Supplementation of EGF, IGF-1, and Connexin 37 in IVM Medium Significantly Improved the Maturation of Bovine Oocytes and Vitrification of Their IVF Blastocysts
Abstract
1. Introduction
2. Materials and Methods
2.1. IVM of Bovine Oocytes
2.2. IVF of Oocytes
2.3. qRT-PCR of Candidate Genes in Oocytes and Blastocysts
2.4. Examination of Total Nuclear Cell per Blastocyst
2.5. Vitrification of Blastocysts
2.6. Analysis of TZP Intensity in Oocytes
2.7. GSH Assay in Oocytes
2.8. Analysis of ROS Level in Oocytes
2.9. ATP Content of Oocytes
2.10. Statistical Analysis
3. Results
3.1. Effect of EGF and IGF-1 on the Maturation and Developmental Ability of Bovine Oocytes
3.2. Effect of EGF and IGF-1 on the Maturation and Developmental Ability of Bovine Oocytes without Gonadotropins
3.3. Effect of Cx37 on the Maturation and Developmental Ability of Bovine Oocytes
3.4. Effect of the Combination Treatment of EGF, IGF-1, and Cx37 on TZPs in Bovine Oocytes
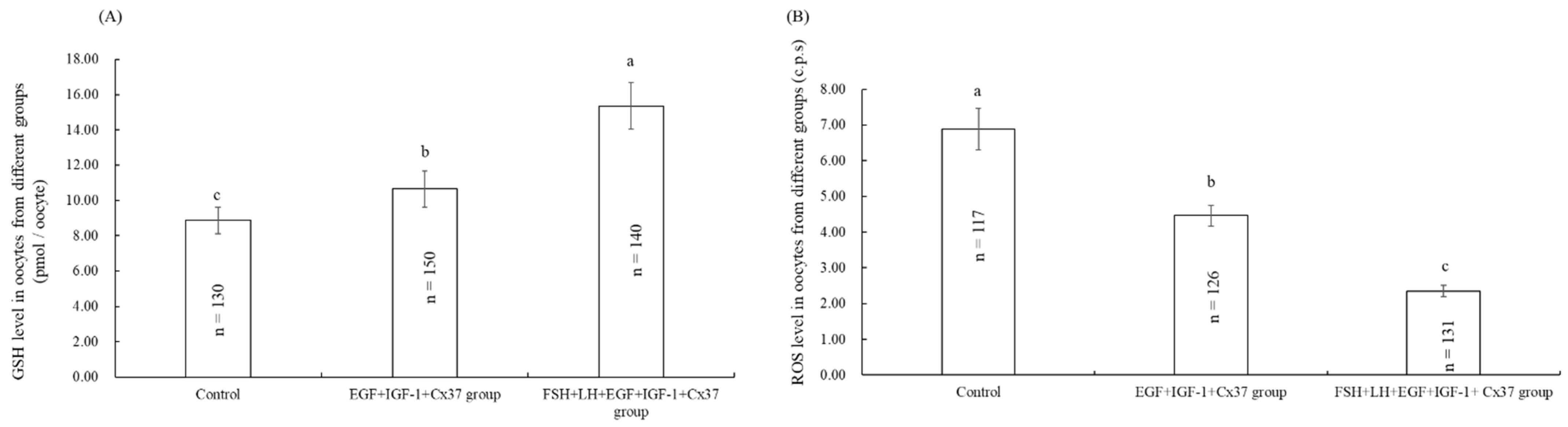
3.5. Effect of the Combination Treatment of EGF, IGF-1, and Cx37 on GSH, Reactive Oxygen Species (ROS) Level in Bovine Oocytes
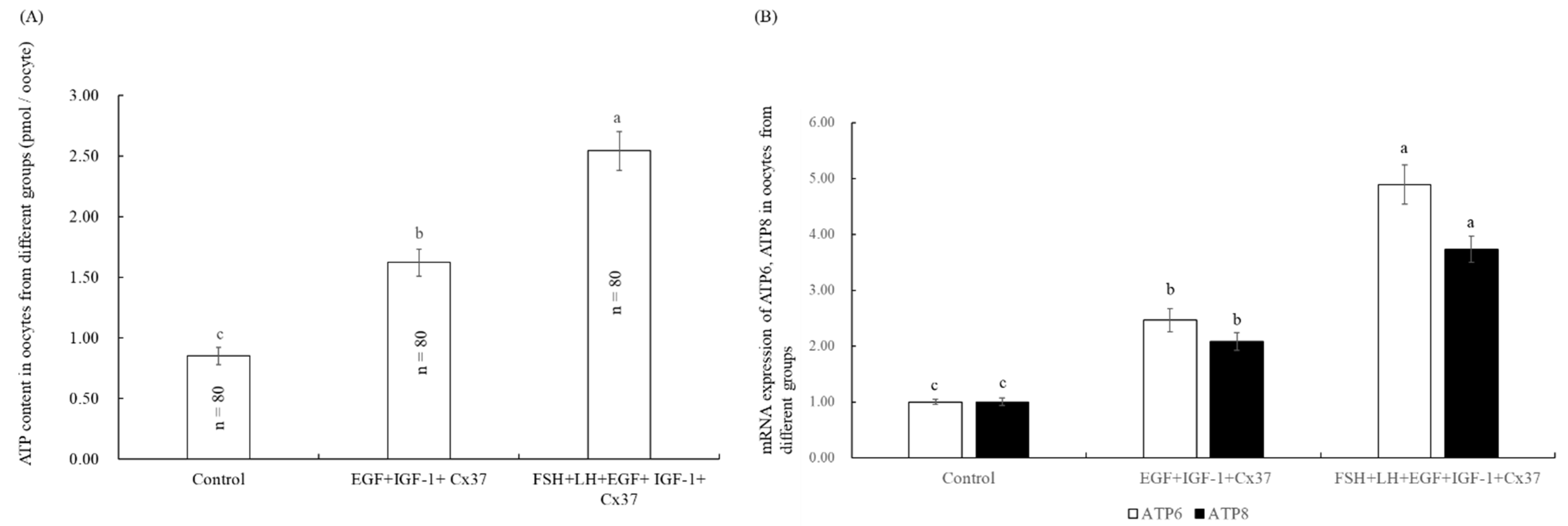
3.6. Effect of the Combination Treatment of EGF, IGF-1, and Cx37on Mitochondrial Function in Bovine Oocytes
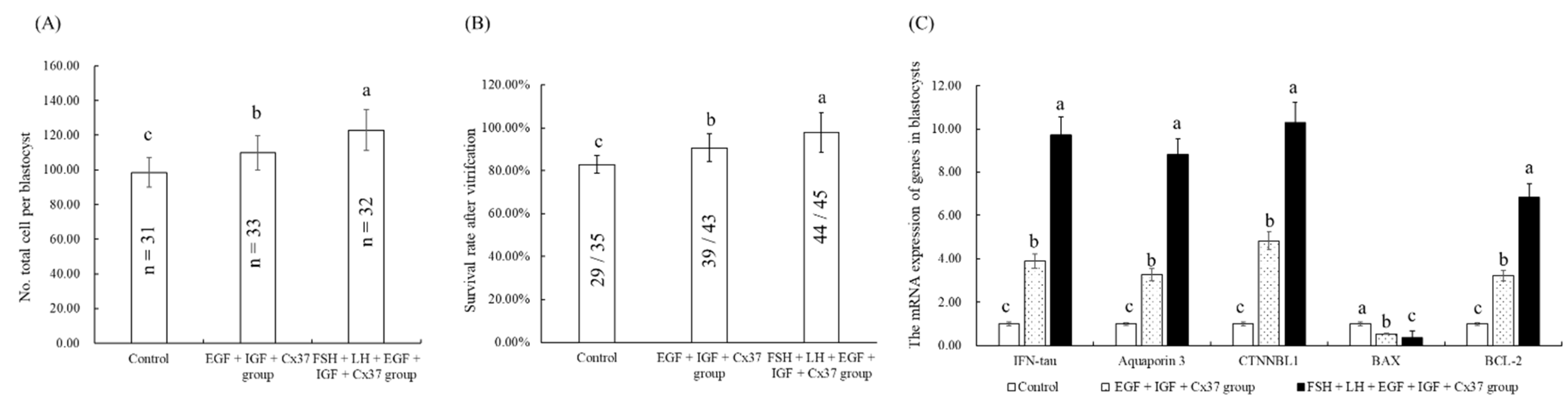
3.7. Effect of the Combination Treatment of EGF, IGF-1, and Cx37on the Maturation and Developmental Ability of Bovine Oocytes
3.8. Effect of the Combination Treatment of EGF, IGF-1, and Cx37on the Quality of IVF Blastocysts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, J.G.; Gilchrist, R.B. Pioneering contributions by Robert Edwards to oocyte in vitro maturation (IVM). Mol. Hum. Reprod. 2013, 19, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.D.; Tan, S.L. In vitro maturation of oocytes. Semin. Reprod. Med. 2005, 23, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Krisher, R.L. In vitro maturation (IVM) of porcine oocytes. Methods. Mol. Biol. 2012, 825, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Salilew-Wondim, D.; Schellander, K.; Hoelker, M.; Tesfaye, D. Oviductal, endometrial and embryonic gene expression patterns as molecular clues for pregnancy establishment. Anim. Reprod. Sci. 2012, 134, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Block, J.; Hansen, P.J.; Loureiro, B.; Bonilla, L. Improving post-transfer survival of bovine embryos produced in vitro: Actions of insulin-like growth factor-1, colony-stimulating factor-2 and hyaluronan. Theriogenology 2011, 76, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.G. Are minimal stimulation IVF and IVM set to replace routine IVF? Reprod. Biomed. Online 2007, 14, 267–270. [Google Scholar] [CrossRef]
