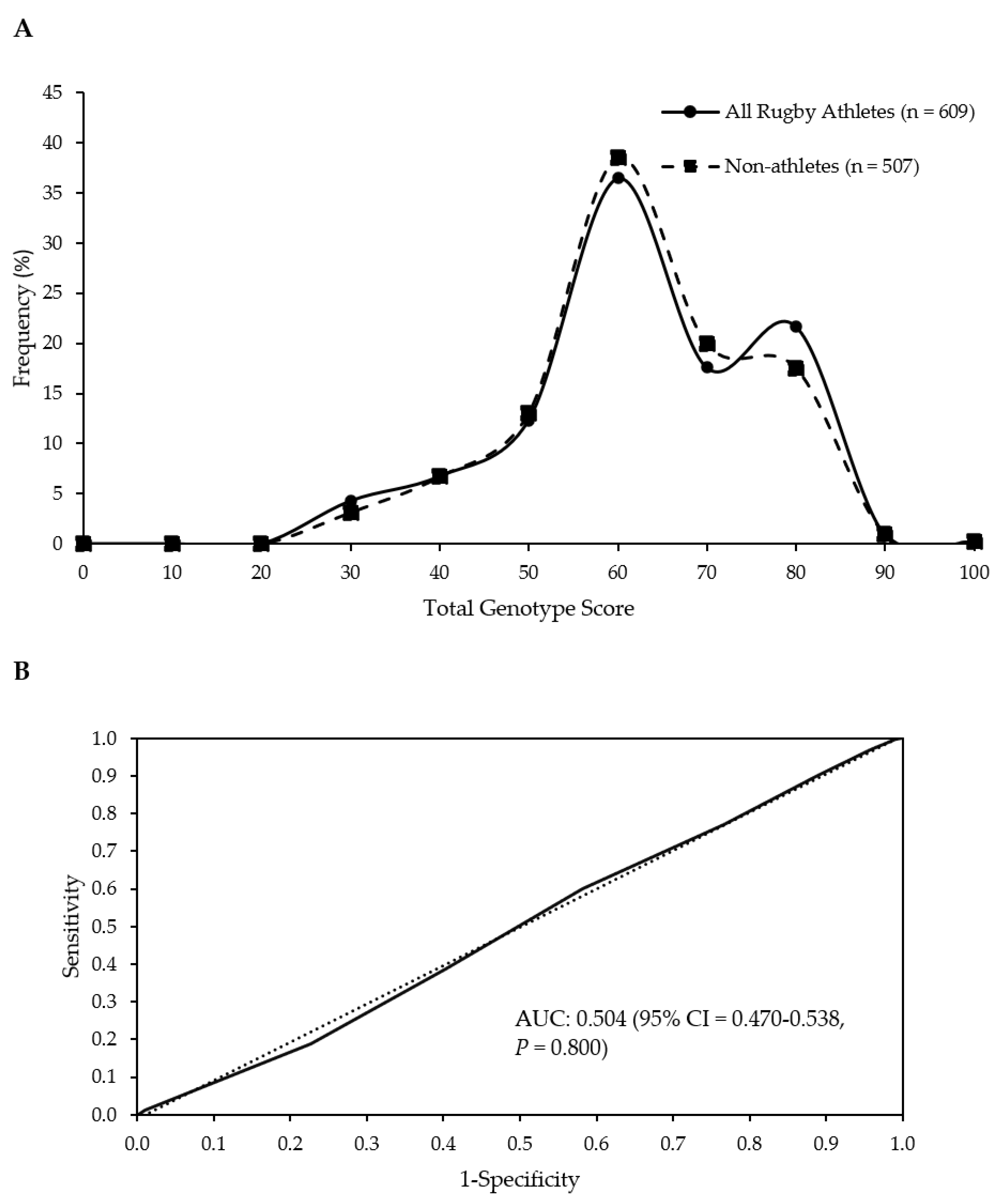
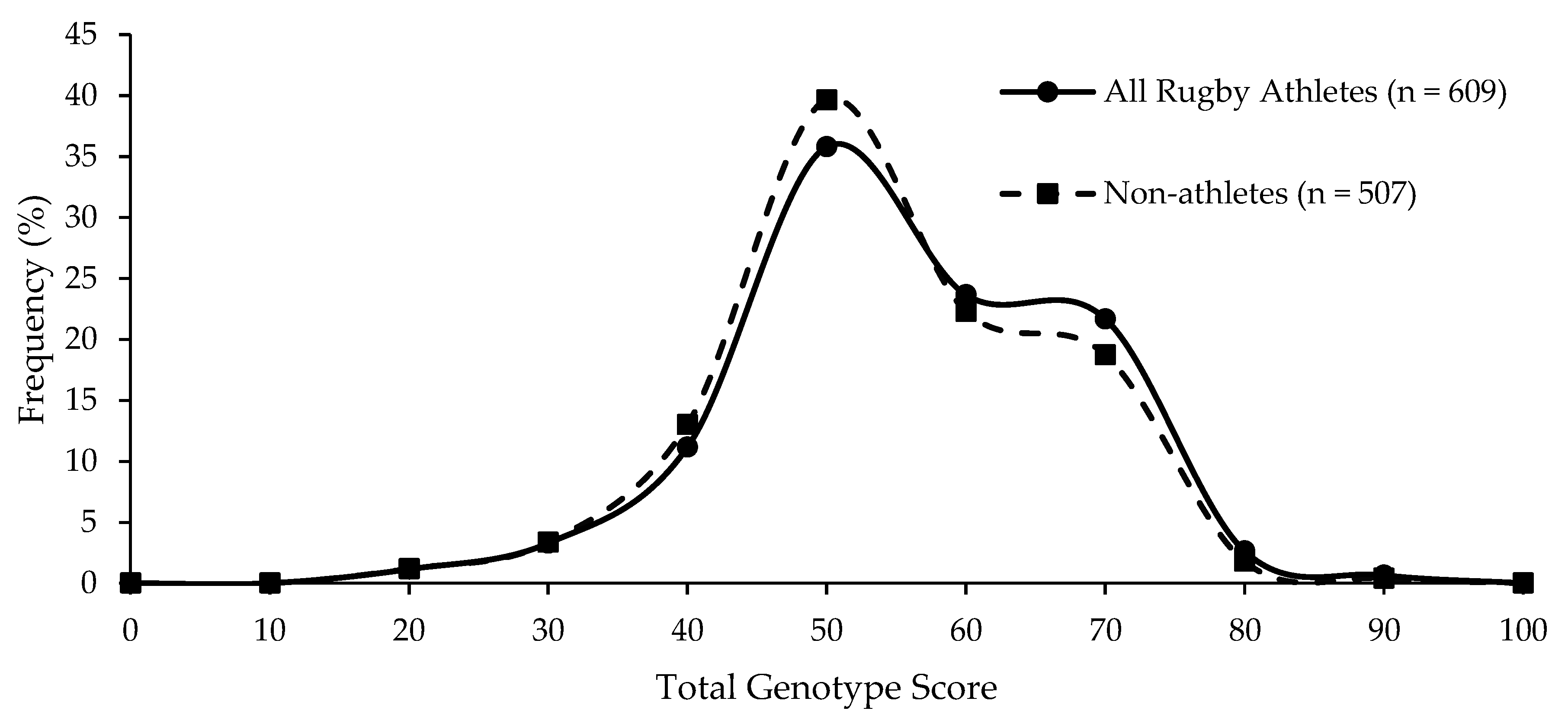
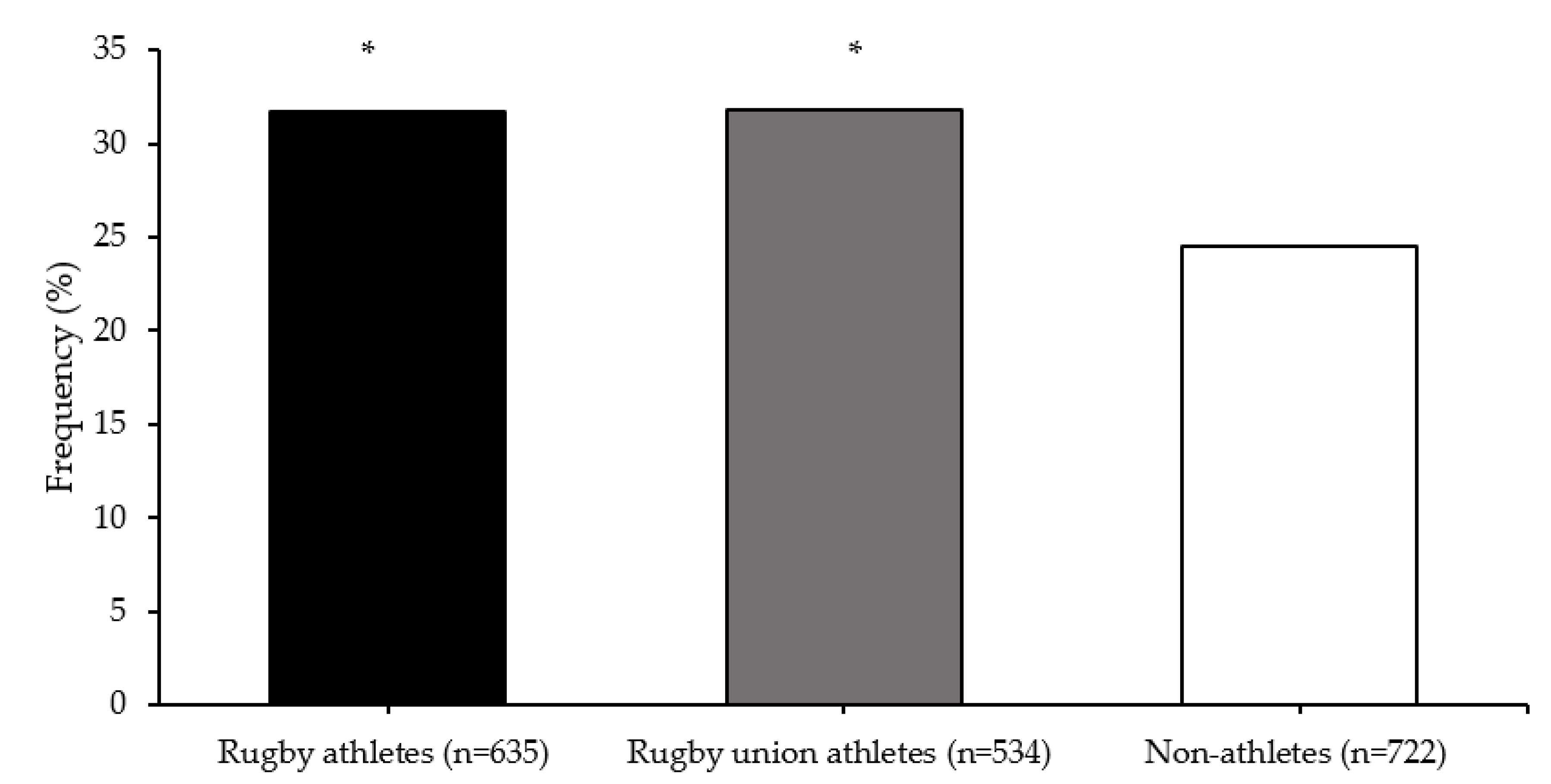
| Group | Mean (SD) TGS | Mean (SE) Kurtosis | p-Value Athlete Group vs. Non-Athletes | p-Value Top Quartile vs. Bottom Quartile TGS | ROC Curve Analysis AUC (95% CI) | p-Value AUC |
|---|---|---|---|---|---|---|
| Non-athletes | 56.4 (12.8) | −0.403 (0.217) | ||||
| All Rugby Athletes | 56.5 (13.6) | −0.506 (0.198) | 0.797 | 0.349 | 0.504 (0.470–0.538) | 0.800 |
| RU Athletes | 56.4 (13.4) | −0.490 (0.215) | 0.828 | 0.415 | 0.504 (0.468–0.539) | 0.830 |
| RL Athletes | 56.9 (14.7) | −0.617 (0.488) | 0.821 | 0.444 | 0.507 (0.440–0.575) | 0.823 |
| RU Forwards | 56.3 (13.3) | −0.384 (0.283) | 0.934 | 0.678 | 0.502 (0.460–0.544) | 0.935 |