- Gilchrist, R.; Smitz, J.; Thompson, J. Current status and future trends of the clinical practice of human oocyte in vitro maturation. Hum. Assist. Reprod. Technol. Future Trends Lab. Clin. Pract. 2011, 38, 186–198. [Google Scholar] [CrossRef]
- Son, W.-Y.; Henderson, S.; Cohen, Y.; Dahan, M.; Buckett, W. Immature Oocyte for Fertility Preservation. Front. Endocrinol. 2019, 10, 464. [Google Scholar] [CrossRef]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef]
- Lonergan, P.; Fair, T. Maturation of Oocytes in Vitro. Annu. Rev. Anim. Biosci. 2016, 4, 255–268. [Google Scholar] [CrossRef]
- Crozet, N.; Ahmed-Ali, M.; Dubos, M.P. Developmental competence of goat oocytes from follicles of different size categories following maturation, fertilization and culture in vitro. J. Reprod. Fertil. 1995, 103, 293–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romero, S.; Sánchez, F.; Lolicato, F.; Van Ranst, H.; Smitz, J. Immature Oocytes from Unprimed Juvenile Mice Become a Valuable Source for Embryo Production When Using C-Type Natriuretic Peptide as Essential Component of Culture Medium. Biol. Reprod. 2016, 95, 64. [Google Scholar] [CrossRef] [PubMed]
- Arias-Álvarez, M.; García-García, R.M.; López-Tello, J.; Rebollar, P.G.; Gutiérrez-Adán, A.; Lorenzo, P.L. In vivo and in vitro maturation of rabbit oocytes differently affects the gene expression profile, mitochondrial distribution, apoptosis and early embryo development. Reprod. Fertil. Dev. 2017, 29, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Crosier, A.E.; Farin, P.W.; Dykstra, M.J.; Alexander, J.E.; Farin, C.E. Ultrastructural morphometry of bovine blastocysts produced in vivo or in vitro. Biol. Reprod. 2001, 64, 1375–1385. [Google Scholar] [CrossRef]
- Walls, M.L.; Hunter, T.; Ryan, J.P.; Keelan, J.A.; Nathan, E.; Hart, R.J. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: A comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum. Reprod. 2015, 30, 88–96. [Google Scholar] [CrossRef]
- Sutton, M.L.; Gilchrist, R.B.; Thompson, J.G. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Hum. Reprod. Update 2003, 9, 35–48. [Google Scholar] [CrossRef]
- Soares, A.C.S.; Marques, K.N.G.; Bragança, L.G.M.; Lodde, V.; Luciano, A.M.; Buratini, J. Synchronization of germinal vesicle maturity improves efficacy of in vitro embryo production in Holstein cows. Theriogenology 2020, 154, 53–58. [Google Scholar] [CrossRef]
- Richani, D.; Gilchrist, R.B. The epidermal growth factor network: Role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Hernandez, E.R.; Resnick, C.E.; Svoboda, M.E.; Van Wyk, J.J.; Payne, D.W.; Adashi, E.Y. Somatomedin-C/insulin-like growth factor I as an enhancer of androgen biosynthesis by cultured rat ovarian cells. Endocrinology 1988, 122, 1603–1612. [Google Scholar] [CrossRef]
- Lorenzo, P.L.; Illera, M.J.; Illera, J.C.; Illera, M. Enhancement of cumulus expansion and nuclear maturation during bovine oocyte maturation in vitro by the addition of epidermal growth factor and insulin-like growth factor I. J. Reprod. Fertil. 1994, 101, 697–701. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, L.; Kozhevnikova, V.; Brusentsev, E.; Jänsch, S.; Amstislavsky, S.; Jewgenow, K. IGF-I Medium Supplementation Improves Singly Cultured Cat Oocyte Maturation and Embryo Development In Vitro. Animals 2021, 11, 1909. [Google Scholar] [CrossRef]
- Xie, L.; Tang, Q.; Yang, L.; Chen, L. Insulin-like growth factor I promotes oocyte maturation through increasing the expression and phosphorylation of epidermal growth factor receptor in the zebrafish ovary. Mol. Cell Endocrinol. 2016, 419, 198–207. [Google Scholar] [CrossRef]
- Niemann, H.; Wrenzycki, C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: Implications for subsequent development. Theriogenology 2000, 53, 21–34. [Google Scholar] [CrossRef]
- Richard, S.; Baltz, J.M. Prophase I arrest of mouse oocytes mediated by natriuretic peptide precursor C requires GJA1 (connexin-43) and GJA4 (connexin-37) gap junctions in the antral follicle and cumulus-oocyte complex. Biol. Reprod. 2014, 90, 137. [Google Scholar] [CrossRef]
- Fortune, J.E.; Hansel, W. Concentrations of steroids and gonadotropins in follicular fluid from normal heifers and heifers primed for superovulation. Biol. Reprod. 1985, 32, 1069–1079. [Google Scholar] [CrossRef]
- Combelles, C.M.; Carabatsos, M.J.; Kumar, T.R.; Matzuk, M.M.; Albertini, D.F. Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Mol. Reprod. Dev. 2004, 69, 347–355. [Google Scholar] [CrossRef]
- Buratini, J.; Soares, A.C.S.; Barros, R.G.; Dellaqua, T.T.; Lodde, V.; Franciosi, F.; Dal Canto, M.; Renzini, M.M.; Luciano, A.M. Physiological parameters related to oocyte nuclear differentiation for the improvement of IVM/IVF outcomes in women and cattle. Reprod. Fertil. Dev. 2021, 34, 27–35. [Google Scholar] [CrossRef]
- Keefer, C.L.; Stice, S.L.; Dobrinsky, J. Effect of follicle-stimulating hormone and luteinizing hormone during bovine in vitro maturation on development following in vitro fertilization and nuclear transfer. Mol. Reprod. Dev. 1993, 36, 469–474. [Google Scholar] [CrossRef]