| RU Backs | 56.5 (13.5) | −0.613 (0.328) | 0.769 | 0.326 | 0.507 (0.460–0.554) | 0.772 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antrobus, M.R.; Brazier, J.; Callus, P.C.; Herbert, A.J.; Stebbings, G.K.; Khanal, P.; Day, S.H.; Kilduff, L.P.; Bennett, M.A.; Erskine, R.M.; et al. Concussion-Associated Polygenic Profiles of Elite Male Rugby Athletes. Genes 2022, 13, 820. https://doi.org/10.3390/genes13050820
Antrobus MR, Brazier J, Callus PC, Herbert AJ, Stebbings GK, Khanal P, Day SH, Kilduff LP, Bennett MA, Erskine RM, et al. Concussion-Associated Polygenic Profiles of Elite Male Rugby Athletes. Genes. 2022; 13(5):820. https://doi.org/10.3390/genes13050820
Chicago/Turabian StyleAntrobus, Mark R., Jon Brazier, Peter C. Callus, Adam J. Herbert, Georgina K. Stebbings, Praval Khanal, Stephen H. Day, Liam P. Kilduff, Mark A. Bennett, Robert M. Erskine, and et al. 2022. "Concussion-Associated Polygenic Profiles of Elite Male Rugby Athletes" Genes 13, no. 5: 820. https://doi.org/10.3390/genes13050820
APA StyleAntrobus, M. R., Brazier, J., Callus, P. C., Herbert, A. J., Stebbings, G. K., Khanal, P., Day, S. H., Kilduff, L. P., Bennett, M. A., Erskine, R. M., Raleigh, S. M., Collins, M., Pitsiladis, Y. P., Heffernan, S. M., & Williams, A. G. (2022). Concussion-Associated Polygenic Profiles of Elite Male Rugby Athletes. Genes, 13(5), 820. https://doi.org/10.3390/genes13050820