- Akaki, Y.; Yoshioka, K.; Noguchi, M.; Hoshi, H.; Funahashi, H. Successful piglet production in a chemically defined system for in-vitro production of porcine embryos: Dibutyryl cyclic amp and epidermal growth factor-family peptides support in-vitro maturation of oocytes in the absence of gonadotropins. J. Reprod. Dev. 2009, 55, 446–453. [Google Scholar] [CrossRef]
- Redel, B.K.; Spate, L.D.; Yuan, Y.; Murphy, C.N.; Roberts, R.M.; Prather, R.S. Neither gonadotropin nor cumulus cell expansion is needed for the maturation of competent porcine oocytes in vitro†. Biol. Reprod. 2021, 105, 533–542. [Google Scholar] [CrossRef]
- Brackett, B.G.; Oliphant, G. Capacitation of rabbit spermatozoa in vitro. Biol. Reprod. 1975, 12, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrans, C.F., Jr.; First, N.L. Effect of free amino acids and vitamins on cleavage and developmental rate of bovine zygotes in vitro. J. Anim. Sci. 1994, 72, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Zhang, P.; Hao, H.; Du, W.; Pang, Y.; Zhao, S.; Zou, H.; Zhu, H.; Yu, W.; Li, S.; et al. The combination treatment of cholesterol-loaded methyl-β-cyclodextrin and methyl-β-cyclodextrin significantly improves the fertilization capacity of vitrified bovine oocytes by protecting fertilization protein JUNO. Reprod. Domest. Anim. 2021, 56, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Du, W.H.; Wang, D.; Hao, H.S.; Liu, Y.; Qin, T.; Zhu, H.B. Recovery of mitochondrial function and endogenous antioxidant systems in vitrified bovine oocytes during extended in vitro culture. Mol. Reprod. Dev. 2011, 78, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Spate, L.D.; Redel, B.K.; Tian, Y.; Zhou, J.; Prather, R.S.; Roberts, R.M. Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation. Proc. Natl. Acad. Sci. USA 2017, 114, E5796–E5804. [Google Scholar] [CrossRef]
- Ozawa, M.; Hirabayashi, M.; Kanai, Y. Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or in vitro. Reprod.-Camb. 2002, 124, 683–689. [Google Scholar] [CrossRef]
- Rahimi, G.; Isachenko, E.; Sauer, H.; Isachenko, V.; Wartenberg, M.; Hescheler, J.; Mallmann, P.; Nawroth, F. Effect of different vitrification protocols for human ovarian tissue on reactive oxygen species and apoptosis. Reprod. Fertil. Dev. 2003, 15, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J.; Davis, P.W.; Lee, J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum. Reprod. 1995, 10, 415–424. [Google Scholar] [CrossRef]
- Rieger, D.; Luciano, A.M.; Modina, S.; Pocar, P.; Lauria, A.; Gandolfi, F. The effects of epidermal growth factor and insulin-like growth factor I on the metabolic activity, nuclear maturation and subsequent development of cattle oocytes in vitro. J. Reprod. Fertil. 1998, 112, 123–130. [Google Scholar] [CrossRef][Green Version]
- Sakaguchi, M.; Dominko, T.; Yamauchi, N.; Leibfried-Rutledge, M.L.; Nagai, T.; First, N.L. Possible mechanism for acceleration of meiotic progression of bovine follicular oocytes by growth factors in vitro. Reprod.-Camb. 2002, 123, 135–142. [Google Scholar] [CrossRef]
- Purohit, G.N.; Brady, M.S.; Sharma, S.S. Influence of epidermal growth factor and insulin-like growth factor 1 on nuclear maturation and fertilization of buffalo cumulus-oocyte complexes in serum-free media and their subsequent development in vitro. Anim. Reprod. Sci. 2005, 87, 229–239. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Baxter, G.; Hogg, C.O.; Woad, K.J. Insulin-like growth factor (IGF) system in the oocyte and somatic cells of bovine preantral follicles. Reprod.-Camb. 2002, 123, 789–797. [Google Scholar] [CrossRef]
- Stoecklein, K.S.; Ortega, M.S.; Spate, L.D.; Murphy, C.N.; Prather, R.S. Improved cryopreservation of in vitro produced bovine embryos using FGF2, LIF, and IGF1. PLoS ONE 2021, 16, e0243727. [Google Scholar] [CrossRef]
- Gallaher, B.W.; Hille, R.; Raile, K.; Kiess, W. Apoptosis: Live or die-hard work either way! Horm. Metab. Res. 2001, 33, 511–519. [Google Scholar] [CrossRef]
- Alam, M.H.; Miyano, T. Interaction between growing oocytes and granulosa cells in vitro. Reprod. Med. Biol. 2020, 19, 13–23. [Google Scholar] [CrossRef]
- Winterhager, E.; Kidder, G.M. Gap junction connexins in female reproductive organs: Implications for women’s reproductive health. Hum. Reprod. Update 2015, 21, 340–352. [Google Scholar] [CrossRef]
- Gittens, J.E.; Kidder, G.M. Differential contributions of connexin37 and connexin43 to oogenesis revealed in chimeric reaggregated mouse ovaries. J. Cell Sci. 2005, 118, 5071–5078. [Google Scholar] [CrossRef]
- Simon, A.M.; Goodenough, D.A.; Li, E.; Paul, D.L. Female infertility in mice lacking connexin 37. Nature 1997, 385, 525–529. [Google Scholar] [CrossRef]
- Eppig, J.J. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays 1991, 13, 569–574. [Google Scholar] [CrossRef]
- Clarke, H.J. Regulation of germ cell development by intercellular signaling in the mammalian ovarian follicle. Wiley Interdiscip Rev. Dev. Biol. 2018, 7, e294. [Google Scholar] [CrossRef]
- Macaulay, A.D.; Gilbert, I.; Scantland, S.; Fournier, E.; Ashkar, F.; Bastien, A.; Saadi, H.A.; Gagné, D.; Sirard, M.A.; Khandjian, É.W.; et al. Cumulus Cell Transcripts Transit to the Bovine Oocyte in Preparation for Maturation. Biol. Reprod. 2016, 94, 16. [Google Scholar] [CrossRef]
- You, J.; Kim, J.; Lim, J.; Lee, E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology 2010, 74, 777–785. [Google Scholar] [CrossRef]
- de Matos, D.G.; Furnus, C.C.; Moses, D.F. Glutathione synthesis during in vitro maturation of bovine oocytes: Role of cumulus cells. Biol. Reprod. 1997, 57, 1420–1425. [Google Scholar] [CrossRef]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal. Res. 2016, 60, 132–141. [Google Scholar] [CrossRef]
- Kalay, Z.; Cevher, S.C. Oxidant and antioxidant events during epidermal growth factor therapy to cutaneous wound healing in rats. Int. Wound J. 2012, 9, 362–371. [Google Scholar] [CrossRef]
- Tang, X.; Liu, B.; Wang, X.; Yu, Q.; Fang, R. Epidermal Growth Factor, through Alleviating Oxidative Stress, Protect IPEC-J2 Cells from Lipopolysaccharides-Induced Apoptosis. Int. J. Mol. Sci. 2018, 19, 848. [Google Scholar] [CrossRef]
- Hsieh, R.H.; Au, H.K.; Yeh, T.S.; Chang, S.J.; Cheng, Y.F.; Tzeng, C.R. Decreased expression of mitochondrial genes in human unfertilized oocytes and arrested embryos. Fertil. Steril. 2004, 81 (Suppl. 1), 912–918. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Min, J.-T.; Du, W.-H.; Hao, H.-S.; Liu, Y.; Qin, T.; Wang, D.; Zhu, H.-B. Melatonin enhances the in vitro maturation and developmental potential of bovine oocytes denuded of the cumulus oophorus. Zygote 2015, 23, 525–536. [Google Scholar] [CrossRef]
- Contreras-Solís, I.; Catalá, M.; Soto-Heras, S.; Roura, M.; Paramio, M.T.; Izquierdo, D. Effect of follicle size on hormonal status of follicular fluid, oocyte ATP content, and in vitro embryo production in prepubertal sheep. Domest. Anim. Endocrinol. 2021, 75, 106582. [Google Scholar] [CrossRef]
- Nemerovsky, L.; Bar-Joseph, H.; Eldar-Boock, A.; Miller, I.; Ben-Ami, I.; Shalgi, R. Pigment epithelium-derived factor negates oxidative stress in mouse oocytes. FASEB J. 2021, 35, e21637. [Google Scholar] [CrossRef]
- Ascari, I.J.; Alves, N.G.; Jasmin, J.; Lima, R.R.; Quintão, C.C.R.; Oberlender, G.; Moraes, E.A.; Camargo, L.S.A. Addition of insulin-like growth factor I to the maturation medium of bovine oocytes subjected to heat shock: Effects on the production of reactive oxygen species, mitochondrial activity and oocyte competence. Domest. Anim. Endocrinol. 2017, 60, 50–60. [Google Scholar] [CrossRef]
- Yoon, J.D.; Hwang, S.U.; Kim, M.; Lee, G.; Jeon, Y.; Hyun, S.H. GDF8 enhances SOX2 expression and blastocyst total cell number in porcine IVF embryo development. Theriogenology 2019, 129, 70–76. [Google Scholar] [CrossRef]
- Andreas, E.; Reid, M.; Zhang, W.; Moley, K.H. The effect of maternal high-fat/high-sugar diet on offspring oocytes and early embryo development. Mol. Hum. Reprod. 2019, 25, 717–728. [Google Scholar] [CrossRef]
- Wooldridge, L.K.; Nardi, M.E.; Ealy, A.D. Zinc supplementation during in vitro embryo culture increases inner cell mass and total cell numbers in bovine blastocysts1. J. Anim. Sci. 2019, 97, 4946–4950. [Google Scholar] [CrossRef]
- Yousefian, I.; Zare-Shahneh, A.; Goodarzi, A.; Baghshahi, H.; Fouladi-Nashta, A.A. The effect of Tempo and MitoTEMPO on oocyte maturation and subsequent embryo development in bovine model. Theriogenology 2021, 176, 128–136. [Google Scholar] [CrossRef]
- Yao, N.; Wan, P.C.; Hao, Z.D.; Gao, F.F.; Yang, L.; Cui, M.S.; Wu, Y.; Liu, J.H.; Liu, S.; Chen, H.; et al. Expression of interferon-tau mRNA in bovine embryos derived from different procedures. Reprod. Domest. Anim. 2009, 44, 132–139. [Google Scholar] [CrossRef]
- Edashige, K. The movement of water and cryoprotectants across the plasma membrane of mammalian oocytes and embryos and its relevance to vitrification. J. Reprod. Dev. 2016, 62, 317–321. [Google Scholar] [CrossRef]
- Renault, T.T.; Dejean, L.M.; Manon, S. A brewing understanding of the regulation of Bax function by Bcl-xL and Bcl-2. Mech. Ageing Dev. 2017, 161, 201–210. [Google Scholar] [CrossRef]

| Gene | Primers (5′–3′) | Size (bp) | GenBank Accession No. | Annealing Temperature (°C) |
|---|---|---|---|---|
| IFN- tau | F: GCTCCAGAAGGATCAGGCTATC | 95 | AF238611 | 60 |
| R: TGTTCCAAGCAGCAGACGAGT | ||||
| CTNNBL1 | F: GTTCCTGCCTAATGCTGAGTTCC | 191 | NM_174637.3 | 60 |
| R: GGTCCGTAAGCCAAGAATGTCA | ||||
| AQP3 | F: AACCCTGCTGTGACCTTTGCTA | 230 | AF123316 | 60 |
| R: TTGACCATGTCCAAGTGTCCAG | ||||
| BAX | F: TTTCTGACGGCAACTTCAACTG | 83 | NM_173894.1 | 60 |
| R: GGTGCACAGGGCCTTGAG | ||||
| BCL-2 | F: TCAATTGTCGTGGCATCAAAA | 249 | NM_001077486.2 | 60 |
| R: CCCCCGACACCTGTTAGCTT | ||||
| ATP6 | F: GAACACCCACTCCACTAATCCCAAT | 147 | AF493542 | 60 |
| R: GTGCAAGTGTAGCTCCTCCGATT | ||||
| ATP8 | F: CACAATCCAGAACTGACACCAACAA | 129 | AF493542 | 60 |
| R: CGATAAGGGTTACGAGAGGGAGAC | ||||
| Β-ACTIN | F: TCCTGGGCATGGAATCCTG | 177 | NM_173979 | 60 |
| R: GGCGCGATGATCTTGATCTTC |
| Groups | No. COCs | No. Metaphase (M) II Oocytes | No. Cleavage Embryos | No. Blastocysts |
|---|---|---|---|---|
| FSH+LH+EGF | 187 | 159 (85.03 ± 5.04%) b | 128 (80.50 ± 7.21%) b | 39 (30.47 ± 2.71%) b |
| FSH+LH+IGF-1 | 194 | 163 (84.02 ± 4.93%) b | 132 (80.98 ± 6.19%) b | 41 (31.06 ± 2.63%) b |
| FSH+LH+EGF+IGF-1 | 210 | 194 (92.38 ± 2.18%) a | 174 (89.69 ± 4.71%) a | 71 (40.80 ± 3.59%) a |
| Control | 291 | 238 (81.79 ± 1.39%) b | 176 (73.95 ± 8.63%) b | 51 (28.98 ± 2.45%) b |
| Groups | No. COCs | No. MII Oocytes | No. Cleavage Embryos | No. Blastocysts |
|---|---|---|---|---|
| EGF+IGF-1 | 243 | 203 (83.54 ± 4.92%) a | 143 (70.44 ± 9.83%) a | 44 (30.77 ± 3.09%) a |
| EGF | 251 | 172 (68.53 ± 5.89%) b | 93 (54.07 ± 4.34%) b | 18 (19.35 ± 1.67%) b |
| IGF-1 | 288 | 200 (69.44 ± 8.44%) b | 110 (55.00 ± 5.89%) b | 23 (20.91 ± 2.39%) b |
| -FSH-LH | 371 | 208 (56.06 ± 5.23%) c | 94 (45.19 ± 4.47%) c | 14 (14.89 ± 1.93%) c |
| Control | 202 | 162 (80.20 ± 6.81%) a | 122 (75.31 ± 5.17%) a | 38 (31.15 ± 2.68%) a |
| Groups | No. Cocs | No. MII Oocytes | No. Cleavage Embryos | No. Blastocysts |
|---|---|---|---|---|
| 12.5 μg/mL Cx37 | 102 | 84 (82.35 ± 7.82%) a | 65 (77.38 ± 6.91%) b | 19 (29.23 ± 2.13%) b |
| 25 μg/mL Cx37 | 116 | 96 (82.76 ± 7.39%) a | 83 (86.46 ± 8.08%) a | 32 (38.55 ± 2.59%) a |
| 50 μg/mL Cx37 | 105 | 86 (81.90 ± 7.13%) a | 68 (79.07 ± 7.12%) b | 21 (30.88 ± 2.79%) b |
| Control | 94 | 76 (80.85 ± 6.34%) a | 58 (76.32 ± 4.07%) b | 17 (29.31 ± 2.62%) b |
| Groups | No. COCs | No. MII Oocytes | No. Cleavage Embryos | No. Blastocysts |
|---|---|---|---|---|
| EGF+ IGF-1+Cx37 | 350 | 306 (87.43 ± 6.86%) b | 250 (81.70 ± 7.65%) b | 100 (40.00 ± 3.87%) b |
| FSH+LH+EGF+ IGF-1+Cx37 | 279 | 262 (93.91 ± 7.84%) a | 237 (90.46 ± 8.28%) a | 120 (50.63 ± 4.75%) a |
| Control | 383 | 310 (80.94 ± 6.64%) c | 234 (75.48 ± 6.82%) c | 73 (31.20 ± 3.05%) c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Yang, Y.; Hao, H.; Du, W.; Pang, Y.; Zhao, S.; Zou, H.; Zhu, H.; Zhang, P.; Zhao, X. Supplementation of EGF, IGF-1, and Connexin 37 in IVM Medium Significantly Improved the Maturation of Bovine Oocytes and Vitrification of Their IVF Blastocysts. Genes 2022, 13, 805. https://doi.org/10.3390/genes13050805
Yang S, Yang Y, Hao H, Du W, Pang Y, Zhao S, Zou H, Zhu H, Zhang P, Zhao X. Supplementation of EGF, IGF-1, and Connexin 37 in IVM Medium Significantly Improved the Maturation of Bovine Oocytes and Vitrification of Their IVF Blastocysts. Genes. 2022; 13(5):805. https://doi.org/10.3390/genes13050805
Chicago/Turabian StyleYang, Sha, Yuze Yang, Haisheng Hao, Weihua Du, Yunwei Pang, Shanjiang Zhao, Huiying Zou, Huabin Zhu, Peipei Zhang, and Xueming Zhao. 2022. "Supplementation of EGF, IGF-1, and Connexin 37 in IVM Medium Significantly Improved the Maturation of Bovine Oocytes and Vitrification of Their IVF Blastocysts" Genes 13, no. 5: 805. https://doi.org/10.3390/genes13050805
APA StyleYang, S., Yang, Y., Hao, H., Du, W., Pang, Y., Zhao, S., Zou, H., Zhu, H., Zhang, P., & Zhao, X. (2022). Supplementation of EGF, IGF-1, and Connexin 37 in IVM Medium Significantly Improved the Maturation of Bovine Oocytes and Vitrification of Their IVF Blastocysts. Genes, 13(5), 805. https://doi.org/10.3390/genes13050805






